Abstract
Background and Aim
The prevalence of allergic disorders, including asthma, atopic dermatitis, and allergic rhinitis has been increasing, and the prevalence of Helicobacter pylori (H. pylori) infection has been decreasing. Chronic bacterial infection during childhood is reported to protect the development of allergic diseases. The aim of the present study was to identify whether H. pylori infection influences the prevalence of allergic rhinitis, which has become a serious social problem, especially in the developed countries.
Methods
We initially investigated the association between the prevalence of H. pylori and pollinosis symptoms in 97 healthy volunteers.We had investigated the association between the serum H. pylori–immunoglobulin (Ig) G antibodies and specific IgE antibodies for pollen, mites, and house dust in 211 consecutive patients.
Results
There were 52.2% (36/69) of H. pylori-negative volunteers with allergic symptoms, which was significantly higher than H. pylori-positive volunteers (14.3%, 4/28, P <0.05). The risk of pollinosis symptoms by H. pylori infection was 0.148 (95% confidence interval): 0.046–0.475, P <0.05). The prevalence of H. pylori infection increased according to age, whereas that of specific IgE-positive patients gradually decreased. Among the IgE-positive patients, the prevalence of H. pylori-negative patients was significantly higher than H. pylori-positive patients who were younger in age (P <0.05).
Conclusion
H. pylori infection decreased the pollinosis effects, especially among the younger volunteers. However, the prevalence of pollinosis in patients who were 50 years or older were almost same between H. pylori-positive and H. pylori-negative patients; therefore, the recent increase of pollinosis might relate to not only H. pylori infection, but also change in social environment.
Keywords: allergic rhinitis, Helicobacter pylori, pollinosis
Introduction
Helicobacter pylori (H. pylori) colonizes in gastric mucosa in human populations, with a prevalence ranging from approximately 25% in developed countries, to as high as 80–90% in developing countries.1–4 Chronic H. pylori infection cause the development of gastroduodenal diseases, such as peptic ulcers, gastric cancer, gastric mucosa-associated lymphoid tissue lymphoma, and extra-gastroduodenal diseases, such as idiopathic thrombocytopenic purpura, chronic idiopathic urticaria, and iron-deficiency anemia.5–10 In recent times, however, the prevalence of H. pylori infection has been decreasing in developed countries,11–13 whereas new problems, such as Barrett’s esophageal cancer, metabolic syndrome, and allergic disorders, are increasing.14–16 Possible hypotheses to explain the recent decrease of H. pylori include increased antibiotic usage in children, the consumption of clean water, a decrease in the H. pylori infection rate in parents, a decrease of premasticate food by H. pylori-positive patients, and smaller families.
Allergic diseases, such as asthma and atopic dermatitis, are becoming more common in developed countries.17 A change in environmental exposure and situation could be considered a major cause of their rapid increase. The phenomenon that microbial infections during early childhood could prevent or diminish atopic sensitization and asthma is known as the ‘hygiene hypothesis’.18 In particular, inadequate microbial stimulation of gut-associated lymphoid tissue, a critical site for the maturation of mucosal immunity, could be relevant to this mechanism.19 An antigenic-rich environment could be essential for normal immune maturation, thereby decreasing allergic disorders.
H. pylori occurs in early childhood, usually after the first year of life,20 and the infection persists at least for decades or for the full life of its host, if not treated with antibiotics, including eradication therapy. The recent decrease in H. pylori infection could result in an increase in the prevalence of allergic diseases. For example, colonization with the CagA-positive strain, which has higher virulent ability, was reported to relate inversely to ever having had asthma, especially in younger adults.21 Colonization with H. pylori was also inversely related to allergic rhinitis, allergic symptoms, and skin sensitization due to pollens and molds.21 There seems to exist a relationship between H. pylori infection and the development of chronic urticaria and atopic dermatitis, since allergic symptoms are apparent throughout the year.21 However, it is still unclear whether season-related diseases, especially pollinosis, whose symptoms are manifested in spring/autumn, are related to chronic H. pylori infection.13 We therefore examined whether pollinosis is influenced by the H. pylori infection rate in relation to status or grade of allergic rhinitis-related symptoms, and whether the different allergens of allergic rhinitis and the Ig (Ig) E level is associated with H. pylori infection among the Japanese population, who have been reported to have CagA-positive H. pylori strains if infected.22
Methods
In study 1, we enrolled 97 healthy Japanese individuals working at Nara City Hospital, who agreed to participate in the present study, in March 2004. H. pylori infection was evaluated by measuring the H. pylori antibody in urine (Urineriza, Ohtsuka, Tokushima, Japan). We determined the individuals who had constant pollinosis-related symptoms (e.g. sniveling, sneezing, and itchy eyes) during spring, when pollen is most common, as symptom-positive patients. We determined patients with no symptoms as symptom-negative patients, and as mild for patients with symptoms but without anti-allergic medication. The grade of symptom in patients who needed anti-allergic medication for controlling pollinosis-related symptoms were determined as moderate, and patients refractory to medication were considered severe.
