Abstract
The stress-activated protein kinase p38 is often induced by cytotoxic agents, but its contribution to cell death is ill defined. In Rat-1 cells, we found a strong correlation between activation of p38 and induction of c-Myc–dependent apoptosis. In cells with deregulated c-Myc expression but not in control cells, cis-diamminedichloroplatinum induced p38 activity and typical features of apoptosis, including internucleosomal DNA degradation, induction of caspase activities, and both nuclear (nuclear condensation and fragmentation) and extranuclear (cell blebbing) morphological alterations. The pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone did not block p38 activation and the p38 inhibitor SB203580 had no detectable effect on the activation of caspases or the in vivo cleavage of several caspase substrates, suggesting that p38 and caspase activation can contribute distinct features of apoptosis. Accordingly, we found that cell blebbing was independent of caspase activity and, rather, depended on p38-sensitive changes in microfilament dynamics likely mediated by heat shock protein 27 phosphorylation. Furthermore, p38 activity contributed to both caspase-dependent and caspase-independent nuclear condensation and fragmentation, suggesting a role in an early event triggering both mechanisms of apoptosis or sensitizing the cells to the action of both types of apoptosis executioners. Inhibiting p38 also resulted in a significant enhancement in cell survival estimated by colony formation. This capacity to modulate the sensitivity to apoptosis in cells with deregulated c-Myc expression suggests an important role for p38 in tumor cell killing by chemotherapeutic agents.
INTRODUCTION
Apoptosis is an active form of cell death that plays an essential role in physiological and pathological conditions throughout the development and adult life of multicellular organisms, eliminating damaged cells or cells with defects in key-regulated processes such as growth (Kerr et al., 1972; Wyllie et al., 1980; Ellis and Horvitz, 1986). Not surprisingly, several tumors emerge with mutations in genes conferring apoptosis resistance (Kerr et al., 1994; Reed, 1999) allowing them to continue uncontrolled growth under conditions that would be proapoptotic to normal cells. Resistance to apoptosis may also contribute to drug resistance, inasmuch as several anticancer drugs induce apoptosis (Kaufmann and Earnshaw, 2000).
Apoptosis is highly regulated. At the morphological level, it is characterized by membrane blebbing, cell shrinkage, chromatin condensation, nuclear/cytoplasmic fragmentation, and formation of dense bodies that are quickly removed via phagocytosis by neighboring cells. Apoptosis can be induced through receptor-mediated mechanisms and as a consequence of stresses, such as growth factor withdrawal (Frisch and Francis, 1994; Xia et al., 1995) or exposures to cytotoxic drugs (Hale et al., 1996; Muschel et al., 1998). Once triggered, the apoptotic program involves activation of a series of biochemical events comprising most of the times the release of proteins from the mitochondria into the cytoplasm and the nucleus. The best characterized execution pathway of apoptosis involves the release of cytochrome c that leads, in sequence, to the activation of caspases, the proteolytic degradation of specific substrates, the activation of nucleases, and the internucleosomal DNA fragmentation (Villa et al., 1997). Whereas caspases undoubtedly play an important role in apoptosis, some cytoplasmic and nuclear hallmarks of apoptosis can also occur independently of caspases. One caspase-independent mechanism involves the release of apoptosis-inducing factor (AIF) from the mitochondria and its translocation to the nucleus where it contributes in an unknown manner to trigger nuclear condensation (Susin et al., 1999, 2000).
The stress-activated protein kinases Jun N-terminal kinase (JNK) and p38 are induced in many cell lines when treated with toxic agents, and their activation has been repeatedly associated with induction of apoptosis. However, their role, particularly that of p38, is poorly defined. In principle, activation of the p38-signaling pathway during toxic aggression may aim at initiating either a defense or a homeostatic mechanism and therefore contribute to cell survival or, alternatively, may contribute to the signaling or execution of some of the apoptotic events. There is some evidence for a role of p38 in both directions. Overexpressing an active form of the p38 activator MKK6 protects cardiac myocytes from treatment with anisomycin, expression of active MEKK1, or β-adrenergic receptor-mediated apoptosis. The protection is blocked by the p38 inhibitor SB203580 (Zechner et al., 1998; Communal et al., 2000). Similarly, early activation of p38 is necessary and sufficient to protect Kym cells from tumor necrosis factor-α–mediated apoptosis (Roulston et al., 1998), and expression of the p38 β-isoform attenuates cell death induced by Fas ligand and UV light (Nemoto et al., 1998). p38 phosphorylates and activates mitogen-activated protein kinase-activated protein kinase-2 (MAPKAP kinase-2), leading to the phosphorylation of HSP27, a heat shock protein involved in phosphorylation-dependent protection against stress (Rouse et al., 1994; Huot et al., 1995; Lavoie et al., 1995). Activation of p38 may also protect through the down-regulation of the Fas receptor expression (Ivanov and Ronai, 2000). There are even more reports concerning a proapoptotic function of p38. p38 is proapoptotic in spontaneous apoptosis of neutrophils (Aoshiba et al., 1999) and apoptosis induced by withdrawal of trophic factors (Kummer et al., 1997), glutamate (Kawasaki et al., 1997), and sodium salicylate (Schwenger et al., 1997). Also, a p38 inhibitor blocks apoptosis induced by UV light, cis-diamminedichloroplatinum (cDDP or cisplatin), hyperosmolarity, and sphingosine (Frasch et al., 1998; Bulavin et al., 1999; Assefa et al., 2000; Sanchez-Prieto et al., 2000), and early membrane blebbing during oxidative stress-induced apoptosis is tightly regulated by p38-mediated actin organization (Huot et al., 1998). Such opposite effects on apoptosis are not unique to p38. Many growth-promoting pathways can be either pro- or antiapoptotic, depending on the cellular context (Thompson, 1998; Joneson and Bar-Sagi, 1999). Similar opposite effects were also found for the other stress-activated protein kinase JNK. Whereas in most studies JNK activation was necessary for apoptosis (Xia et al., 1995; Cahill et al., 1996; Frisch et al., 1996; Verheij et al., 1996; Zanke et al., 1996; Mosser et al., 1997; Toyoshima et al., 1997; Kim et al., 1999; Srivastava et al., 1999), in others, JNK was either without any effect or even protective (Potapova et al., 1997; Sanchez-Perez et al., 1998; Yujiri et al., 1999). The opposing effects on apoptosis observed for p38 probably reflect the multiple and complex activities of this signaling pathway, which acts on different targets at once and thus can yield distinct overall effects depending on the cellular context. Thus, identifying the specific targets of p38 during apoptosis is of major importance.
We used Rat-1 cells with deregulated expression of c-Myc as a model to study the role of the p38 pathway in the induction of apoptosis by anticancer drugs. Cells expressing deregulated c-Myc are hypersensitive to induction of apoptosis by a number of distinct stresses such as serum or growth factor starvation, exposures to cancer chemotherapeutic drugs, radiation, hypoxia, and death receptor activation (Askew et al., 1991; Evan et al., 1992; Graeber et al., 1996; Guo et al., 1997; Han et al., 1997; Klefstrom et al., 1997; Yu et al., 1997; Rupnow et al., 1998; Fulda et al., 1999). In the present study we show that activation of p38 is part of the apoptotic program elicited by expression of c-Myc in Rat-1 cells exposed to cisplatin and contributes importantly to cell blebbing and nuclear condensation. Blocking p38 activity antagonized these manifestations of apoptosis and resulted in a significant increase in cell resistance to the drug.
MATERIALS AND METHODS
Materials
[γ-32P]ATP (3000 Ci/mmol) was purchased from NEN Life Science Products (Boston, MA). Cisplatin, etoposide, cycloheximide, sodium arsenite, 4-hydroxytamoxifen (OHT), and cytochalasin D were from Sigma Chemicals (St. Louis, MO). SB203580 was obtained from Calbiochem (La Jolla, CA), N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD-fmk) from Enzyme Systems Products (Livermore, CA), and acetyl-aspartyl-glutamyl-valyl-aspartyl-amino-4-methylcoumarin was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). Recombinant Chinese hamster HSP27 and ATF2-glutathione S-transferase (GST) were purified from Escherichia coli transformed with appropriate plasmids (Landry et al., 1992; Dérijard et al., 1995). Chemicals for electrophoresis were purchased from Bio-Rad (Hercules, CA) and Fisher Scientific (Pittsburgh, PA). SB203580, zVAD-fmk, and cytochalasin D were diluted in dimethyl sulfoxide (DMSO) to make stock solution of 40, 20, and 1 mM, respectively. Cisplatin was diluted in water, and OHT was diluted in ethanol. Zero-concentration controls always included solvent-only solutions.
