Abstract
Objective
To identify the main clinical and epidemiological features of ALS in a large cohort of African American (AA) patients and compare them to Caucasian (CA) patients in a clinic-based population.
Methods
We retrospectively identified 207 patients who were diagnosed with ALS based on the revised El Escorial criteria (60 AA and 147 CA subjects). Patients were seen in the Neuromuscular Division at the University Medical Center. We compared epidemiological and clinical features of these two groups, focusing on age of onset and diagnosis, clinical presentation and survival.
Results
AA patients had a significantly younger age of disease onset (55 years v. 61 for CA, p=0.011) and were diagnosed at an earlier age (56 v. 62, p=0.012). In younger ALS patients (<45 years old), there was a significant difference in gender frequency, with females predominating in the AA population and males in the CA population (p = 0.025). In a multivariable Cox proportional hazard model, survival rates were not different between the groups. In both groups, survival significantly increased with younger age.
Conclusion
AA patients presented at an earlier age, but there was no difference in survival compared to CA patients. A gender reversal occurred in younger ALS patients, with AA patients more likely to be female and CA patients more likely to be male.
Search Terms: motor neuron disease, race, phenotype, gender effect
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentless and fatal disease characterized by progressive weakness, muscle wasting, and spasticity (1). Epidemiologic studies addressing disease variations between African American (AA) and Caucasian (CA) patients with ALS have been limited (2, 3). Some population-based studies suggest lower mortality rates and a decreased incidence among AA, but provide little insight into disease features and survival, indicating a need for further study (3–5). Systematic study of ALS in AA patients is hampered by lower population numbers, possibly a lower incidence, and/or a decreased access to health care (5, 6). The aim of this study was to compare the main clinical and epidemiological features of ALS in AA versus CA patients in a large clinic-based population.
Methods
We identified 236 consecutive patients from September, 1998 to July, 2011 who were diagnosed with ALS based on revised El Escorial criteria. This database includes patients from the UAB-served county hospital which provides free medical care to the uninsured. The study was approved by the UAB Institutional Review Board with a waiver of consent. Complete clinical records were available on 207 (88%) patients: 60 AAs and 147 CAs. Of the excluded records (due to incomplete clinical information), 22% were AA and 78% CA. The Age of death was confirmed with the Social Security Death Index. Patients were stratified into three age groups (at the time of diagnosis) based on a prior study (7): ≤44, 45–64 and ≥ 65 years old. Continuous variables were assessed using t-tests and categorical using chi-square tests. The multivariable Cox proportional hazard model was used to assess the risk of mortality by subgroup and Kaplan-Meier survival curves were used to compare resulting survival (SAS version 9.2). Gender frequency by age group was assessed using the chi-squared test. Patients were classified into two groups: predominant upper motor neuron (p-UMN) ALS and classic ALS based on criteria established elsewhere (8). Briefly, patients in the p-UMN group presented with prominent pyramidal signs but with definite signs of lower motor neuron impairment from onset of disease. Classic ALS was defined by presentation of mainly lower motor neuron signs with slight to moderate UMN signs, and was subdivided into site of onset (spinal versus bulbar).
Results
Sixty patients were AA (29.0%) and 147 (71%) were CA (table 1). The percent of AA in our study matched closely that of the AA population in Alabama based on the national census bureau (26.2%; http://2010.census.gov/2010census/data/). AA patients were significantly younger than CA patients at age of onset (55 v. 61 years, p = 0.011) and diagnosis (56 v. 62 years, p = 0.012). There was no significant difference in the interval from symptom onset to diagnosis (AAs, 19 months; CAs, 16 months, p = 0.515). After stratifying patients by age, the majority of young CA patients (< 45 y) were male (5.8 to 1) consistent with prior reports (8, 9). In AA patients, the ratio was reversed with females exceeding males (1 to 0.8). This gender reversal was statistically significant (p = 0.025). We found no significant differences in clinical features, including bulbar versus spinal presentation or classic versus predominantly upper motor neuron (p-UMN) ALS in any age group. The overall frequency of bulbar presentation (26%) and its low occurrence in young patients (11%) is consistent with prior report (9, 10). A p-UMN presentation occurred in 37% of patients in the younger group (table 1), with all being male in the CA group and 33% female in the AA group (data not shown).
Table 1.