In study 2, we enrolled 211 consecutive Japanese patients who visited Nara City Hospital for the investigation of dyspeptic symptoms between 1 March and 15 April 2004, and who agreed to participate in the present study. In study 2, we did not include the 97 volunteers recruited in study 1. H. pylori infection was evaluated by measuring the serum H. pylori antibody (Determinar H. pylori antibody enzyme immunoassay kit; Kyowa Medex, Tokyo, Japan).We measured the IgE antibody specific for cedar pollen, as well as most well-known allergens common in spring, and IgE antibodies common throughout the year in allergens, including mites and house dust (all from UniCAP, Pharmacia Diagnostics AB, Uppsala, Sweden). Serum IgE antibodies less than 5 IU/mL, 5.1–20 IU/mL, and more than 20 IU/mL were determined as mild, moderate, and severe, respectively. IgE and specific IgE were presented as total IgE containing antibodies against all allergens and specific IgE for cedar pollen, mites, and/or house dust.
In both studies, no patient had received previous treatment for H. pylori infection. Informed consent was obtained from all patients, and the protocol was approved by the local hospital’s ethics committee.
Statistical differences in demographic characteristics, including positive and negative H. pylori status of allergic symptoms, were determined by χ2-test. The effects of H. pylori infection on the risk of developing pollinosis and symptoms in patients were expressed as odds ratios (OR) with 95% confidence intervals (CI) in H. pylori-negative patients, and adjusted by age and sex. A P-value of less than 0.05 was accepted as statistically significant. Calculations were carried out using statistical software StatView 5.0 (SAS institute, Cary, NC, USA).
Results
Study 1
The prevalence of H. pylori infection in healthy volunteers aged between 25 and 40 years was 28.9% (28/97) (Table 1). The percentage of H. pylori-negative patients with typical allergic symptoms, such as conjunctivitis, sneezing, and sniveling, was 52.2% (36/69), which was significantly higher than that of H. pylori-positive individuals (14.3%, 3/28, P = 0.003) (Table 1). After dividing the volunteers into two groups according to age (under 30 and older than 30), the symptom-positive rates in H. pylori-positive and H. pylori-negative individuals were 20% and 56% in the under-30 group. There was no significant difference in the grade of pollinosis symptoms between H. pylori-positive and H. pylori-negative individuals (Table 1). The H. pylori IgG level did not relate to status and the grade of pollinosis symptoms.
Table 1.
Demographic characteristics of Japanese healthy volunteers enrolled in study 1
|
Helicobacter pylori positivity |
Helicobacter pylori negativity |
Total | P-value | ||
|---|---|---|---|---|---|
| n | 28 | 69 | 97 | ||
| Sex | Male | 60.7% | 66.7% | 64.9% | 0.58 |
| Age | (Mean ± SD) | 32.5 ± 1.0 | 32.7 ± 0.5 | 32.7 ± 0.5 | 0.84 |
| Symptom | Negative | 24 (85.7%) | 33 (47.8%) | 57 (58.8%) | <0.01 |
| Positive | 4 (14.3%) | 36 (52.2%) | 40 (41.2%) | ||
| Symptom grade | None | 24 (85.6%) | 33 (47.2%) | 57 (58.8%) | <0.01 |
| Mild | 2 (7.2%) | 14 (20.9%) | 16 (16.5%) | ||
| Moderate | 2 (7.2%) | 22 (31.9%) | 24 (24.7%) |
P-values analyze demographic data between Helicobacter pylori-positive patients and Helicobacter pylori-negative patients.