Antibodies
Anti-poly(ADP-ribose) polymerase (PARP) C2–10 is a monoclonal antibody raised against the amino acids 216–375 of PARP (Lamarre et al., 1988). Anti-MAPKAP kinase-2 was raised in rabbit against a GST fusion protein containing the 223 C-terminal amino acids of Chinese hamster MAPKAP kinase-2 (Huot et al., 1995). Anti-p38 is a rabbit polyclonal antibody raised against the C-terminal sequence PPLQEEMES of murine p38 (Huot et al., 1997). The anti-caspase-3 MF393 antibody recognizes procaspase-3 and its cleaved p17 active subunit (Mancini et al., 1998). Anti-lamins A/C (131C3) recognizes both lamins A and C and their cleavage products p47 and p37, respectively (Pugh et al., 1997). Anti-focal adhesion kinase (FAK) and anti-MCH-3/caspase-7 are monoclonal antibodies generated from chicken FAK and human MCH-3, respectively (Transduction Laboratories, Mississauga, Ontario, Canada). Anti-phospho p38 was purchased from New England Biolabs (Beverly, MA).
Cells
Rat-1/MycERTM cells express a human c-Myc protein that becomes active in the presence of OHT. In the control cell line Rat-1/ΔMycERTM, c-Myc has been replaced by a nonfunctional deletant of c-Myc (Littlewood et al., 1995). The cells were maintained in α-modified Eagle's medium containing NaHCO3 (2.2 g/l) supplemented with 10% fetal bovine serum. For stock maintenance cultures, the selection pressure was maintained with puromycin (5 μg/ml). c-Myc was activated by adding OHT to the medium at the final concentration of 100 nM for 16 h. The Chinese hamster CCL39 cell lines B12, V, and 3 were described before (Huot et al., 1995; Lavoie et al., 1995). B12 cells express 4.8 ng/μg human HSP27. Clone V expresses 3.3 ng/μg of a nonphosphorylatable form of human HSP27. Clone 3 expresses only the selection gene neo. CCL39 cell lines were maintained in DMEM containing NaHCO3 (2.2 g/l) and glucose (4.5 g/l) and supplemented with 5% fetal bovine serum. HeLa/p38(AGF) cells, expressing a nonactivable/phosphorylatable mutant form of p38α, and their parental cell line HeLa/HIVcat were described before (Taher et al., 1999). They were maintained in DMEM containing NaHCO3 (2.2 g/l) and glucose (4.5 g/l) and supplemented with 10% fetal bovine serum. Selection was maintained in stock cultures by adding geneticin (200 μg/ml) and hygromycin (200 μg/ml). All cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Transient Transfection Assay
Expression of wild-type HA-tagged p38 and mutant FLAG-tagged p38(AGF) was achieved by transfection of the plasmids pcDNA3-HA-p38 (Berra et al., 1998) and pCMV-FLAG-p38(AGF) (Raingeaud et al., 1995), respectively. DNA was introduced into Rat-1 cells by lipofection with the use of Effectene (Quiagen, Mississauga, ONT) according to the manufacturer's instructions. The cells were seeded at a density 2 × 105 per 25-cm2 flask and exposed 24 h later to a DNA/lipid mixture containing 1 μg of the plasmid and 25 μl of lipofection reagent in the cell culture medium for 24 h. OHT was added 8 h after the lipofection medium was washed out, and the cells were used another 16 h later (48 h after beginning of transfection).
Immunoprecipitation
The cells were scraped and extracted in lysis buffer containing 20 mM morpholinopropanesulfonic acid, pH 7.0, 10% glycerol, 80 mM β-glycerophosphate, 5 mM EGTA, 0.5 mM EDTA, 1 mM Na3VO4, 5 mM Na4P2O7, 50 mM NaF, 1% Triton X-100, 1 mM benzamidine, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. The extracts were vortexed and centrifuged at 17,000 × g for 12 min at 4°C. The clarified supernatants were immediately used for immunoprecipitation or were stored at −80°C. The succeeding steps were done at 4°C. The clarified supernatant was diluted four times in buffer I (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 1 mM EGTA, 1 mM MgCl2, 1 mM Na3VO4, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride). Anti-p38 or anti-MAPKAP kinase-2 antibodies were added in limiting concentrations, and the mixtures were incubated for 1 h. Protein A Sepharose (10–15 μl, 50%, vol/vol; Amersham Pharmacia Biotech, Piscataway, NJ) in buffer I were added, and the mixtures were incubated for 30 min. Samples were centrifuged for 15 s and washed three times with 300 μl of buffer I. Immunoprecipitates were used directly for kinase assays.
Kinase Assay
p38 and MAPKAP kinase-2 activities were assayed in immune complexes. MAPKAP kinase-2 was measured with the use of recombinant HSP27 as substrate (Huot et al., 1995). The assays were done in 25 μl of kinase buffer containing 100 μM ATP, 3 μCi of [γ32P]ATP, 40 mM p-nitrophenyl phosphate, 20 mM morpholinopropanesulfonic acid, pH 7.0, 10% glycerol, 15 mM MgCl2, 0.05% Triton X-100, 1 mM dithiothreitol, 1 μM leupeptin, 0.1 mM phenylmethylsulfonyl fluoride, and 0.3 μg of protein kinase A inhibitor. The kinase activity was assayed for 30 min at 30°C and was stopped by the addition of 10 μl of SDS sample buffer. Immunoprecipitated p38 was assayed analogously with ATF2-GST as substrate in a kinase assay buffer containing 50 μM ATP, 3 μCi of [γ32P]ATP, 50 mM HEPES, pH 7.4, 50 mM β-glycerophosphate, 50 mM MgCl2, 0.2 mM Na3VO4, and 4 mM dithiothreitol (Guay et al., 1997). The amount of radioactivity incorporated in the substrates was determined after electrophoresis with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Electrophoresis and Western blot were done essentially as described previously (Huot et al., 1995; Guay et al., 1997).
Morphological Features of Apoptosis
After treatments, the cells were fixed with 3.7% formaldehyde and permeabilized with 0.1% saponin in phosphate-buffered saline (PBS), pH 7.5. To visualize nuclear apoptosis, the cells were stained for 1 h with 4,6-diamidino-2-phenylindole (DAPI, 100 μg/ml) diluted in PBS. The percentage of cells with a condensed or fragmented nucleus was estimated with an Eclipse 600 epifluorescence microscope (Nikon, Melville, NY) by counting >500 different cells in random microscopic fields. Membrane blebbing was counted directly in live cells under Hoffman contrast microscopy or in fixed cells under standard phase contrast microcopy, with a Nikon Diaphot-TDM microscope equipped with a 40× objective. The percentage of cells with membrane blebbing was determined by counting >300 different cells in random microscopic fields. Pictures were taken with a Micromax CCD camera (Princeton Instruments, Trenton, NJ).
Internucleosomal DNA Fragmentation
After treatments, floating and adherent cells were washed with PBS, pooled, and then lysed at 37°C for 16 h in a buffer containing 10 mM Tris, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% SDS, and 0.2 mg/ml proteinase K. After phenol-chloroform extraction, DNA was precipitated with ethanol and suspended in 10 mM Tris, pH 8.0, 1 mM EDTA, and 0.5 mg/ml RNase. The DNA was separated into a 1.0% agarose gel.
Caspase Activities and Protein Cleavages
DEVDase activity in cell extract was determined essentially as described by Enari et al. (1996) with some modifications. After treatments, floating and adherent cells were washed with PBS and pooled. Cytosolic extracts were prepared by 13 repeated cycles of freezing and thawing in 100 μL of extraction buffer containing 50 mM morpholinopropanesulfonic acid, pH 7.0, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 20 μM cytochalasin D, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 50 μg/ml antipain. Extracts were clarified by centrifugation for 12 min in a microfuge at 4°C. For the assay, the protein extracts were mixed with 500 μl of the reaction buffer containing 100 mM HEPES-KOH, pH 7.5, 10% sucrose, 0.1% 3-[(cholamidopropyl)dimethylammonio]-1-propanesulfonic acid, 10 mM dithiothreitol, 0.1 mg/ml ovalbumin, and 1 μM caspase-3-like substrate acetyl-aspartyl-glutamyl-valyl-aspartyl-amino-4-methylcoumarin. After an incubation at 30°C for 30 min, the released fluorogenic substrate methylcoumarin was detected by excitation at 380 nm and emission at 460 nm with a luminescence spectrometer (model LS50B, Perkin Elmer-Cetus, Norwalk, CT). DEVDase activities was corrected for protein concentrations and normalized to the activity of the control sample.