Demographic and clinical features of ALS patients
| AA | CA | Overall | P Valuea | |
|---|---|---|---|---|
| Total sample (%) | 60 (29%) | 147 (71%) | 207 | |
| Mean age of onset, y (95% CI) | 55 (51, 59) | 61 (59, 63) | 59 (57, 61) | 0.011 |
| Mean age at diagnosis, y (95% CI) | 56 (53, 60) | 62 (60, 64) | 60 (59, 62) | 0.012 |
| Onset to diagnosis, m (95% CI) | 19 (10, 27) | 16 (13, 18) | 17 (14, 19) | 0.515 |
| Male: female (n) | ||||
| <45 y | 0.8:1 (14) | 5.5:1 (13) | 1.7:1 (27) | 0.025 |
| 45 to 64 y | 1.8:1 (25) | 1.2:1 (68) | 1.2:1 (93) | 0.282 |
| ≥ 65 y | 0.9:1 (21) | 0.9:1 (66) | 0.9:1 (87) | 0.945 |
| Overall | 1.1:1 | 1.2:1 | 1.1:1 | |
| Region of onset (%) | ||||
| Bulbar | 13 (22%) | 41 (28%) | 54 (26%) | 0.388 |
| Spinal | 47 (78%) | 106 (72%) | 153 (74%) | |
| Upper limb only | 27 (45%) | 54 (37%) | 81 (39%) | |
| Lower limb only | 16 (27%) | 47 (32%) | 63 (30%) | |
| Bulbar < 45 yb | 1 (7%) | 2 (15%) | 3 (11%) | |
| Clinical type (%) | ||||
| p–UMN | 15 (25%) | 48 (33%) | 63 (30%) | 0.320 |
| Classic | 45 (75%) | 99 (67%) | 144 (70%) | |
| P-UMN <45 yb | 6 (43%)b | 4 (31%) | 10 (37%) | |
Abbreviations: AA = African American; CA = Caucasian; y = years; CI = confidence interval; m = months; p-UMN = predominant upper motor neuron.
Comparison between AA and CA.
Data are expressed as a percentage of the <45 y group.
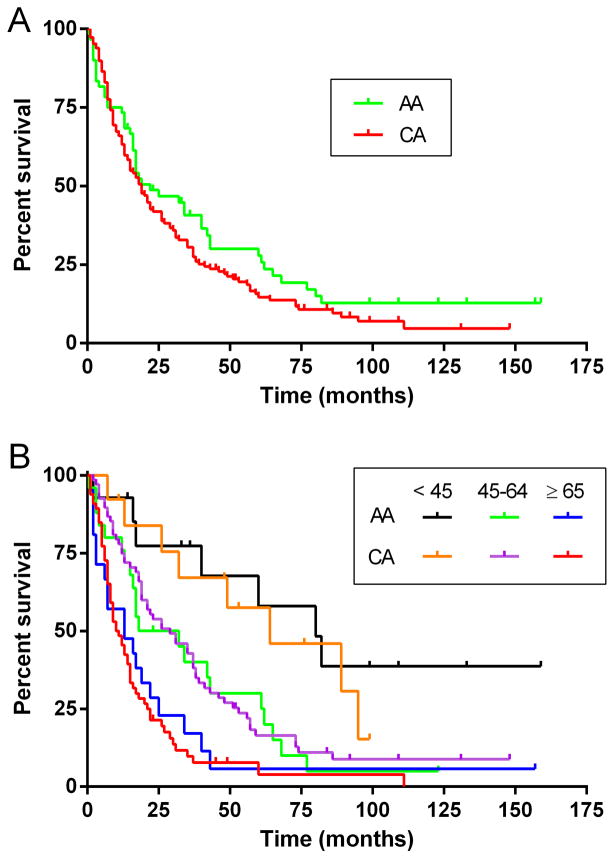
We next compared survival rates (table 2 and Figure 1). As of July, 2012, 78% of the AA cohort and 86% of the CA cohort had died. The difference in overall median survival was not significantly different (AA group, 22 months vs. CA group, 19 months; hazard ratio = 0.79, and 0.91 after adjusting for age). Kaplan-Meier survival curves are presented in Figure 1. In both ethnicities, younger patients had significantly prolonged survival. For AA patients, median survival for the <45 year old group was 80 months compared to 32 months in the 45–64 year old group (hazard ratio = 0.34, p = 0.002)) and 13 months in the ≥ 65 year old group (hazard ratio = 0.21, p = 0.011). Survival in the 45–64 year age group was prolonged compared to the ≥ 65 group, and close to significance (p = 0.119). CA patients followed a similar pattern with the <45 year age group having a median survival of 64 months compared to the 45–64 year age group (29 months, hazard ratio = 0.21) and the ≥ 65 year age group (11 months, hazard ratio = 0.49). The 45–64 year age group had significantly prolonged survival compared to the ≥ 65 year group (hazard ratio = 0.45, p =0.001). There was no difference in survival between AAs and CAs within each of the age groups.