The risk of pollinosis symptoms for H. pylori infection, adjusted by age and sex, was 0.148 (95% CI: 0.046–0.475, P = 0.0013), which suggested that chronic H. pylori infection significantly decreased the presence of pollinosis symptoms. The risk of moderate rhinitis-related symptoms for H. pylori infection was 0.121 (95% CI: 0.026–0.571, P = 0.008).
Study 2
The H. pylori infection rate for consecutive patients was 44.8% (99/221) (Table 2). The sex and mean age ratio significantly differed between the H. pylori-positive and H. pylori-negative groups (P = 0.02 and P <0.001, respectively). The percentage of serum IgE-positive patients among the H. pylori-negative patients was 67.9% (76/112), which was significantly higher than those among the H. pylori-positive individuals (33.3%, 32/99, P <0.001) (Table 2). However, there was no significant difference in the IgE level (mild, moderate, or severe) between H. pylori-positive and H. pylori-negative individuals (P = 0.18) (Table 2). The specific IgE levels for pollen, as a spring allergen, and mites and house dust, as allergens throughout the year in H. pylori-negative patients, were 67.9%, 36.6%, and 39.3%, respectively, which were significantly higher than those in H. pylori-positive patients (Table 2). The H. pylori IgG level did not relate to H. pylori-positive patients and the specific IgE level for pollen, irrespective of the patients’ age.
Table 2.
Demographic characteristics of Japanese patients enrolled in study 2
| Helicobacter pylori positivity |
Helicobacter pylori negativity |
Total | P-value | ||
|---|---|---|---|---|---|
| n | 99 | 112 | 211 | ||
| Sex | Male | 36.4% | 51.8% | 44.5% | 0.02 |
| Age | (Mean ± SD) | 58.0 ± 1.8 | 40.5 ± 1.9 | 49.8 ± 1.4 | <0.01 |
| IgE | Negative | 67 (67.7%) | 36 (32.1%) | 103 (48.8%) | <0.01 |
| Positive | 32 (33.3%) | 76 (67.9%) | 108 (51.2%) | ||
| IgE level | Mild | 16 (16.1%) | 52 (46.4%) | 68 (32.2%) | 0.18 |
| Moderate | 9 (9.1%) | 12 (10.7%) | 21 (10.0%) | ||
| Severe | 7 (7.0%) | 12 (10.7%) | 19 (9.7%) | ||
| Allergen | Pollen | 32 (32.2%) | 76 (67.9%) | 108 (51.2%) | <0.01 |
| Mites | 19 (19.2%) | 41 (36.6%) | 60 (28.4%) | <0.01 | |
| House dust | 16 (16.2%) | 44 (39.3%) | 60 (28.4%) | <0.01 |
P-values analyze demographic data between Helicobacter pylori-positive patients and Helicobacter pylori-negative patients. IgE, immunoglobulin E.