The presence of cleaved proteins in situ was evaluated by Western blotting with specific antibodies. After treatments, floating and adherent cells were washed in PBS, pooled, and then solubilized in buffer containing 62.5 mM Tris, pH 6.8, 2% SDS, 6 M urea, 10% glycerol, 0.00125% bromophenol blue, and 720 mM β-mercaptoethanol. Proteins were separated on SDS electrophoresis and transferred onto nitrocellulose membrane. After reacting the membrane with specific antibodies, proteins were detected with an ECL detection kit (Amersham Pharmacia Biotech) or by iodinated secondary antibodies and quantified with a PhosphorImager.
Clonogenic Survival
Cells were treated in their exponential phase of growth. Immediately after treatments, they were trypsinized and plated at appropriate dilutions in triplicate to have approximately 50–200 viable cells per dish (Huot et al., 1996). Relative survival was calculated from the number of single cells that formed colonies of >50 cells within 12 d. The survival data were corrected for the plating efficiency of the appropriate controls.
RESULTS
c-Myc-dependent Apoptosis Is Associated with p38 Activation
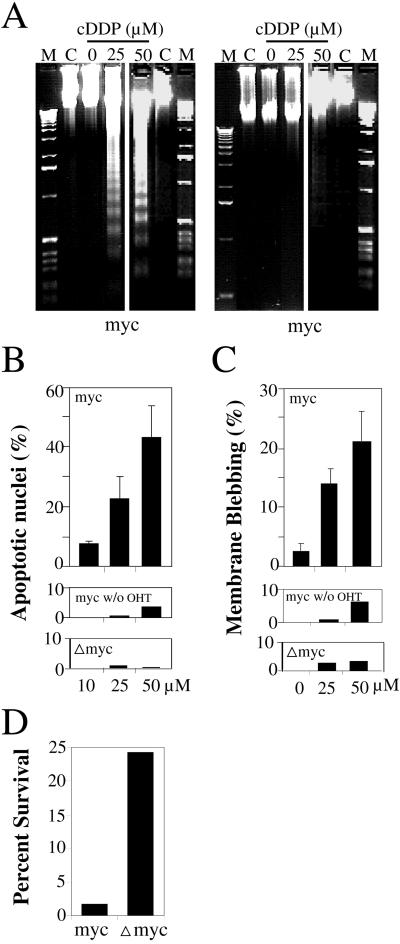
Activating c-Myc by addition of OHT to Rat-1 cells expressing MycERTM (Littlewood et al., 1995) made the cells highly sensitive to apoptosis induction by cisplatin. Under conditions of deregulated c-Myc expression, cisplatin induced in a dose-dependent manner several features of apoptosis, including internucleosomal DNA degradation (Figure 1A), nuclear condensation and fragmentation (Figure 1B), cell blebbing (Figure 1C), DEVDase activity, and the cleavage of several caspase substrates (Figure 3). All these features of apoptosis were absent or were induced at very low levels in Rat-1/MycERTM not pre-exposed to OHT or in Rat-1/Δmyc-ERTM cells, which expressed a nonfunctional deletant of c-Myc. The effect of c-Myc was not just to accelerate apoptosis and resulted in a real decrease in long-term cell survival as evaluated by the colony formation. Typically, deregulated expression of c-Myc caused a 10-fold decrease in the number of colonies that grew after exposure to cisplatin for 1 h at 25 μM (Figure 1D).
Figure 1.
Deregulated expression of c-Myc elicits apoptosis induction by cisplatin. Exponentially growing Rat-1/MycERTM (myc) or Rat-1/ΔMycERTM (Δmyc) cells were exposed to OHT for 16 h or left untreated (lanes C in panel A, middle panels in B anc C) and then exposed to cisplatin (cDDP) for 3 (C), or 6 h (A, B) at the indicated concentration of 0, 10, 25, or 50 μM, or for 1 h at a concentration of 25 μM (D). Lanes M contain DNA markers. Internucleosomal DNA degradation (A), condensed and fragmented nuclei (B), cell blebbing (C), and clonogenic survival (D) were evaluated as described in MATERIALS AND METHODS. The means and SDs were calculated from at least three separate experiments.
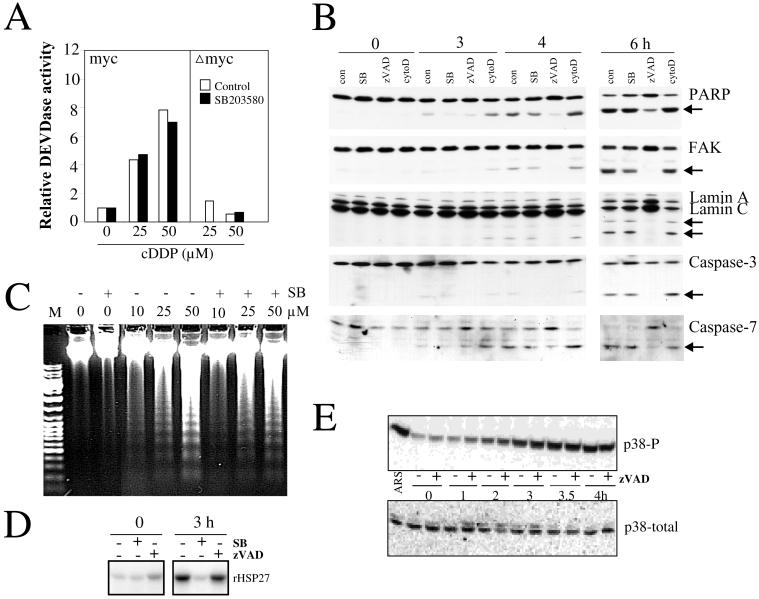
Figure 3.
p38 and caspases are activated in independent pathways. Rat-1/MycERTM (myc) or Rat-1/ΔMycERTM (Δmyc) cells were exposed to OHT for 16 h and then treated as indicated. (A) DEVDase activity: The cells were treated with cisplatin (cDDP, 0, 25, or 50 μM) in the presence of SB203580 (5 μM added 1 h before) or the vehicle only (control, 0.25% DMSO). Floating and adherent cells were pooled and processed to determine DEVDase activity in cell extracts as described in MATERIALS AND METHODS. (B) Cleaved protein substrates: Rat-1/MycERTM cells were pretreated with vehicle only (con, 0.5% DMSO), SB203580 (SB, 5 μM), zVAD-fmk (zVAD, 100 μM), or cytochalasin D (cytoD, 10 nM) for 1 h and then treated with cisplatin (50 μM) for the time indicated (0, 3, 4, or 6 h). Cell extracts were prepared, and after electrophoresis, blots were probed for PARP, FAK, lamin A/C, caspase-3, and caspase-7 with specific antibodies. Arrows indicate the position of the respective cleavage products. (C) Internucleosomal DNA degradation: Rat-1/MycERTM cells were pretreated with vehicle only (−, 0.25% DMSO) or SB203580 (+, 5 μM) for 1 h and then treated for 6 h with cisplatin at a concentration of 0, 10, 25, or 50 μM. DNA was analyzed as described in MATERIALS AND METHODS. Lane M contains DNA markers. (D and E) Role of caspases in the activation of the p38 kinase pathway. Rat-1/MycERTM were pretreated (+) or not (−) with zVAD-fmk (D, 50 μM; E, 100 μM) or SB203580 (D, 5 μM) and then treated with cisplatin (50 μM) for the time indicated (0–4 h) or with arsenite (ARS, 500 μM) for 1 h. Extracts were processed to determine MAPKAP kinase-2 activity with recombinant HSP27 (rHSP27) as substrate (D) or the levels of total (p38-total) and phosphorylated (p38-P) p38 with specific antibodies (E).
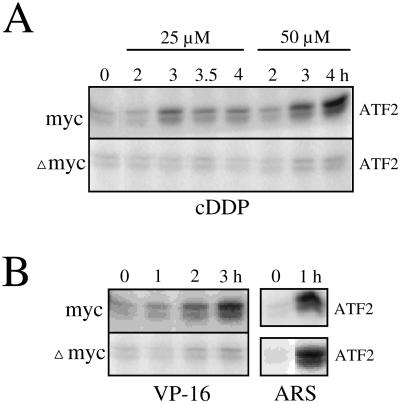
There was a very close relationship between apoptosis induction by cisplatin and p38 activation, which was also strictly dependent on the expression of a functional c-Myc. p38 was immunoprecipitated at various times after treatment with cisplatin, and its activity was determined with ATF2 as substrate. Cisplatin induced p38 only in the OHT-treated MycERTM cells. Activation of p38 was dose dependent and was detectable approximately 3 h after exposure to 25 and 50 μM cisplatin. No activity was induced for up to 4 h in the OHT-treated ΔmycERTM cells (Figure 2A) or in the MycERTM cells not preincubated with OHT (Deschesnes, Huot, and Landry, unpublished results). The lack of activation of p38 in these cells in response to cisplatin was not due to a general lack of responsiveness of the p38 pathway. Sodium arsenite (Figure 2B), H2O2 (Figure 6), and heat shock (Deschesnes, Huot, and Landry, unpublished results) induced p38 equally well in both ΔmycERTM and MycERTM cells exposed to OHT. Furthermore, the requirement for c-Myc in the activation of the p38 pathway was not restricted to cisplatin. Etoposide (Figure 2B) and many other anticancer agents tested, including doxorubicin, daunorubicin, taxol, and cycloheximide (Deschesnes, Huot, and Landry, unpublished results), also induced p38 (and apoptosis) in a c-Myc-dependent manner. The results suggested that activation of p38 by cisplatin in the OHT-treated MycERTM cells occurred as a consequence of the early signaling of apoptosis and thus may contribute specific events in the execution of apoptosis.