Table 2.
Cox proportional hazard models for survival
| Age Group (y) | Median Survival | Hazard Ratio | CI | P Value | |
|---|---|---|---|---|---|
| AA | CA | ||||
| Overall (diagnosis) | 22 | 19 | 0.79 | 0.56 to 1.10 | 0.160 |
| Age adjusted | 0.91 | 0.65 to 1.28 | 0.623 | ||
| Overall (onset) | 41 | 34 | 0.78 | 0.55 to 1.10 | 0.150 |
| Age adjusted | 0.94 | 0.66 to 1.32 | 0.712 | ||
| <45 | 80 | 64 | 0.69 | 0.22 to 2.10 | 0.501 |
| 45–64 | 32 | 29 | 0.94 | 0.56 to 1.54 | 0.814 |
| ≥65 | 13 | 11 | 0.91 | 0.53 to 1.50 | 0.011 |
| AA comparison | |||||
| <45 v ≥65 | 0.34 | 0.13 to 0.79 | 0.002 | ||
| <45 v 45–64 | 0.21 | 0.08 to 0.50 | 0.011 | ||
| 45–64 v ≥65 | 0.58 | 0.30 to 1.13 | 0.119 | ||
| CA comparison | |||||
| <45 v ≥65 | 0.21 | 0.09 to 0.43 | 0.040 | ||
| <45 v 45–64 | 0.49 | 0.21 to 0.96 | 0.004 | ||
| 45–64 v ≥65 | 0.45 | 0.29 to 0.63 | 0.001 | ||
Abbreviations: AA = African American; CA = Caucasian; CI = confidence interval; y = years.
Figure 1.
Comparison of Kaplan-Meier survival curves between African American (AA) and Caucasian (CA) ALS patients. (A) Overall survival from time of diagnosis; (B) survival by age group (<45, 45–64, and ≥ 65 years old) from time of diagnosis.
Discussion
A high density of AAs in Alabama (26.2% of the population) provided a unique opportunity to study epidemiological features of a large cohort of AA with ALS. Additionally, free access to neurological care reduced potential patient selection bias. Indeed, we were reassured that the proportion of AAs in our database was similar to that of the population in Alabama, albeit the study was not designed to assess incidence. From our findings, four noteworthy observations emerged. First, gender ratios were inverted in the younger AA patients, with females slightly exceeding males in the <45 year old group. Although epidemiological studies indicate a trend toward gender equalization in older patients, the young ALS population remains predominately male as seen with the CA cohort here (9, 10). Several studies concluded that African or AA males have a higher incidence of ALS, but did not stratify by age and may have had gender-biased referral patterns (11, 12). Gender inversion in black patients was observed in a population-based study of ALS patients in Cuba, although the study was based on mortality rates derived from government records (13). Our finding could be explained by a reduced susceptibility of young AA males or CA females versus a greater vulnerability to the disease in AA females or CA males. The gender ratio in the AA group more closely matched the overall ratio, suggesting that the differential susceptibility resided in the CA group. Others have commented that the male predominance in young ALS patients likely reflects a genetic influence (8, 9). Alternatively, differential referral patterns could have made the AA group more representative than the CA group. The second observation is the younger age of presentation in AA patients. This finding is likely related to the overrepresentation of patients in the <45 age range (23% for the AA cohort versus 9% for the CA cohort). Population and clinic based studies have suggested an earlier onset of ALS in patients from Africa or of African descent (2, 9, 11, 14). The younger age of AA patients in our study may reflect a confluence of genetic, socioeconomic factors, and/or environmental exposures. Third, there was no significant difference in survival overall or when stratified by age. Several studies from Africa have suggested a better prognosis in patients of African descent (11, 14) whereas other studies, performed in other countries using different methodologies, have shown no differences (13, 15). One of those latter studies, however, did find decreased mortality rates in patients with mixed ancestry (13). In AA patients, Lee et al. reported a trend toward improved survival but it was not statistically significant (6). That study had smaller numbers of AAs and did not stratify by age. We found that the survival advantage of being young occurred in AA patients equivalent to that of CA patients. Within the AA group, a survival advantage was seen in the 45–64 year AA group (compared to the ≥65 group) although not statistically significant. This could be related to lower sample size, or other factors such as comorbidities, motivation or compliance with treatment. One study, for example, showed that AA patients had significantly higher odds of undergoing emergency placement of a gastrostomy tube, suggesting less insight, understanding, or guidance regarding disease course (16). The final observation was the similarity of clinical features, including region of onset, subtype, and time to diagnosis. Since these features directly influence disease outcome (1), the phenotypic similarity supports our observation that AA and CA patients had equivalent survival rates.