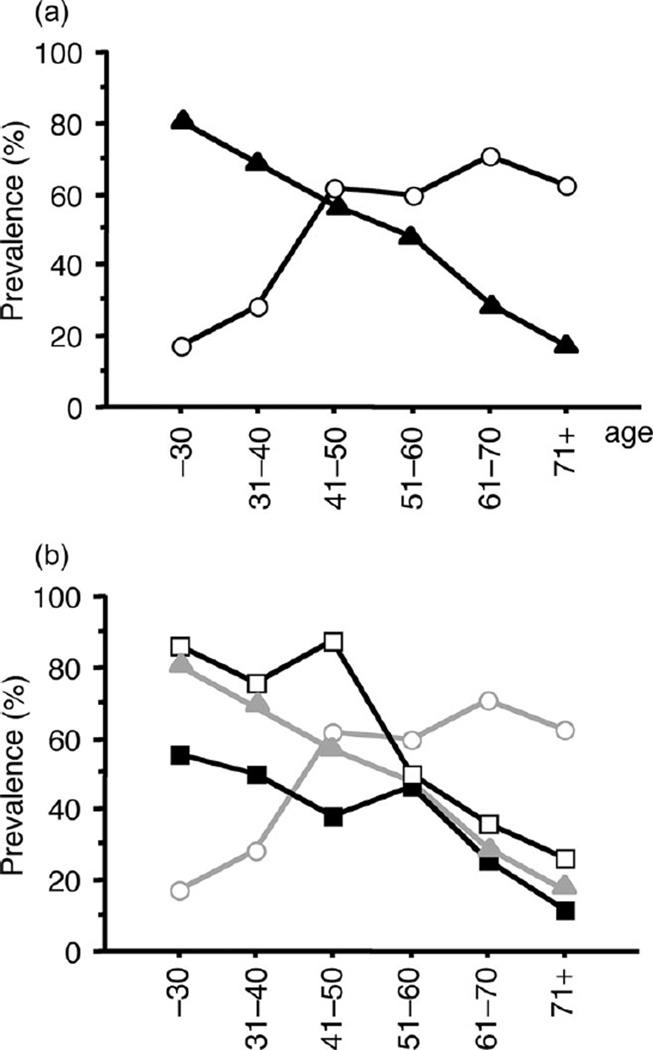
When we divided the patients into age-dependent groups, the H. pylori infection rate increased according to age, whereas the IgE-positive rate decreased according to age (Fig. 1a). The prevalence of H. pylori infection was inversely related to the presence of IgE antibodies. The IgE-positive rate in H. pylori-negative patients was higher than that in H. pylori-positive patients, especially among the younger patients (Fig. 1b). These differences between H. pylori-negative and H. pylori-positive patients decreased according to age, and in patients 50 years or older, the IgE-positive rate was independent of H. pylori status (Fig. 1b).
Figure 1.
(a) Helicobacter pylori (H. pylori) infection rate and specific immunoglobulin E (IgE)-positive rate in different age-dependent groups. H. pylori infection rate increased according to age, whereas the specific IgE-positive rate decreased. (b) Specific IgE-positive rate in H. pylori-positive patients and H. pylori-negative patients. (○) H. pylori-positive patients; (▲) specific IgE positivity; (■) specific IgE positivity, and H. pylori-positive patients; (□) specific IgE positivity, and H. pylori-negative patients.
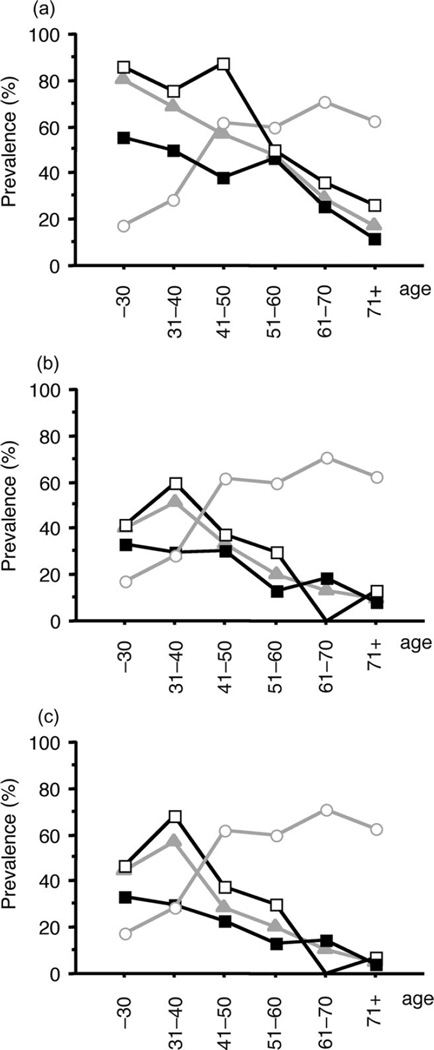
The specific IgE-positive rate for pollen, mites, and house dust in H. pylori-negative patients was higher than that of H. pylori-positive patients, irrespective of age (Fig. 2). However, the differences in the specific IgE-positive rate for the three antigens between both groups decreased according to age (Fig. 2). The differences in the specific IgE-positive rate for pollen between both groups were also compared (Fig. 2). In the patients who were 50 years or older, there was no difference in the specific IgE-positive rate between both groups (Fig. 2).
Figure 2.
Helicobacter pylori (H. pylori) infection rate and specific immunoglobulin E (IgE)-positive rate for pollen (a), mites (b), and house dust (c) in different age-dependent groups. H. pylori infection rate increased according to age, whereas the specific IgE-positive rate decreased. (○) H. pylori-positive patients; (▲) specific IgE positivity; (■) specific IgE positivity, and H. pylori-positive patients; (□) specific IgE positivity, and H. pylori-negative patients.