Figure 2.
Deregulated expression of c-Myc modulates induction of p38 by cisplatin and etoposide but not arsenite. Rat-1/MycERTM (myc) or Rat-1/ΔMycERTM (Δmyc) cells were exposed to OHT for 16 h and then treated for the time indicated (hours) with 25 or 50 μM cisplatin (A), 10 μg/ml etoposide (VP-16; B), or 500 μM arsenite (ARS; B). p38 kinase activities were measured in immunocomplexes with the use of ATF2-GST as substrate.
Figure 6.
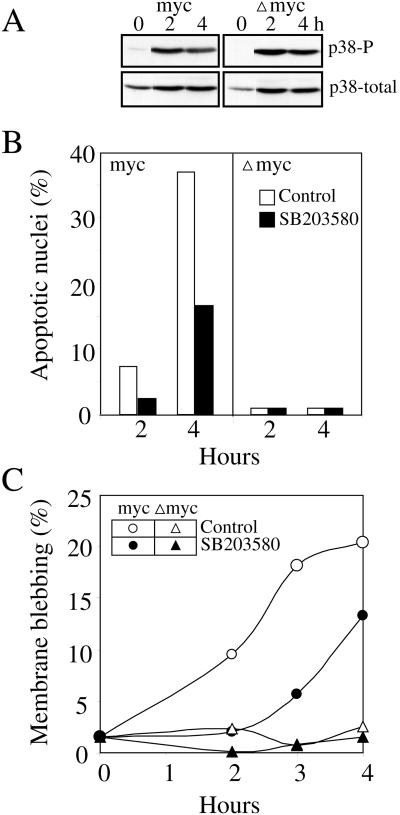
Induction of p38, apoptotic nuclei and membrane blebbing by H2O2. Rat-1/MycERTM (myc) or Rat-1/ΔMycERTM (Δmyc) cells were exposed to OHT for 16 h and then treated for the time indicated (hours) with 250 μM H2O2 in the presence or absence of SB203580 (5 μM). At the time indicated, cell extracts were prepared to determine the levels of total (p38-total) and phosphorylated (p38-P) p38 with specific antibodies (A) or the cells were fixed and, after staining with DAPI, the percentage of cells with an apoptotic nucleus (condensed or fragmented) (B) or with membrane blebbing (C) was determined by epifluorescence microscopy and Hoffman contrast, respectively.
Caspase and p38 Activities Are Induced Independently of Each Other
Caspases were also activated by cisplatin only in the apoptosis-prone cells. Extracts of OHT-activated MycERTM cells treated with cisplatin for 6 h contained four to eight times more DEVDases activity than similarly treated ΔmycERTM cells (Figure 3A) or cisplatin-treated MycERTM cells not pretreated with OHT (Deschesnes, Huot, and Landry, unpublished results). Activation of the caspases was reflected by the progressive cleavage of a number of caspase substrates, including caspase-3, caspase-7, PARP, FAK, lamin A, and lamin C (Figure 3B). We investigated whether p38 was activated upstream or downstream of caspases using the p38 inhibitor SB203580 (Cuenda et al., 1995) and the pan-caspase inhibitor zVAD-fmk. Used at a concentration of 5 μM, SB203580 totally blocked cisplatin-induced activation of MAPKAP kinase-2 (Figure 3D) and phosphorylation of HSP27 downstream of p38 (Deschesnes, Huot, and Landry, unpublished results); however, it had no or little effect on activation of DVEDase activities (Figure 3A) and on the extent of cleavage of the caspase substrates investigated, i.e., caspase-3, caspase-7, PARP, FAK, lamin A, or lamin C (Figure 3B). Also, SB203580 had no major effect on cisplatin-induced DNA internucleosomal degradation (Figure 3C), which occurs downstream of caspase-3 (Liu et al., 1997; Sakahira et al., 1998). Similarly, at a concentration that inhibited the caspase-dependent cleavage of protein substrates (Figure 3B) and the DEVDase activity in vitro (Figure 3A), zVAD-fmk had no effect on either the phosphorylation of p38 (measured with a phosphorylation-specific antibody) or the activation of MAPKAP kinase-2 (HSP27 kinase activity of immunoprecipitated MAPKAP kinase-2), measured at different times during cisplatin treatments (Figure 3, D and E). Thus, p38 and caspases are activated downstream of a common c-Myc-dependent event that also signals apoptosis; however, the activation is mostly independent. This suggested the possibility that p38 might contribute some caspase-independent features of apoptosis.
Involvement of p38 in Caspase-independent Cell Blebbing
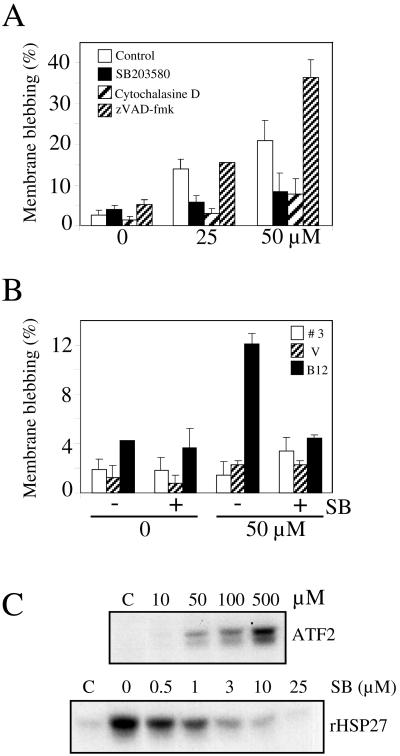
Apoptosis-prone Rat-1 cells were exposed to graded concentrations of cisplatin and examined under the microscope to determine the extent of cell blebbing. A 3-h treatment with cisplatin induced intense blebbing activities in up to 20% of the cells (Figure 4A). Cell blebbing did not require caspase activities and instead was enhanced by >50% in the presence of the pan-caspase inhibitor zVAD-fmk. This enhancement likely resulted from a block in the apoptotic process allowing the accumulation of blebbing cells, as previously observed in other cell systems (McCarthy et al., 1997; Huot et al., 1998; Mills et al., 1998). Consistent with a role of actin polymerization activity in cell blebbing, we found that a very low concentration of the inhibitor of actin polymerization cytochalasin D drastically reduced cell blebbing. In contrast to the effect of zVAD-fmk, SB203580 efficiently antagonized cisplatin-induced cell blebbing, reducing the frequency of cell blebbing close to the background level observed in the absence of cisplatin treatment.
Figure 4.
Cisplatin-induced membrane blebbing requires p38 activity and can be modulated by HSP27 phosphorylation and concentration. (A) Rat-1 cells. OHT-treated Rat-1/MycERTM cells were preincubated for 1 h with vehicle only (Control, 0.5% DMSO), SB203580 (5 μM), cytochalasin D (10 nM), or zVAD-fmk (100 μM) and then treated with cisplatin at the indicated concentrations for 3 h. (B) CCL39 cells. CCL39 clone B12 (overexpressing HSP27), clone V (overexpressing a phosphorylation mutant of HSP27), and clone 3 (control cell line) were pretreated (+) or not (−) for 1 h with SB203580 (SB, 5 μM) and then exposed to cisplatin (0, 50 μM) for 3 h. The percentage of cells showing membrane blebbing was counted under Hoffman contrast microscopy in live cells immediately after the treatments. The means and SDs are from at least three separate experiments. (C) SB203580 blocked cisplatin-induced p38 activity in CCL39 cells. CCL39 cells were exposed to cisplatin at the indicated concentrations (10, 50 100, or 500 μM) or to cisplatin at 500 μM in the presence of SB203580 at the indicated concentrations (0, 0.5, 1, 3, 10, or 25 μM). After 3-h exposures, p38 or MAPKAP kinase-2 were immunoprecipitated and their activities measured with the use of ATF2 or HSP27 as substrate, respectively.
