Abstract
Dendritic cells (DCs) play a vital role in initiating robust immunity against pathogens as well as maintaining immunological tolerance to self antigens. However, the intracellular signaling networks that program DCs to become tolerogenic remain unknown. We report here that the Wnt–β-catenin signaling in intestinal dendritic cells regulates the balance between inflammatory versus regulatory responses in the gut. β-catenin in intestinal dendritic cells was required for the expression of anti-inflammatory mediators such as retinoic acid–metabolizing enzymes, interleukin-10, and transforming growth factor–β, and the stimulation of regulatory T cell induction while suppressing inflammatory effector T cells. Furthermore, ablation of β-catenin expression in DCs enhanced inflammatory responses and disease in a mouse model of inflammatory bowel disease. Thus, β-catenin signaling programs DCs to a tolerogenic state, limiting the inflammatory response.
A central question in immunology is how the immune system launches robust immunity against invading pathogens maintaining tolerance to self antigens. This particular importance in the intestine because trillions of commensal microorganisms and antigens that confront the intestinal immune every day. Antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages are specialized immune cells that play a vital role in stimulating immune responses (1, 2). Recent evidence suggests that DCs are also critical in suppressing immune responses, through the generation of T regulatory cells (Treg cells) (2, 3). DCs express several pathogen-recognition receptors (PRRs), which are capable of sensing highly conserved structural motifs in pathogens (4–6) and initiating downstream signaling cascades that are important for controlling the innate and adaptive immune responses (2, 7). Triggering PRRs on DCs can activate DCs and determine the types of cytokines they produce. Such cytokines regulate the differentiation fate of naïve CD4+ T cells into inflammatory cells [T helper 1 (TH1) or TH17] or Treg cells, regulating the balance between immunity and tolerance. In the intestine, emerging evidence indicates that DCs and macrophages play a pivotal role in mediating mucosal tolerance against commensals and dietary antigens while mounting inflammatory responses against harmful pathogens (8–12). The signaling pathways that program DCs into a tolerogenic or inflammatory state, however, are poorly understood.
To address this, we analyzed gene expression profiles using microarray analysis of purified intestinal lamina propria DCs (LP-DCs) and compared it with those of splenic DCs (spl-DCs) from mice (13). Several Wnt-ligands were up-regulated in LP-DCs as compared with spl-DCs. Consistent with this, reverse transcriptase polymerase chain reaction (RT-PCR) analysis showed that LP-DCs constitutively expressed several Wnt-ligands as compared with that of spl-DCs in the steady state (fig. S1). β-catenin, a central component in Wnt-signaling, is widely expressed in hematopoietic stem cells, macrophages, DCs, and lymphocytes (14). Furthermore, the Wnt–β-catenin pathway has been implicated in the differentiation of DCs from hematopoietic stem cells (15, 16).
Activation of Wnt-Frizzled (fzd) receptor signaling causes translocation of β-catenin from the cytoplasm to the nucleus, where it interacts with T cell factor/lymphoid enhancer factor (TCF/LEF) family members and regulates transcription of several target genes (14, 17). Thus, we assessed whether the β-catenin pathway was active in LP-DCs using the TCF/LEF–lacZ reporter mice (18). In contrast to spl-DCs, LP-DCs from TCF-reporter mice showed strong β-galactosidase expression, suggesting that β-catenin signaling pathway is constitutively active in intestinal DCs (fig. S2). Apart from Wnt signaling, disruption of homotypic E-cadherin interactions in DCs results in β-catenin activation and impairs their immune stimulatory capacity (19). Whether the constitutive β-catenin signaling in intestinal DCs programs them to induce tolerogenic responses, however, is unknown.