The risk of IgE-positive H. pylori infection, adjusted by age and sex, was 0.39 (95% CI: 0.16–0.57, P = 0.004), suggesting that H. pylori infection significantly decreased the presence of serum IgE in allergens (Table 3). The risk of specific IgE-positive H. pylori infection for pollen, mites, and house dust significantly decreased (0.26 [0.13–0.40], 0.41 [0.22–0.77], and 0.39 [0.16–0.57], respectively). However, when adjusted by age and sex, the risk of specific IgE-positive H. pylori infection for pollen was 0.39 (95% CI: 0.16–0.57, P = 0.004), whereas the risk of specific IgE-positive H. pylori infection for mites and house dust decreased (Table 3).
Table 3.
Risk of immunoglobulin E positivity for Helicobacter pylori-positive patients
| Allergen | Odds ratio | 95% confidence interval | P-value | |
|---|---|---|---|---|
| Total | Pollen | 0.23 | 0.13–0.40 | <0.01 |
| Mites | 0.41 | 0.22–0.77 | <0.01 | |
| House dust | 0.39 | 0.16–0.57 | <0.01 | |
| Age and sex adjusted | Pollen | 0.39 | 0.20–0.74 | <0.01 |
| Mites | 0.74 | 0.36–1.52 | 0.41 | |
| House dust | 0.61 | 0.29–1.30 | 0.20 |
Discussion
Pollinosis is an important social problem in Japan that occurs throughout the year, so much so that cedar pollen forecasting is reported in weather reports in the media. In this study, we demonstrated that patients with pollinosis-related symptoms and with serum total IgE positivity or pollen-specific IgE positivity had a lower rate of H. pylori infection than those without, especially in younger adults. Exposure to chronic infections, such as H. pylori, in early life is necessary for the normal maturation of immune response, so as to achieve a balance between T-helper type 1 (Th1; protective immunity) and T-helper type 2 (Th2; immunity for allergic diseases, including asthma and pollinosis) cytokine responses. In recent times, particular allergic conditions have been increasing because of Th1- and Th2-type immune response imbalances due to modern lifestyles.18,23 Lifestyle changes can increase the risk of developing allergic diseases, especially in developed countries.24
The immune system is activated after the establishment of H. pylori colonization in gastric mucosa. H. pylori infection, therefore, was expected to have a particularly marked effect on the developing immune system and to reduce the risk of atopic dermatitis, allergic rhinitis, and asthma by approximately 30%.25 In Finland, the prevalence of atopic dermatitis was significantly higher than in Russia, and this difference was explained, at least in part, by significantly higher H. pylori seropositivity in Russia compared to Finland.26 H. pylori was also inversely associated with the onset of asthma in not only adults, but also children.21,27 Among children aged less than 20 years, the presence of H. pylori was inversely related to ever having had asthma (OR: 0.69, 95% CI: 0.45–1.06).27 H. pylori infection was also related to a decreasing risk of food allergies.28 However, there are only a few studies about H. pylori infection and allergic rhinitis.21 Colonization with H. pylori was inversely associated with currently (OR: 0.77, 95% CI: 0.62–0.96) or ever (OR: 0.77, 95% CI: 0.62–0.94) having a diagnosis of allergic rhinitis, especially with a childhood onset (OR: 0.55, 95% CI: 0.37–0.82).21 In the present study, the risk of pollinosis symptoms for H. pylori infection was 0.148 (95% CI: 0.046–0.475, P = 0.0013), suggesting that chronic H. pylori infection significantly decreases the presence of pollinosis symptoms, as observed in other allergic disorders.