Regulation of actin dynamics is one of the characterized functions of the p38 pathway. After activation by p38, MAPKAP kinase-2 phosphorylates HSP27, a protein that can modulate actin polymerization. (Lavoie et al., 1993, 1995; Guay et al., 1997; Huot et al., 1997; Piotrowicz and Levin, 1997; Rousseau et al., 1997; Landry and Huot, 1999). Rat-1 cells express constitutively high levels of HSP27 (Deschesnes, Huot, and Landry, unpublished results). To confirm that HSP27 phosphorylation can mediate bleb formation in response to cisplatin, we used CCL39 cells, a Chinese hamster fibroblast cell line that expresses little HSP27, and three previously established CCL39-derived cell lines (Lavoie et al., 1995), which express wild-type human HSP27 (clone B12), a nonphosphorylatable mutant of HSP27 (clone V) or the neo gene only (clone 3). In spite of a strong SB203580-inhibitable p38 activity induced by cisplatin in CCL39 cells (Figure 4C), no cell blebbing was induced in clone 3 or in clone V. Blebbing was induced only in B12 cells and was inhibited by SB203580 (Figure 4B). Thus, membrane blebbing in these cells was highly dependent on the overexpression of a phosphorylatable HSP27 and on p38 activity.
Involvement of p38 in Nuclear Apoptosis
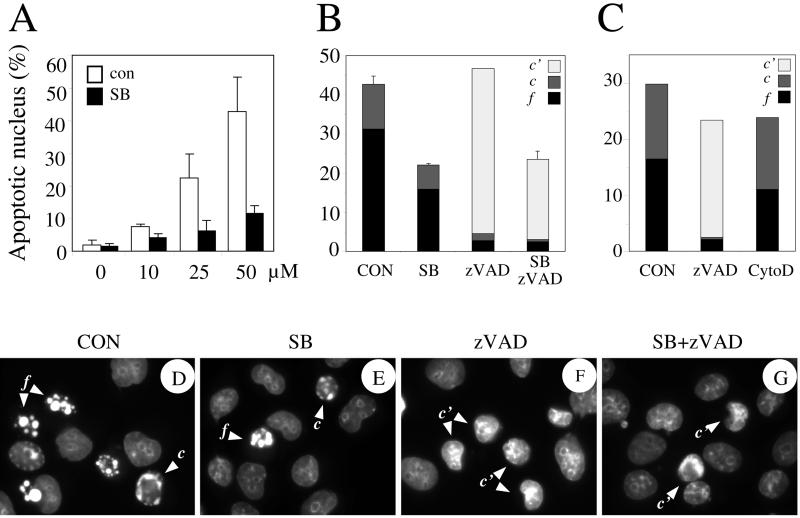
Cisplatin-induced alteration in the nuclear morphology was dose dependent, and after 6 h of treatment at 50 μM, highly condensed (labeled c in Figure 5) or fragmented nuclei (labeled f) were observed in approximately 30–40% of the cells (Figure 5, A, B, and D). In the presence of zVAD-fmk, severe nuclear condensation and fragmentation were almost totally inhibited; however, the percentage of cells with altered nuclear morphology was not reduced significantly, the cells seemingly accumulating in a distinctive caspase-independent morphological phase of nuclear alteration (labeled c′), characterized by deformation and condensation in the central part of the nuclei (Figure 5, B, C, and F). Adding SB203580 to zVAD-fmk reduced the number of cells with this altered nuclear morphology typically by twofold (Figure 5, B and G). Interestingly, SB203580 alone also antagonized by twofold the caspase-dependent nuclear fragmentation, but in contrast to the zVAD-fmk, the nuclei were left with a totally normal morphology (Figure 5, A, B, and E). Thus, p38 partially antagonized the whole process, whereas zVAD-fmk caused a total inhibition of only some of the features. The results suggested the involvement of p38 in an early event that promoted both caspase-dependent and caspase-independent nuclear apoptosis.
Figure 5.
SB203580 antagonizes cisplatin-induced nuclear condensation and fragmentation. OHT-treated Rat-1/MycERTM cells were preincubated for 1 h with vehicle only (CON), cytochalasin D (CytoD, 10 nM), SB203580 (SB, 5 μM), zVAD-fmk (zVAD, 100 μM), or both SB203580 and zVAD-fmk (SB+zVAD) and then treated with cisplatin for 6 h at varying concentrations as indicated (A: 0, 10, 25, or 50) or at 50 μM (all data in B–G). After staining with DAPI, the percentage of cells with an apoptotic nucleus was determined by epifluorescence microscopy. Nuclei showing condensation (c or c′) or fragmentation (f) were considered apoptotic and counted as a single group of apoptotic cells (A) or kept in separate groups (B and C).
To determine whether the effect of inhibiting p38 on the nuclear morphology might be a consequence of inhibiting blebbing, we looked at the effect of cytochalasin D. At a concentration that blocked cell blebbing, cytochalasin D did not affect significantly nuclear apoptotic morphology (Figure 5C), nor did it affected the cleavage of the caspase substrates (Figure 3B). Hence, there was no causal link between the morphological changes observed at the level of the plasma membrane and the nucleus.
p38 also Contributes to but Is Not Sufficient for H2O2-induced Cell Blebbing and Nuclear Condensation
As mentioned before, we found that, in contrast to cisplatin, H2O2 induced p38 activity in both MycERTM and ΔmycERTM cells (Figure 6A), indicating that deregulated c-Myc expression was not required for activation of p38 by H2O2. Deregulated c-Myc expression was required, however, for H2O2-induced nuclear condensation and fragmentation and membrane blebbing (Figure 6, B and C). In ΔmycERTM cells, blebbing and nuclear alterations remained at normal background levels for up to 4 h of exposure to H2O2, in spite of a strong activation of p38. In MycERTM cells, H2O2 induced very strongly those two characteristic features of apoptosis. Inhibiting p38 activity with SB203580 reduced apoptosis by ∼50%.
p38 Also Contributes to Cisplatin-induced Cell Blebbing and Nuclear Condensation in HeLa Cells
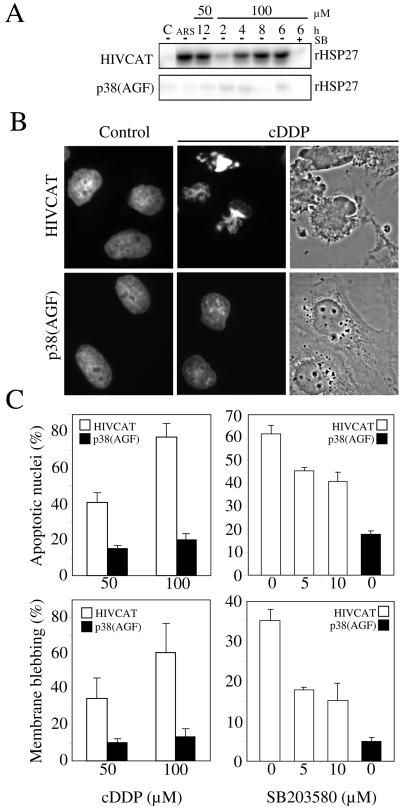
The role of p38 activity in the induction of blebbing and alterations of nuclear morphology was confirmed in another cellular context by comparing the response of parental HeLa cells to cisplatin in the presence or absence of SB203580 with that of HeLa cells expressing an interfering mutant of p38 [HeLa-p38(AGF); Taher et al., 1999]. The expression of p38(AGF) as well as the presence of SB203580 efficiently inhibited p38 activity, yielding very little in situ activation of MAPKAP kinase 2, after either cisplatin or arsenite treatment (Figure 7A). Cisplatin induced dose-dependent membrane blebbing and nuclear apoptosis in control HeLa cells. Inhibiting p38 significantly reduced cell blebbing and nuclear apoptosis. The inhibition obtained with SB203580 was similar to that obtained in Rat-1 cells. For reasons that were not investigated, p38(AGF) was even more efficient than the chemical inhibitor and reduced cell blebbing and nuclear apoptosis by >75% (Figure 7, B and C).
Figure 7.