Some limitations of our study should be noted. Differences in methodologies of retrospective analyses and population-based studies, combined with the rareness of the disease, can produce disparate findings and preclude overarching conclusions. As in our study, clinical populations of tertiary referral centers may have selection biases acting on the races differentially. Despite the density of AAs in Alabama, the catchment of a single institution remains relatively small. A national registry can mitigate against these limitations (10), but until that is initiated and matured (which will take years), studies such as ours provide important clues to racial differences in the onset and course of the disease.
Acknowledgments
Funding: National Institutes of Health, NS064133 (PHK) and NS057664 (LL); Department of Veterans Affairs (PHK).
Footnotes
Disclosure of Interests: None.
References
- 1.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–49. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 2.Marin B, Kacem I, Diagana M, Boulesteix M, Gouider R, Preux PM, et al. Juvenile and adult-onset ALS/MND among Africans: incidence, phenotype, survival: a review. Amyotroph Lateral Scler. 2012;13:276–83. doi: 10.3109/17482968.2011.648644. [DOI] [PubMed] [Google Scholar]
- 3.McGuire V, Nelson LM. Epidemiology of ALS. In: Mitsumoto H, Przedborksi S, Gordon PH, editors. Amyotrophic lateral sclerosis. New York and London: Taylor & Francis; 2006. pp. 17–41. [Google Scholar]
- 4.Noonan CW, White MC, Thurman D, Wong L-Y. Temporal and geographic variation in United States motor neuron disease mortality, 1969–1998. Neurology. 2005;64:1215–21. doi: 10.1212/01.WNL.0000156518.22559.7F. [DOI] [PubMed] [Google Scholar]
- 5.Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology. 2007;68:1002–7. doi: 10.1212/01.wnl.0000258551.96893.6f. [DOI] [PubMed] [Google Scholar]
- 6.Lee JR, Annegers JF, Appel SH. Prognosis of amyotrophic lateral sclerosis and the effect of referral selection. J Neurol Sci. 1995;132:207–15. doi: 10.1016/0022-510x(95)00154-t. [DOI] [PubMed] [Google Scholar]
- 7.del Aguila MA, Longstreth WT, Jr, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60:813–9. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 8.Sabatelli M, Madia F, Conte A, Luigetti M, Zollino M, Mancuso I, et al. Natural history of young-adult amyotrophic lateral sclerosis. Neurology. 2008;71:876–81. doi: 10.1212/01.wnl.0000312378.94737.45. [DOI] [PubMed] [Google Scholar]
- 9.Turner MR, Barnwell J, Al-Chalabi A, Eisen A. Young-onset amyotrophic lateral sclerosis: historical and other observations. Brain. 2012;135:2883–91. doi: 10.1093/brain/aws144. [DOI] [PubMed] [Google Scholar]
- 10.Logroscino G, Traynor BJ, Hardiman O, Chiò A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81:385–90. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osuntokun BO, Adeuja AO, Bademosi O. The prognosis of motor neuron disease in Nigerian africans. A prospective study of 92 patients. Brain. 1974;97:385–94. doi: 10.1093/brain/97.1.385. [DOI] [PubMed] [Google Scholar]
- 12.Breland AE, Jr, Currier RD. Multiple sclerosis and amyotrophic lateral sclerosis in Mississippi. Neurology. 1967;17:1011–6. doi: 10.1212/wnl.17.10.1011. [DOI] [PubMed] [Google Scholar]
- 13.Zaldivar T, Gutierrez J, Lara G, Carbonara M, Logroscino G, Hardiman O. Reduced frequency of ALS in an ethnically mixed population. Neurology. 2009;72:1640–5. doi: 10.1212/WNL.0b013e3181a55f7b. [DOI] [PubMed] [Google Scholar]
- 14.Abdulla MN, Sokrab TE, el Tahir A, Siddig HE, Ali ME. Motor neurone disease in the tropics: findings from Sudan. East African medical journal. 1997;74:46–8. [PubMed] [Google Scholar]
- 15.Loureiro MP, Gress CH, Thuler LC, Alvarenga RM, Lima JM. Clinical aspects of amyotrophic lateral sclerosis in Rio de Janeiro/Brazil. J Neurol Sci. 2012;316:61–6. doi: 10.1016/j.jns.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Tsou AY, Karlawish J, McCluskey L, Xie SX, Long JA. Predictors of emergent feeding tubes and tracheostomies in amyotrophic lateral sclerosis (ALS) Amyotroph Lateral Scler. 2012;13:318–25. doi: 10.3109/17482968.2012.662987. [DOI] [PMC free article] [PubMed] [Google Scholar]