In those aged 30 years or over, the positive rates of allergen-specific IgE and total IgE levels increased.29 The prevalence of positive IgE among a younger population (15–24 years) was found to increase from 11% to 38% (3.5-fold increase) in a 30-year timeframe in Finland.29 Serum IgE responses were also found to increase in a cohort study of young Japanese girls from 1978 to 1991.30 In that study, IgE responses for 16 allergens tested increased from 21% to 39%, and the allergens found to have most significantly increased were timothy, sweet vernal grass, house dust, and Japanese pollen.30 The percentage of IgE-positive Japanese mainly increased in the subgroup with no H. pylori antibodies.30 In the present study, the specific IgE-positive rate for pollen, mites, and house dust was related to H. pylori status, and differences in specific IgE-positive rates between H. pylori-negative and H. pylori-positive patients significantly differed. However, the differences decreased according to age. In patients aged 50 years or over, there was no difference in the IgE-positive rate. Patients aged 50 years or over who were not infected had a higher IgE-positive rate for allergens like their younger counterparts. This suggests that the continuing decline of H. pylori in Japanese is related to the increase of pollinosis. The prevalence of allergic diseases among older populations might be lower because more bacterial and viral infections were common 40–50 years ago. The fact that H. pylori infection and allergies decrease with age in this study might suggest that other environment factors, such as improving hygiene, were related to the prevention of allergic response in patients aged 50 years and older, irrespective of H. pylori infection. In fact, it has been suggested that measles,31 hepatitis A,32 Toxoplasma gondii,33 and Mycobacterium tuberculosis34 infections, and the development of delayed hypersensitivity to tuberculin,35 decrease allergic diseases. Therefore, although H. pylori infection was considered to decrease allergic diseases, including pollinosis, a recent increase of pollinosis could be caused by the decrease of not only H. pylori, but also other bacteria and viruses.
Chen et al.21 reported that CagA-positive H. pylori was inversely associated with current allergic rhinitis (OR: 0.77, 95% CI: 0.62–0.96), especially with a childhood onset (OR: 0.55, 95% CI: 0.37–0.82). However, there was no increased risk of allergic rhinitis in CagA-negative H. pylori-infected patients.21 More than 90–95% of H. pylori strains isolated in East Asian countries carry CagA.36,37 Therefore, in East Asia, the prevalence of allergic disorders might be lower than other populations that have a lower rate of the CagA-positive strain.
In conclusion, we demonstrated that H. pylori infection might play an important role in protecting against pollinosis development in Japanese, especially among younger patients. There are several limitations of the present study, including an insufficient sample size, especially in study 1. Moreover, this epidemiological study on the association between pollinosis and H. pylori infection has not directly proven that the relationship between the decrease of H. pylori infection and the increase of pollinosis is a causal one. Therefore, that the effects of allergic diseases by H. pylori eradication therapy and the solution to H. pylori and pollinosis mechanisms in relation to age require further study.
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development, Medical Research Service Department of Veterans Affairs; NIH grant DK 62813 (toYY); and Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
References
- 1.Perez-Perez GI, Taylor DN, Bodhidatta L, et al. Seroprevalence of Helicobacter pylori infections in Thailand. J. Infect. Dis. 1990;161:1237–1241. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- 2.Rocha GA, Queiroz DM, Mendes EN. Indirect immunofluorescence determination of the frequency of anti-H. pylori antibodies in Brazilian blood donors. Braz. J. Med. Biol. Res. 1992;25:683–689. [PubMed] [Google Scholar]
- 3.Souto FJ, Fontes CJ, Rocha GA, de Oliveira AM, Mendes EN, Queiroz DM. Prevalence of Helicobacter pylori infection in a rural area of the state of Mato Grosso, Brazil. Mem. Inst. Oswaldo. Cruz. 1998;93:171–174. doi: 10.1590/s0074-02761998000200006. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin. Microbiol. Infect. 2009;10:1227–1236. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 7.Wotherspoon AC, Doglioni C, de Boni M, Spencer J, Isaacson PG. Antibiotic treatment for low-grade gastric MALT lymphoma. Lancet. 1994;343:1503. [PubMed] [Google Scholar]
- 8.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 9.Tebbe B, Geilen CC, Schulzke JD, Bojarski C, Radenhausen M, Orfanos CE. Helicobacter pylori infection and chronic urticaria. J. Am Acad. Dermatol. 1996;34:685–686. doi: 10.1016/s0190-9622(96)80086-7. [DOI] [PubMed] [Google Scholar]