Overexpression of a dominant-negative p38 antagonizes cisplatin-induced membrane blebbing, nuclear condensation, and fragmentation. (A) HeLa/HIVCAT and HeLa/p38(AGF) cells were treated for varying periods of time as indicated (in hours) with cisplatin (50 or 100 μM) or for 1 h with arsenite (ARS, 500 μM), which was used as a positive control. The inhibitory effect of SB203580 (SB+, 5 μM) was tested during a 6-h exposure to cisplatin. Extracts were prepared and processed to determine MAPKAP kinase-2 activity with the use of recombinant HSP27 (rHSP27) as substrate. (B) HeLa/HIVCAT and HeLa/p38(AGF) cells were treated with cisplatin (100 μM) for 12 h, fixed, and stained with DAPI. The microphotographs illustrate DAPI-stained nuclei for both cell types, treated (cDDP) or untreated (Control) and phase contrast morphology of the treated cells. (C) The cells were treated with cisplatin at 50 or 100 μM (cDDP) in the absence of SB203580 (left) or with cisplatin at 100 μM in the presence of varying concentration of SB203580 as indicated. The percentage of cells with an apoptotic nucleus and membrane blebbing was determined with epifluorescence of DAPI-stained cells and phase contrast microscopy, respectively.
p38 Contributes to Cisplatin-induced Cell Death
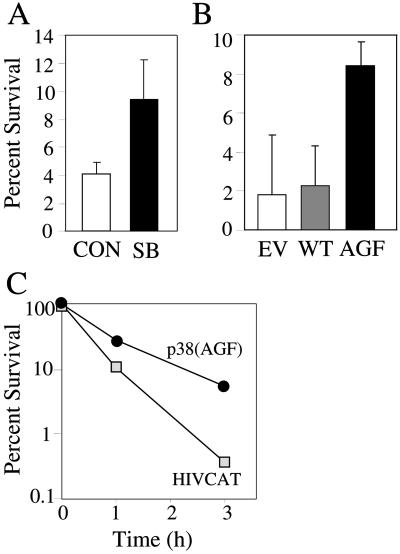
Colony formation assays were used to determine whether activation of p38 contributed significantly to cell death. Rat-1 cells were exposed to cisplatin for 1 h at a concentration of 25 μM and plated at low concentrations to determine the proportion of cells capable of forming colonies. The cells were transfected before treatment with either wild-type p38, an empty vector used as controls, or with the kinase inactive mutant of p38. In other experiments, SB203580 was added 1 h before cisplatin and left during treatments. Both approaches to block p38 resulted in a two- to fourfold increase in cell survival (Figure 8, A and B), in spite of the fact that only ∼50% of the cells expressed the mutant in the case of transfection. Not surprisingly, an even more spectacular effect was obtained comparing control and HeLa-p38(AGF) cells, the latter surviving cisplatin exposure ∼10 times better than the former (Figure 8C). Hence, p38 activity significantly affected the probability of the cells to survive cisplatin treatments.
Figure 8.
p38 activity contributes to clonogenic cell death. (A) OHT-treated Rat-1/MycERTM cells were incubated with cisplatin (25 μM) for 1 h in the presence or absence of SB203580 (SB, 5 μM) added 1 h before treatment. (B) Rat-1/MycERTM were transfected with either pcDNA3 (EV, empty vector), pcDNA3-HA-p38 (WT, wild-type p38), or pCMV-Flag-p38(AGF) (AGF, dominant-negative p38), activated with OHT and treated with cisplatin (25 μM) for 1 h. (C) HeLa/HIVCAT and HeLa/p38(AGF) cells were treated with cisplatin (10 μM) for up to 3 h. After the treatments, survival was determined by colony formation. One hundred percent is defined relative to the number of colonies obtained with the same cells untreated with cisplatin.
DISCUSSION
c-Myc is essential for induction of apoptosis in many experimental systems. c-Myc null fibroblasts or fibroblasts with low levels of c-Myc expression are resistant to apoptosis (Chang et al., 2000), whereas cells with deregulated expression of Myc become sensitive to apoptosis induction by a number of conditions, including growth factor deprivation, inhibition of protein synthesis, and treatments with cancer chemotherapeutic agents (Askew et al., 1991; Evan et al., 1992; Graeber et al., 1996; Guo et al., 1997; Han et al., 1997; Klefstrom et al., 1997; Yu et al., 1997; Nesbit et al., 1998; Rupnow et al., 1998; Fulda et al., 1999). In Rat-1 cells, we found here that all cisplatin-induced features of apoptosis and also p38 activation were c-Myc dependent. A similar sensitization to apoptosis by c-Myc was found for several other cancer chemotherapeutic agents, including etoposide, methotrexate, taxol, doxorubicin, colchicine, and 5-fluorouracil (Deschesnes, Huot, and Landry, unpublished results). c-Myc-sensitization to apoptosis has previously been mechanistically associated with sensitization to caspase activation (Kagaya et al., 1997; McCarthy et al., 1997; Kangas et al., 1998). One mechanism of action of c-Myc is to facilitate the release of cytochrome c from mitochondria, which triggers activation of caspases (Juin et al., 1999; Kennedy et al., 1999; Conzen et al., 2000). Consistent with a proximal action of c-Myc at the level of the mitochondria, we found that caspases were strongly activated early during cisplatin treatments. Here we propose that sensitization to p38 activation is also involved in c-Myc sensitization to apoptosis, contributing to both caspase-independent membrane blebbing and to caspase-dependent and caspase-independent nuclear apoptosis.
It is intriguing that p38 can be induced selectively in cells with deregulated expression of c-Myc. p38 is activated in a cascade of kinase reactions that can involve the two MAP kinase kinases MKK3 and MKK6 (Dérijard et al., 1995; Han et al., 1996; Moriguchi et al., 1996; Raingeaud et al., 1996) and several different MAP kinase kinase kinases such as MLK2/3, MEKK1, ASK1, and TAK1 (Moriguchi et al., 1996; Tibbles et al., 1996; Ichijo et al., 1997; Cuenda and Dorow, 1998). In addition to genotoxic agents, p38 can be activated in mammalian cells by a vast array of agents, including chemical and physical stresses such as heat shock, oxidants, hyperosmolarity, and numerous cytokines and growth factor agonists (reviewed by Widmann et al., 1999). The particular MAP kinase kinases and MAP kinase kinase kinases that are used to activate p38 probably vary depending on the nature of the triggering signal. In the case of H2O2 or tumor necrosis factor-α, for example, ASK1 seems to be the MAP kinase kinase kinase involved in the activation of p38. The signal is generated through the oxidative stress sensor thioredoxin, which acts as a regulator of ASK1 (Gotoh and Cooper, 1998; Saitoh et al., 1998). Little is known concerning the mechanism of activation of p38 by genotoxic agents. In the particular case of cisplatin, damage to DNA may trigger p38 activation through activation of the tyrosine kinase c-abl (Pandey et al., 1996). ASK1 also has been shown to be involved in cisplatin-induced p38 activation, suggesting a pathway from c-abl to ASK1 to p38 (Chen et al., 1999). How c-Myc expression can act as an essential factor in this pathway is unknown. We observed that activation of p38 by arsenite and also H2O2 in Rat-1 cells is not dependent on c-Myc expression, suggesting that c-Myc acts upstream of ASK1 to elicit activation of p38 by cisplatin. Furthermore, a number of other agents tested, such as etoposide and cycloheximide, also require deregulated c-Myc expression for the activation of p38 (Deschesnes, Huot, and Landry, unpublished results), indicating that the site of action of c-Myc is not in the part of the p38-signaling pathway that is specific to cisplatin. It is likely that c-Myc does not play a direct role in the p38 pathway but rather that p38 becomes activable as a consequence of c-Myc transformation. The finding that p38 activation is not dependent on caspase activation suggests that the activation is not a consequence of the stressful conditions generated by apoptosis but likely depends, like the caspase activation, on the action of c-Myc at a proximal point in the apoptosis-signaling pathway. One intriguing possibility is that the signal for p38 activation during cisplatin also originates from a c-Myc-dependent alteration at the level of mitochondria. It would be of interest to determine whether cisplatin-induced p38 activation in other cell lines such as CCL39 or HeLa also depends on an altered regulation of c-Myc or on other oncogenic events deregulating growth in these cells.