To directly assess the role of β-catenin specifically in DC function, we crossed floxed β-catenin allele mice (β-catfl/fl) (20) with transgenic mice (CD11c-cre) expressing the cre enzyme under the control of the CD11c promoter (21). This specifically abrogated β-catenin expression in DCs (fig. S3A). Distinct subsets of intestinal DCs and macrophages can be distinguished by the expression patterns of CD11c and CD11b (CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c− CD11b+ macrophages) (11). β-catenin was highly expressed and constitutively active in LP-DC subsets and LP-macrophages in the small and large intestine (Fig. 1A and fig. S3B). In β-catDC−/− mice, β-catenin expression was abrogated in the CD11c+CD11b− and CD11c+CD11b+ DCs but only partially abrogated in CD11c− CD11b+ macrophages (fig S3B).
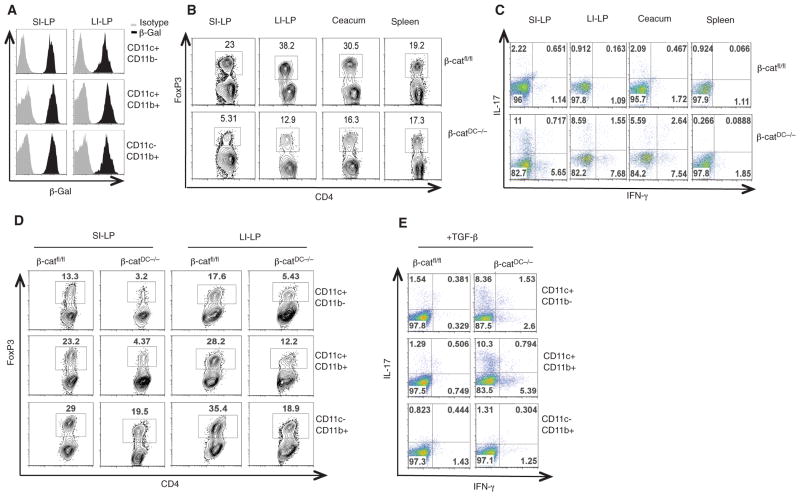
Fig. 1.
β-catenin signaling is constitutively active in intestinal APCs and regulates the induction of Treg cells and effector T cells. (A) Expression of β-galactosidase (representing β-catenin activity) by intestinal DCs and macrophages from SI-LP and LI-LP of TCF-reporter mice. (B and C) Fluorescence-activated cell sorting (FACS) plots representing percentages of CD4+ T cells positive for FoxP3 (Treg cells), IL-17 (TH17), and IFN-γ (TH1) isolated from SI-LP, LI-LP, ceacum, and spleen of β-catfl/fl and β-catDC−/− mice. (D and E) Intracellular expression of FoxP3, IL-17, and IFN-γ in naïve CD4+OT-II T cells stimulated to differentiate in vitro by intestinal APCs (CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c− CD11b+ macrophages) isolated from β-catfl/fl or β-catDC−/− mice, in the presence of TGF-β (1 ng/ml). Numbers in FACS plots represent percentage of cells positive for the indicated protein. Data are from one experiment representative of three.
We next determined whether β-catenin signaling in DCs is critical for intestinal homeostasis. We compared the frequencies of Treg cells and TH17/TH1 cells in the intestine of β-catDC−/− and β-catfl/fl mice because intestinal DCs and macrophages play an important role in the induction of these subsets (10–12, 22). β-catDC−/− mice had lower frequencies of Treg cells in the small intestine (SI)–LP, the large intestine (LI)–LP, and caecum, but not the spleen, as compared with that of β-catfl/fl mice (Fig. 1B and fig S4A). To determine whether the reduced frequencies of Treg cells observed in the LP of β-catDC−/− mice was due to increased frequencies of effector CD4 cells, we examined the frequencies of TH1 and TH17 in the intestine and periphery. We observed higher frequencies of TH17 and TH1 cells in the SI-LP and LI-LP but not in the spleen of β-catDC−/− mice as compared with that of the β-catfl/fl mice (Fig. 1C and fig. S4, B and C). Consistent with this, CD4+ T cells isolated from SI-LP and LI-LP of β-catDC−/− mice showed elevated expression of the TH17 cell–associated mRNAs interleukin (IL)–17, IL-21, Rorγt, and the TH1 cell–associated mRNA interferon (IFN)–γ (23, 24) as compared with the CD4+ T cells isolated from β-catfl/fl mice (fig. S5). Thus, β-catenin signaling in intestinal DCs is critical in maintaining the balance between Treg cells and CD4+ T effector populations.