- 10.Annibale B, Marignani M, Monarca B, et al. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann. Intern. Med. 1999;131:668–672. doi: 10.7326/0003-4819-131-9-199911020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Bu XL, Wang QY, Hu PJ, Chen MH. Decreasing seroprevalence of Helicobacter pylori infection during 1993–2003 in Guangzhou, southern China. Helicobacter. 2007;12:164–169. doi: 10.1111/j.1523-5378.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 12.Yim JY, Kim N, Choi SH. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 13.Shiotani A, Miyanishi T, Kamada T, Haruma K. Helicobacter pylori infection and allergic diseases: epidemiological study in Japanese university students. J. Gastroenterol. Hepatol. 2008;23:e29–e33. doi: 10.1111/j.1440-1746.2007.05107.x. [DOI] [PubMed] [Google Scholar]
- 14.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin. Radiat. Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Burr ML, Wat D, Evans C, Dunstan FD, Doull IJ. Asthma prevalence in 1973, 1988 and 2003. Thorax. 2006;61:296–299. doi: 10.1136/thx.2005.045682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frei T, Gassner E. Trends in prevalence of allergic rhinitis and correlation with pollen counts in Switzerland. Int. J. Biometeorol. 2008;52:841–847. doi: 10.1007/s00484-008-0178-z. [DOI] [PubMed] [Google Scholar]
- 17.Anderson HR, Ayres JG, Sturdy PM. Bronchodilator treatment and deaths from asthma: case-control study. BMJ. 2005;330:117. doi: 10.1136/bmj.38316.729907.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J. Allergy Clin. Immunol. 2006;117:969–977. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kelly D, Conway S, Aminov R. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 2005;26:326–333. doi: 10.1016/j.it.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Malaty HM, El-Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–935. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch. Intern. Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 22.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J. Clin. Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354(Suppl. 2):SII12–SII15. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 24.Busse WW, Lemanske RF., Jr Asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 25.McCune A, Lane A, Murray L, et al. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. Eur. J Gastroenterol. Hepatol. 2003;15:637–640. doi: 10.1097/00042737-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 26.von Hertzen LC, Laatikainen T, Makela MJ. Infectious burden as a determinant of atopy—a comparison between adults in Finnish and Russian Karelia. Int. Arch. Allergy Immunol. 2006;140:89–95. doi: 10.1159/000092251. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J. Infect. Dis. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konturek PC, Rienecker H, Hahn EG, Raithel M. Helicobacter pylori as a protective factor against food allergy. Med. Sci. Monit. 2008;14:CR452– CR458. [PubMed] [Google Scholar]
- 29.Kosunen TU, Hook-Nikanne J, Salomaa A, Sarna S, Aromaa A, Haahtela T. Increase of allergen-specific immunoglobulin E antibodies from 1973–1994 in a Finnish population and a possible relationship to Helicobacter pylori infections. Clin. Exp. Allergy. 2002;32:373–378. doi: 10.1046/j.1365-2222.2002.01330.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakagomi T, Itaya H, Tominaga T, Yamaki M, Hisamatsu S, Nakagomi O. Is atopy increasing? Lancet. 1994;343:121–122. doi: 10.1016/s0140-6736(94)90854-0. [DOI] [PubMed] [Google Scholar]
- 31.Shaheen SO, Aaby P, Hall AJ, et al. Measles and atopy in Guinea–Bissau. Lancet. 1996;347:1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 32.Matricardi PM, Rosmini F, Ferrigno L, et al. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ. 1997;314:999–1003. doi: 10.1136/bmj.314.7086.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matricardi PM, Rosmini F, Riondino S, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320:412–417. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Hertzen L, Klaukka T, Mattila H, Haahtela T. Mycobacterium tuberculosis infection and the subsequent development of asthma and allergic conditions. J. Allergy Clin. Immunol. 1999;104:1211–1214. doi: 10.1016/s0091-6749(99)70015-1. [DOI] [PubMed] [Google Scholar]
- 35.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 36.Yamaoka Y, El-Zimaity HM, Gutierrez O, et al. Relationship between the cagA 3′ repeat region of Helicobacter pylori gastric histology, and susceptibility to low pH. Gastroenterology. 1999;117:342–349. doi: 10.1053/gast.1999.0029900342. [DOI] [PubMed] [Google Scholar]
- 37.Yamaoka Y, Orito E, Mizokami M, et al. Helicobacter pylori in North and South America before Columbus. FEBS. Lett. 2002;517:180–184. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]