Membrane blebbing was caspase independent and appeared as one major consequence of cisplatin-induced p38 activation downstream of c-Myc. Not only was cell blebbing not inhibited in the presence of caspase inhibitors but it was increased, likely as a result of the accumulation of the cells in an unfinished state of apoptosis (McCarthy et al., 1997; Mills et al., 1998). Cell blebbing involves intense membrane movement powered by actin filament dynamics and, accordingly, was associated before with signal transduction pathways regulating actin dynamics (Mills et al., 1999). It has been shown that actin polymerization activities can be generated downstream of p38 by the phosphorylation of HSP27, a protein that regulates actin polymerization activity in vitro (Lavoie et al., 1993, 1995; Benndorf et al., 1994; Guay et al., 1997; Huot et al., 1997; Piotrowicz and Levin, 1997; Rousseau et al., 1997; Landry and Huot, 1999). This activity has been associated with reorganization of the actin cytoskeleton, cell migration, and protection or stabilization of actin filament during oxidative stress or heat shock. Our results showing that blebbing was inhibited by cytochalasin D and could be modulated by the concentration and phosphorylation of HSP27 strongly suggest that actin polymerization generated by p38 activity in response to cisplatin was responsible for membrane blebbing. How can the same actin polymerization signal pathway lead to microfilament reorganization or cell crawling in some cellular contexts and to blebbing in others? Clearly, as shown here in the case of hydrogen peroxide, p38 activation is not sufficient to induce cell blebbing (or nuclear alterations) and an apoptosis context generated in Rat-1 cells by c-Myc is also required. As previously suggested, it is likely that other alterations generated by cisplatin and hydrogen peroxide in the apoptosis-prone cells prevent the appropriate organization of the newly polymerized filaments, causing their accumulation at the membrane and subsequent blebbing. For example, the coactivation of p38 and the mitogen-activated protein kinase ERK is essential to generate a full reorganization of the microfilaments in endothelial cells in response to hydrogen peroxide. In the absence of ERK activity, hydrogen peroxide generates in these cells p38-dependent cell blebbing instead of microfilament assembly (Huot et al., 1998). It is noteworthy that cisplatin does not induce significant ERK activities in Rat-1 cells (Deschesnes, Huot, and Landry, unpublished results). Perhaps an imbalanced activation of the p38 and ERK pathways can lead to membrane blebbing.
Apoptosis-associated nuclear condensation and fragmentation are generally attributed to caspase activities leading to the cleavage of substrates such as lamins, caspase-activated DNAse, or acinus (Rao et al., 1996; Liu et al., 1997; Sakahira et al., 1998; Sahara et al., 1999). However, nuclear condensation can also proceed in the absence of caspase activity. One proposed mechanism involved the AIF, a mitochondrial oxidoreductase, which after leaking out from the mitochondria can induce nuclear condensation in a caspase-independent manner (Susin et al., 1999, 2000; Daugas et al., 2000). Both caspase-independent and caspase-dependent nuclear apoptosis were induced by cisplatin in Rat-1 cells. Cisplatin-induced severe nuclear fragmentation was totally blocked in the presence of zVAD-fmk; however, some form of nuclear condensation morphologically distinct from that seen in the absence of zVAD-fmk accumulated when caspases were inhibited. These morphological features either represented a short-live intermediate step preceding caspase-dependent fragmentation or a redundant pathway of apoptosis occurring in parallel to and being normally masked by the caspase-dependent features. SB203580 had an antagonistic effect on both of these morphological features of nuclear apoptosis, meaning that p38 acts at an early time, making the cells more sensitive to both caspase-dependent and caspase-independent processes of nuclear condensation.
Very little is known about the proapoptotic events activated downstream of p38 which might contribute to both caspase-dependent and -independent nuclear apoptosis. One possible mechanism is suggested by the recent finding that p38 can regulate the translocation of Bax from the cytoplasm to the mitochondria (Ghatan et al., 2000). Bax-mediated changes in the integrity of the mitochondria can enhance caspase-dependent apoptosis by promoting additional cytochrome c release and caspase activation. Bax could also promote caspase-independent cell death (Ghatan et al., 2000) possibly by enhancing the loss of mitochondrial potential and thereby promoting the release from the mitochondria of factors such as AIF. In such a situation, inhibiting p38 would block these additional effects of Bax on the mitochondria and lead, as observed here, to a partial inhibition of both caspase-dependent and caspase-independent apoptosis. The action of Bax at the level of mitochondria does not need to cause a major increase in caspase activity to be important at the survival level. Regulators acting downstream of cytochrome c release such as the inhibitor of apoptosis proteins are suggested to maintain a threshold of tolerance to caspase activity (Deveraux and Reed, 1999; Salvesen and Dixit, 1999). A very small relative increase of caspase activity over this threshold may be sufficient to produce a large effect on the nuclear fragmentation endpoint while being undetectable in total cell extract. Another possible regulator of caspase activation downstream of cytochrome c is HSP27. HSP27, which is expressed at high basal levels in Rat-1 cells, has recently been described as an inhibitor of caspase-3 activation downstream of cytochrome c (Bruey et al., 2000; Pandey et al., 2000). Phosphorylation of HSP27 mediated by p38 could in theory activate this inhibitory function of HSP27 at the level of the apoptosome, such that the net increase in caspase activity would be negligible in spite of the p38/Bax-stimulated cytochrome c release. Because SB203580 also reduced caspase-dependent nuclear fragmentation, an action of p38 on the caspase-dependent process without affecting caspases would imply the existence of other p38-dependent events that sensitize the cells to caspase-dependent processes. Finally, a totally different action of p38 may be responsible for the effects observed here. p38 can directly or indirectly affect the activity of several transcriptional factors, including p53, a key regulator of c-Myc-dependent apoptosis (Hermeking and Eick, 1994; Bulavin et al., 1999). Through modulation of transcription of specific genes, activation of p38 may make the cells more sensitive to the action of caspases as well as other executioner of apoptosis.
Understanding how drugs induce apoptosis in different cell systems is of utmost importance for the improvement of cancer chemotherapy because it may reveal how apoptosis can be manipulated to increase the sensitivity of tumor cells while reducing that of normal tissue. Rat-1/MycERTM cells represent an interesting experimental system because they can be easily switched from an apoptosis-resistant to an apoptosis-sensitive state. With the use of colony formation assays, we found here that apoptosis contributes in a quantitative manner to cell death in Rat-1/MycERTM cells exposed to cisplatin and, thus, that c-Myc transformation-dependent apoptosis is not just a mechanism for the disposal of doomed cells. We also showed that p38 activation can be selectively activated in c-Myc-transformed cells, suggesting that some pathways of p38 activation, such as apoptosis itself, may be linked to oncogenic transformation in general. p38 appears to play a preeminent role in cell death. In both HeLa and Rat-1 cells, inhibiting p38 activity not only delays apoptosis or alters the morphology of the dying cells from one of apoptosis to one of another form of cell death but really affects cell survival as judged by the long-term capacity of the cisplatin-treated cells to form colonies. Hence, understanding how c-Myc expression affects the sensitivity to p38 activation and identifying more precisely the proapoptotic target molecules of p38 have direct implications on understanding the response of cancer cells to chemotherapeutic agents.
ACKNOWLEDGMENTS
We thank L. Penn for providing the Rat-1 cell lines, J. Moscat and R. Davis for providing the p38 plasmids, and G. Poirier, Y. Raymond, and D. Nicholson for kindly donating the anti-PARP, anti-lamins, and anti-caspase-3 antibodies, respectively. This work was supported by the Canadian Institutes of Health Research. R.G.D. received a FCAR/FRSQ-Santé studentship from the Fonds pour la formation de chercheurs et l'aide à la recherche and the Fonds de recherche en santé du Québec.
Abbreviations used:
- AIF
apoptosis-inducing factor
- cDDP or cisplatin
cis-diamminedichloroplatinum
- DAPI
4,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- FAK
focal adhesion kinase
- GST
glutathione S-transferase
- JNK
Jun N-terminal kinase
- MAP
mitogen-activated protein
- MAPKAP kinase-2
MAP kinase activated protein kinase-2
- OHT
4-hydroxytamoxifen
- PBS
phosphate-buffered saline
- PARP
poly(ADP-ribose) polymerase
- zVAD-fmk
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone. .