Intestinal DCs and macrophages can convert naïve T cells into Treg cells that express the transcription factor Foxp3 and induce effector T cell differentiation (10–12, 22, 25). We thus assessed whether β-catenin signaling in LP-DCs and LP-macrophages is critical for inducing Treg, TH1, and TH17 differentiation of naïve CD4+ T cells in vitro using intestinal APCs (CD11c+CD11b− and CD11c+CD11b+ DCs and CD11b+ macrophages) isolated from β-catfl/fl or β-catDC−/− mice. In the absence or presence of TGF-β, a cytokine important for both Treg and TH17 differentiation, LP-DC subsets from β-catDC−/− were less potent in inducing Treg differentiation as measured with FoxP3 expression, but enhanced TH17 and TH1 cell differentiation as compared with that of DCs from β-catfl/fl mice (Fig. 1, D and E, and figs. S6 and S7). The ability of LP-DCs to induce Treg cells is chiefly mediated by a specialized subset of CD103+ DCs (fig. S8 and S9). Similarly, LP-macrophages of β-catDC−/− mice were somewhat less potent in inducing Treg cells as compared with the β-catfl/fl LP-macrophages (Fig. 1D), which is consistent with the partial deletion of β-catenin in macrophages. The absence of β-catenin in DCs did not completely abrogate their ability to induce Treg cells, suggesting that additional pathways are involved in programming DCs to the tolerogenic state. Thus, β-catenin signaling in DCs is required to induce Treg cells and suppress TH1/TH17 responses.
We next explored the molecular mechanisms by which β-catenin signaling in DCs promoted Treg differentiation and suppressed TH1/TH17 cell differentiation. Retinoic acid (RA, a vitamin A metabolite), IL-10, and TGF-β produced by the intestinal APCs and intestinal epithelial cells are critical for the induction of Treg cells (8, 10, 12, 26). Aldh1a1 and Aldh1a2 encode enzymes that catalyze the conversion of retinal to RA. Furthermore, Toll-like receptor (TLR)–mediated signaling in DCs up-regulates Raldh enzymes and promotes RA production (27). Thus, we assessed the expression levels of vitamin A–metabolizing genes by means of RT-PCR and flow cytometry in APC subsets purified from the LP of β-catfl/fl and β-catDC−/− mice. We observed that LP-DCs and LP-macrophages of β-catDC−/− mice expressed much lower amounts of Aldh1a1 and Aldh1a2 mRNA in the SI (Fig. 2A) and LI (fig S10A) as compared with those of β-catfl/fl LP-DCs and LP-macrophages. The relative expression of Raldh protein was similar to that observed with mRNA (Fig. 2B and fig S10B). Because β-catDC−/− LP-DCs had reduced levels of vitamin A–metabolizing enzymes, we assessed whether addition of exogenous RA can rescue their defect in Treg induction. Addition of exogenous RA to β-catDC−/− LP-DCs did indeed enhance their capacity to induce Treg cells as well as β-catfl/fl DCs (fig S11). Thus, β-catenin–mediated signaling promotes Treg induction through the expression of vitamin A–metabolizing enzymes in DCs.
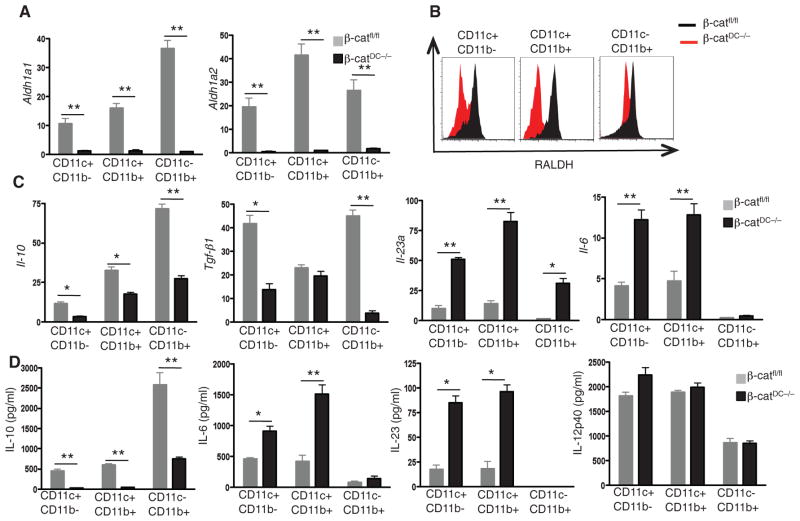
Fig. 2.
β-catenin signaling in intestinal DCs promotes the expression of Raldh and suppresses the expression of proinflammatory cytokines. (A) Expression of Aldh1a1 and Aldh1a2 mRNA in CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c−CD11b+ macrophages isolated from SI-LP of β-catfl/fl or β-catDC−/− mice. (B) Expression of Raldh protein by CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c− CD11b+ macrophages isolated from SI-LP of β-catfl/fl or β-catDC−/− mice, as assessed by in-tracellular staining and flow cytometry. (C) Expression of Il-10, Tgf-β1, Il-23a, and Il-6 mRNAs in CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c−CD11b+ macrophages isolated from SI-LP of β-catfl/fl or β-catDC−/− mice. (D) Cytokine concentrations in the supernatants of CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c−CD11b+ macrophages isolated from SI-LP of β-catfl/fl or β-catDC−/− mice after 24 hours. *P < 0.05; **P < 0.005. Error bars indicate mean ± SEM. Data are representative of three experiments.
Cytokines such as IL-6 and TGF-β promote the differentiation of TH17 cells, whereas IL-23 is thought to regulate the expansion and survival of TH17 cells (24). Thus, we assessed the expression of various pro-inflammatory and anti-inflammatory cytokines in LP-DC subsets isolated from β-catfl/fl and β-catDC−/− mice. β-catDC−/− LP-DCs had higher mRNA levels of the pro-inflammatory cytokines IL-23a and IL-6 and lower mRNA levels of the anti-inflammatory cytokines IL-10 and TGF-β, as compared with that of β-catfl/fl LP-DCs, from the SI and LI (Fig. 2C and fig S10C). Consistent with these results, β-catDC−/− LP-DCs produced higher amounts of IL-6 and IL-23 and lower amounts of IL-10 as compared with that of β-catfl/fl LP-DCs under steady state in both the SI and LI (Fig. 2D and fig. S10D). Thus, β-catenin signaling in LP-DCs is critical for the induction of anti-inflammatory cytokines, which are crucial for the induction of Treg cells and suppression of TH1 and TH17 responses.