REFERENCES
- Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J Immunol. 1999;162:1692–1700. [PubMed] [Google Scholar]
- Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- Assefa Z, Vantieghem A, Garmyn M, Declercq W, Vandenabeele P, Vandenheede JR, Bouillon R, Merlevede W, Agostinis P. p38 mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochrome c release in ultraviolet B radiation-induced apoptosis. J Biol Chem. 2000;275:21416–21421. doi: 10.1074/jbc.M002634200. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Berra E, Diaz-Meco MT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ., Jr Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill MA, Peter ME, Kischkel FC, Chinnaiyan AM, Dixit VM, Krammer PH, Nordheim A. CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene. 1996;13:2087–2096. [PubMed] [Google Scholar]
- Chang DW, Claassen GF, Hann SR, Cole MD. The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol Cell Biol. 2000;20:4309–4319. doi: 10.1128/mcb.20.12.4309-4319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- Communal C, Colucci WS, Singh K. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta-adrenergic receptor-stimulated apoptosis: evidence for Gi-dependent activation. J Biol Chem. 2000;275:19395–19400. doi: 10.1074/jbc.M910471199. [DOI] [PubMed] [Google Scholar]
- Conzen SD, Gottlob K, Kandel ES, Khanduri P, Wagner AJ, O'Leary M, Hay N. Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis. Mol Cell Biol. 2000;20:6008–6018. doi: 10.1128/mcb.20.16.6008-6018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Dorow DS. Differential activation of stress-activated protein kinase kinases SKK4/MKK7 and SKK1/MKK4 by the mixed-lineage kinase-2 and mitogen-activated protein kinase kinase (MKK) kinase-1. Biochem J. 1998;333:11–15. doi: 10.1042/bj3330011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- Dérijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch RJ, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–684. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Frasch SC, Nick JA, Fadok VA, Bratton DL, Worthen GS, Henson PM. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem. 1998;273:8389–8397. doi: 10.1074/jbc.273.14.8389. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun-N-terminal kinase in anoikis: suppression by bcl-2 and crmA. J Cell Biol. 1996;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Lutz W, Schwab M, Debatin KM. MycN sensitizes neuroblastoma cells for drug-induced apoptosis. Oncogene. 1999;18:1479–1486. doi: 10.1038/sj.onc.1202435. [DOI] [PubMed] [Google Scholar]
- Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, Youle RJ, Morrison RS. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000;150:335–347. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Cooper JA. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Guo M, Chen C, Vidair C, Marino S, Dewey WC, Ling CC. Characterization of radiation-induced apoptosis in rodent cell lines. Radiat Res. 1997;147:295–303. [PubMed] [Google Scholar]
- Hale AJ, Smith CA, Sutherland LC, Stoneman VE, Longthorne VL, Culhane AC, Williams GT. Apoptosis: molecular regulation of cell death. Eur J Biochem. 1996;236:1–26. doi: 10.1111/j.1432-1033.1996.00001.x. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Han JW, Dionne CA, Kedersha NL, Goldmacher VS. p53 status affects the rate of the onset but not the overall extent of doxorubicin-induced cell death in rat-1 fibroblasts constitutively expressing c-Myc. Cancer Res. 1997;57:176–182. [PubMed] [Google Scholar]
- Hermeking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Huot J, Lambert H, Lavoie JN, Guimond A, Houle F, Landry J. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur J Biochem. 1995;227:416–427. doi: 10.1111/j.1432-1033.1995.tb20404.x. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten-Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Ronai Z. p38 protects human melanoma cells from UV-induced apoptosis through down-regulation of NF-kappaB activity and Fas expression. Oncogene. 2000;19:3003–3012. doi: 10.1038/sj.onc.1203602. [DOI] [PubMed] [Google Scholar]
- Joneson T, Bar-Sagi D. Suppression of ras-induced apoptosis by the rac GTPase. Mol Cell Biol. 1999;19:5892–5901. doi: 10.1128/mcb.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juin P, Hueber AO, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya S, Kitanaka C, Noguchi K, Mochizuki T, Sugiyama A, Asai A, Yasuhara N, Eguchi Y, Tsujimoto Y, Kuchino Y. A functional role for death proteases in s-Myc- and c-Myc-mediated apoptosis. Mol Cell Biol. 1997;17:6736–6745. doi: 10.1128/mcb.17.11.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas A, Nicholson DW, Hottla E. Involvement of CPP32/caspase-3 in c-Myc-induced apoptosis. Oncogene. 1998;16:387–398. doi: 10.1038/sj.onc.1201779. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis: its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Lee KW, Park BY, Lee JK, Park J, Choi IY, Eom SJ, Chang TS, Kim MJ, Yeom YI, Chang SK, Lee YD, Choi EJ, Han PL. Molecular cloning of multiple splicing variants of JIP-1 preferentially expressed in brain. J Neurochem. 1999;72:1335–1343. doi: 10.1046/j.1471-4159.1999.721335.x. [DOI] [PubMed] [Google Scholar]
- Klefstrom J, Arighi E, Littlewood T, Jaattela M, Saksela E, Evan GI, Alitalo K. Induction of TNF-sensitive cellular phenotype by c-Myc involves p53 and impaired NF-kappaB activation. EMBO J. 1997;16:7382–7392. doi: 10.1093/emboj/16.24.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- Lamarre D, Talbot B, de Murcia G, Laplante C, Leduc Y, Mazen A, Poirier GG. Structural and functional analysis of poly(ADP ribose) polymerase: an immunological study. Biochim Biophys Acta. 1988;950:147–160. doi: 10.1016/0167-4781(88)90007-3. [DOI] [PubMed] [Google Scholar]
- Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat shock protein of 27 kDa (Hsp27) Biochem Soc Symp. 1999;64:79–89. [PubMed] [Google Scholar]
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Mancini M, Nicholson DW, Roy S, Thornberry NA, Peterson EP, Casciola-Rosen LA, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC, Stone NL, Pittman RN. Extranuclear apoptosis: the role of the cytoplasm in the execution phase. J Cell Biol. 1999;146:703–708. doi: 10.1083/jcb.146.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel RJ, Soto DE, McKenna WG, Bernhard EJ. Radiosensitization and apoptosis. Oncogene. 1998;17:3359–3363. doi: 10.1038/sj.onc.1202580. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38beta mitogen-activated protein kinase. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- Nesbit CE, Fan S, Zhang H, Prochownik EV. Distinct apoptotic responses imparted by c-myc and max. Blood. 1998;92:1003–1010. [PubMed] [Google Scholar]
- Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, Weichselbaum R, Kufe D, Kharbanda S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- Pandey P, Raingeaud J, Kaneki M, Weichselbaum R, Davis RJ, Kufe D, Kharbanda S. Activation of p38 mitogen-activated protein kinase by c-Abl-dependent and -independent mechanisms. J Biol Chem. 1996;271:23775–23779. doi: 10.1074/jbc.271.39.23775. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem. 1997;272:25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- Potapova O, Haghighi A, Bost F, Liu C, Birrer MJ, Gjerset R, Mercola D. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J Biol Chem. 1997;272:14041–14044. doi: 10.1074/jbc.272.22.14041. [DOI] [PubMed] [Google Scholar]
- Pugh GE, Coates PJ, Lane EB, Raymond Y, Quinlan RA. Distinct nuclear assembly pathways for lamins A and C lead to their increase during quiescence in Swiss 3T3 cells. J Cell Sci. 1997;110:2483–2493. doi: 10.1242/jcs.110.19.2483. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Dérijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- Roulston A, Reinhard C, Amiri P, Williams LT. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Rupnow BA, Alarcon RM, Giaccia AJ, Knox SJ. p53 mediates apoptosis induced by c-Myc activation in hypoxic or gamma irradiated fibroblasts. Cell Death Differ. 1998;5:141–147. doi: 10.1038/sj.cdd.4400328. [DOI] [PubMed] [Google Scholar]
- Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation [see comments] Nature. 1999;401:168–173. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000;60:2464–2472. [PubMed] [Google Scholar]
- Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA. 1997;94:2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RK, Mi QS, Hardwick JM, Longo DL. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prevost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;192:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Taher MM, Baumgardner T, Dent P, Valerie K. Genetic evidence that stress-activated p38 MAP kinase is necessary but not sufficient for UV activation of HIV gene expression. Biochemistry. 1999;38:13055–13062. doi: 10.1021/bi9902900. [DOI] [PubMed] [Google Scholar]
- Thompson EB. The many roles of c-Myc in apoptosis. Annu Rev Physiol. 1998;60:575–600. doi: 10.1146/annurev.physiol.60.1.575. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR, Lassam NJ. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F, Moriguchi T, Nishida E. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6–p38 pathways independent of CPP32-like proteases. J Cell Biol. 1997;139:1005–1015. doi: 10.1083/jcb.139.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Villa P, Kaufmann SH, Earnshaw WC. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yu K, Ravera CP, Chen YN, McMahon G. Regulation of Myc-dependent apoptosis by p53, c-Jun N-terminal kinases/stress-activated protein kinases, and Mdm-2. Cell Growth Differ. 1997;8:731–742. [PubMed] [Google Scholar]
- Yujiri T, Fanger GR, Garrington TP, Schlesinger TK, Gibson S, Johnson GL. MEK kinase 1 (MEKK1) transduces c-Jun NH2-terminal kinase activation in response to changes in the microtubule cytoskeleton. J Biol Chem. 1999;274:12605–12610. doi: 10.1074/jbc.274.18.12605. [DOI] [PubMed] [Google Scholar]
- Zanke BW, Rubie EA, Winnett E, Chan J, Randall S, Parsons M, Boudreau K, McInnis M, Yan M, Templeton DJ, Woodgett JR. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]
- Zechner D, Craig R, Hanford DS, McDonough PM, Sabbadini RA, Glembotski CC. MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J Biol Chem. 1998;273:8232–8239. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]