Intestinal commensals play an important role in the maintenance of TH17 cells (25, 28, 29), and commensal DNA limits the induction of Treg cells in the gut (30). Moreover, intestinal DCs can directly take up bacteria and are thus constantly exposed to various pathogen-associated molecular patterns. Thus, we determined whether the enhanced frequencies of TH17 and TH1 cells in the β-catDC−/− mice were dependent on the microbiota by treating the mice with an antibiotic cocktail. In β-catfl/fl mice, antibiotic treatment resulted in modestly enhanced frequencies of Treg cells in the LI-LP (fig. S12A). In contrast, antibiotic treatment of β-catDC−/− mice failed to enhance Treg cells in the LI-LP (fig. S12A) but did enhance twofold their frequency in the SI-LP (Fig. 3A). Even with antibiotic treatment, there was a marked reduction in the frequency of Treg cells in β-catDC−/− mice, relative to β-catfl/fl mice, demonstrating that commensals were not essential for β-catenin activity (Fig. 3C and fig. S12C) and for the β-catenin–mediated programming of tolerogenic DCs. We also observed a decrease in the frequency of TH17 cells in the LP of β-catDC−/− and β-catfl/fl mice after antibiotic treatment (Fig. 3B and fig. S12B). Again, even with antibiotic treatment there was a markedly enhanced frequency of TH17 cells in β-catDC−/− mice relative to β-catfl/fl mice (Fig. 3B and fig. S12B), suggesting that commensals are not essential for β-catenin signaling–mediated programming of DCs to induce Treg cells and suppress TH17 responses. Furthermore, in the absence of β-catenin antibiotic treatment resulted in slightly enhanced Treg cells and diminished TH17 responses (Fig. 3, A and B), suggesting that additional β-catenin–independent pathways might be involved in regulating induction of Treg cells and TH17 by DCs.
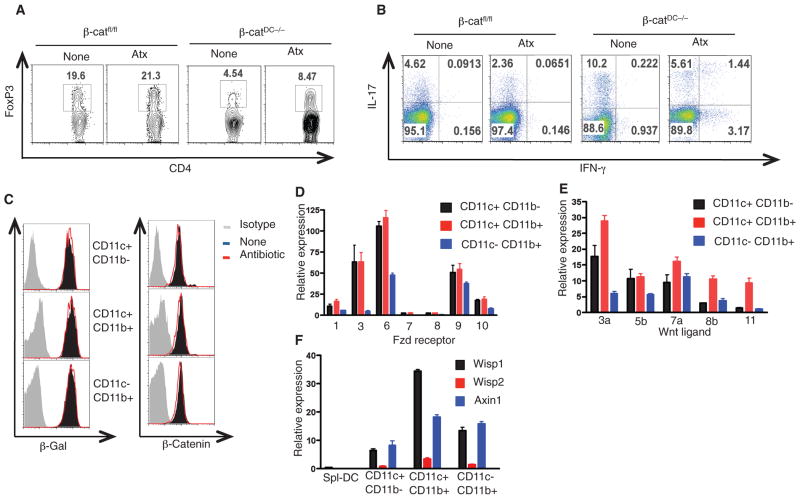
Fig. 3.
β-catenin activation in intestinal DCs is independent of commensals and induced by Wnt-signaling. (A and B) FACS plots representing the percentage of CD4+ T cells that express (A) FoxP3, IL-17, and (B) IFN-γ isolated from SI-LP of β-catfl/fl or β-catDC−/− mice treated with (Atx) or without (None) antibiotics. (C) FACS plots showing the intracellular expression levels of β-galactosidase or β-catenin protein in CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c−CD11b+ macrophages isolated from SI-LP of TCF-reporter mice treated with or without antibiotics. (D and E) mRNA expression of (D) Fzd receptors and (E) Wnt ligands in CD11c+CD11b and CD11c+CD11b+ DCs and CD11c CD11b+ macrophages isolated from SI-LP of β-catfl/fl mice. (F) mRNA expression levels of Wnt–β-catenin target genes Wisp1, Wisp2, and Axin1 in CD11c+CD11b− and CD11c+CD11b+ DCs and CD11c−CD11b+ macrophages isolated from SI-LP of β-catfl/fl mice. Error bars indicate mean ± SEM. Data are from one experiment representative of three.
We next assessed by means of RT-PCR the expression of Wnt ligands and Fzd receptors in intestinal APCs because Wnt-signaling activates β-catenin (17). DCs and macrophage subsets from the SI-LP and LI-LP expressed high amounts of various Fzd receptors and Wnt ligands (Fig. 3, D and E, and fig. S12, D and E). To determine whether Fzd-Wnt receptor signaling is active in intestinal DCs and macrophages, we evaluated the expression levels of several Wnt target genes—specifically Wisp1, Wisp2, and Axin1—that are dependent on β-catenin. Compared with spl-DCs, LP-DCs expressed substantially more Wisp1 and Axin1 mRNA (Fig. 3F and fig. S12F). Next, we tested whether activation of the β-catenin pathway is sufficient to promote Treg differentiation in vitro. In order to test this, we used LiCl treatment to activate β-catenin (fig S13A) (31). Compared with untreated DCs, LiCl-treated DCs induced greater frequencies of Treg cells (fig S13B). Thus, Wnt-mediated signaling activates β-catenin in intestinal DCs and programs them to induce Treg cells.
Lastly, we determined what impact β-catenin deficiency in DCs might have on the onset of autoimmunity in a mouse model of inflammatory bowel disease (IBD) because β-catDC−/− LP-DCs more efficiently promote inflammatory TH17 and TH1 effector cells, which are both implicated in IBD (32, 33) and experimental autoimmune encephalomyelitis pathogenesis (24). Dextran sulfate sodium (DSS) treatment induced drastic body weight loss, which is a characteristic of severe intestinal inflammation, in β-catDC−/− mice relative to β-catfl/fl mice (Fig. 4A). Furthermore, T cells isolated from ceacum and LI-LP of β-catDC−/− mice treated with DSS had higher frequencies of TH17 and TH1 cells and lower frequencies of Treg cells (Fig. 4B and fig. S14) as compared with that of T cells isolated from the colon of β-catfl/fl controls. Consistent with this, histological analyses showed increases in inflammatory cell infiltration, edema, epithelial cell hyperplasia, and loss of goblet cells in the colon of β-catDC−/− mice as compared with β-catfl/fl mice (Fig. 4C). Thus, deletion of β-catenin in DCs induces a severe inflammatory response to enteric bacteria upon DSS treatment. Collectively, this observation suggests that β-catenin signaling in DCs is critical in the regulation of intestinal homeostasis and tolerance. In addition to β-catenin signaling, recent studies have shown that indole-amine 2,3-dioxygenase (IDO) expressed by gut DCs promotes tolerogenic response (34), whereas CD11c+ E-cadherin+ DCs promotes inflammatory response (35). However, the role of β-catenin in the regulation of IDO or CD11c+ E-cadherin+ DCs is currently unknown.
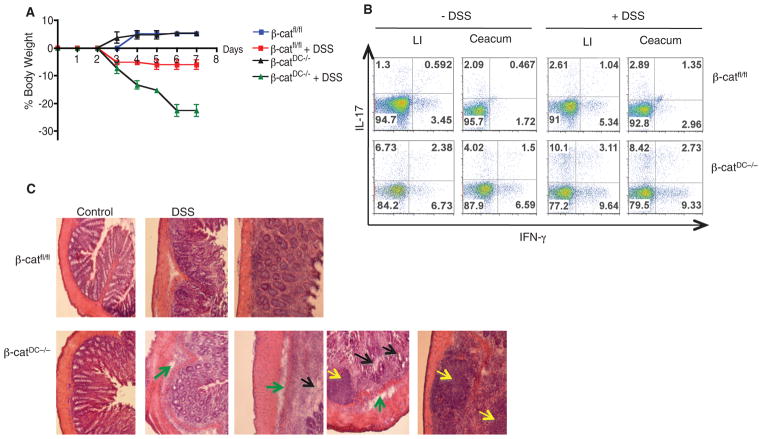
Fig. 4.
β-catenin signaling in LP-DCs regulates intestinal homeostasis. (A) Percent body weight of β-catfl/fl or β-catDC−/− mice treated with 2% DSS for 7 days. Error bars indicate mean ± SD. (B) FACS plot representing percentage of CD4+ cells positive for IL-17 and IFN-γ isolated on day 8, from Ceacum and LI-LP of β-catfl/fl or β-catDC−/− mice treated with DSS for 6 days. Data are from one experiment representative of three. (C) Histopathological changes in colon tissue from β-catfl/fl or β-catDC−/− mice treated with or without 2% DSS treatment for 7 days. Areas of interest are infiltrations, edema (yellow arrows), globlet cells (black arrows), and basement membrane (green arrows). Images are 10× original magnification.
In summary, we have demonstrated that a signaling pathway involving β-catenin in DCs regulates the balance between inflammatory versus regulatory responses in the intestine. The present data demonstrate that β-catenin signaling in DCs is critical for maintaining DCs in tolerogenic state, via induction of various anti-inflammatory factors such as RA, IL-10, and TGF-β. Strategies that can activate β-catenin signaling specifically in DCs may be attractive means of controlling autoimmune diseases such as IBD via the induction of Treg cells and concomitant suppression of inflammatory effector T cells.
Supplementary Material
Acknowledgments
We thank S.A. Mertens and L. Bronner for assistance with cell sorting and D. Levesque (Emory Vaccine Center) and D. Bonenberger for assistance with maintenance of mice strains at the Emory Vaccine Center vivarium. The work in the laboratory of BP was supported by grants U54AI057157, R37AI48638, R01DK057665, U19AI057266, HHSN266 200700006C, NO1 AI50019, and NO1 AI50025 from the National Institutes of Health and a grant from the Bill & Melinda Gates Foundation. Microarray accession number is GSE2213 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE22136).
Footnotes
References and Notes
- 1.Steinman RM, Banchereau J. Nature. 2007;449:419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Pulendran B. Immunol Rev. 2004;199:227. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC. Annu Rev Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. Nat Immunol. 2010;11:373. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Shaw MH, Kim YG, Nuñez G. Annu Rev Pathol. 2009;4:365. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek TB, Gringhuis SI. Nat Rev Immunol. 2009;9:465. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manicassamy S, Pulendran B. Semin Immunol. 2009;21:185. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izcue A, Coombes JL, Powrie F. Annu Rev Immunol. 2009;27:313. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 9.Belkaid Y, Tarbell K. Annu Rev Immunol. 2009;27:551. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 10.Sun CM, et al. J Exp Med. 2007;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Nat Immunol. 2007;8:1086. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 12.Coombes JL, et al. J Exp Med. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Materials and methods are available as supporting material on Science Online.
- 14.Staal FJ, Luis TC, Tiemessen MM. Nat Rev Immunol. 2008;8:581. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 15.Lehtonen A, et al. J Leukoc Biol. 2007;82:710. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Immunity. 2009;30:845. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clevers H. Cell. 2006;127:469. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Maretto S, et al. Proc Natl Acad Sci USA. 2003;100:3299. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang A, et al. Immunity. 2007;27:610. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brault V, et al. Development. 2001;128:1253. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 21.Caton ML, Smith-Raska MR, Reizis B. J Exp Med. 2007;204:1653. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uematsu S, et al. Nat Immunol. 2008;9:769. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov II, et al. Cell. 2006;126:1121. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Korn T, Bettelli E, Oukka M, Kuchroo VK. Annu Rev Immunol. 2009;27:485. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 25.Atarashi K, et al. Nature. 2008;455:808. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 26.Manicassamy S, Pulendran B. Semin Immunol. 2009;21:22. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manicassamy S, et al. Nat Med. 2009;15:401. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov II, et al. Cell Host Microbe. 2008;4:337. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaph C, et al. J Exp Med. 2008;205:2191. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall JA, et al. Immunity. 2008;29:637. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clément-Lacroix P, et al. Proc Natl Acad Sci USA. 2005;102:17406. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho JH, Weaver CT. Gastroenterology. 2007;133:1327. doi: 10.1053/j.gastro.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Rescigno M, Lopatin U, Chieppa M. Curr Opin Immunol. 2008;20:669. doi: 10.1016/j.coi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Matteoli G, et al. Gut. 2010;59:595. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui KR, Laffont S, Powrie F. Immunity. 2010;32:557. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.