Abstract
Recent studies (for review see Ahmed, 2010; 2012) show that when given a mutually exclusive choice between cocaine and food, rats almost exclusively choose food. The present experiment investigated potential shifts in preference between levers associated with either food or cocaine which might occur during extinction (food and cocaine no longer available) and during footshock-induced, cocaine-primed, and food-primed reinstatement. During self-administration sessions where food and cocaine were simultaneously available, rats demonstrated a stable food preference, choosing food over cocaine on 83% of trials. During extinction when neither reinforcer was available, no preference between levers was evident and responding decreased until rats responded on the previously food- and cocaine-associated levers at equally low rates. Footshock resulted in a non-specific reinstatement of responding upon both levers, while cocaine priming resulted in a significant preference for cocaine seeking over food seeking. This suggests that the mechanism underlying footshock-induced reinstatement is distinct from that of cocaine-primed reinstatement. Food priming engendered a mild, non-specific increase in responding on both levers. Although rats generally prefer food over cocaine when presented with a choice between these primary reinforcers, the present results suggest that in certain situations cocaine-seeking behavior prevails over food-seeking behavior.
Keywords: Choice, Cocaine, Rat, Reinstatement, Self-administration, Stress
Previous studies of drug abuse which have utilized animal models have yielded somewhat puzzling findings regarding the reinforcing power of drugs of abuse. While the traditional belief is that drugs of abuse are especially potent reinforcers, recent studies (Cantin et al., 2010; Kerstetter et al., 2012; Lenoir et al., 2007; for reviews see Ahmed, 2010, 2012) have systematically shown that rats demonstrate near-exclusive preference for food over cocaine when given a mutually exclusive choice between these two reinforcers. It appears that this general preference for food occurs regardless of the dose of cocaine used, the nutritive value of the food alternative (Lenoir et al., 2007), or the heaviness of past cocaine use in the animal, and is not dependent upon the animal being food- or water-restricted (Cantin et al., 2010; Kerstetter et al., 2012). Similar outcomes have been obtained in recent behavioral economic studies where demand for cocaine vs. food was systematically investigated. These studies indicated that rats made much more effort to defend their consumption of food as compared to cocaine with increases in the number of responses required to earn reinforcement (Christensen et al., 2008a; Christensen et al., 2008b; Christensen et al., 2009). These studies showing that cocaine is a relatively weak reinforcer has led Ahmed to suggest there is a “validation crisis” in animal models of drug addiction (Ahmed, 2010). Interestingly, some human studies have also provided evidence that drugs do not serve as particularly potent primary reinforcers. For example, observations that human cocaine abusers will forego an opportunity to consume a drug reinforcer when a non-drug alternative such as a small sum of money (e.g. $1–2) or tokens exchangeable for snacks and entertainment privileges are available (e.g. Foltin & Fischman, 1994; Higgins, Roll, & Bickel, 1996). Indeed, there appears to be a disconnection between the reports of a low reward value of drug reinforcers from laboratory studies and the persistent and compulsive drug-taking behavior which defines addiction.
While food may be generally preferred over drugs, there may be specific situations in which animals demonstrate a reversal of preference from non-drug to drug reinforcers. A study by Ahmed and Koob (1997) suggests that stress-induced reinstatement may be one such situation. In their study, rats in separate groups were first trained to lever press for either cocaine infusions or for food pellets. In a second phase, rats in both groups were exposed to extinction, where lever pressing no longer resulted in reinforcement. This phase continued until lever pressing declined to low levels in both groups. Animals were then exposed to an additional extinction session which was preceded by exposure to a stressor in the form of 15 minutes of intermittent footshock. This treatment caused a strong reappearance of lever pressing in the group of rats that had previously lever pressed for cocaine. In contrast, no reappearance of lever pressing was observed in the group of rats that had been trained to respond for food. The selective footshock-induced reinstatement of drug seeking, but not food seeking, has since been replicated in studies where the drug was cocaine (Mantsch & Goeders, 1999), nicotine (Buczek et al., 1999) or alcohol (Lê et al., 1998). These findings from animal models of stress-induced reinstatement suggest that reinstatement of reinforcer seeking after exposure to a stressor may be a phenomenon which is unique to certain drugs of abuse and thus, may indicate one way in which drug reinforcers differ from non-drug reinforcers in their effect upon behavior (Kearns, Gomez-Serrano, & Tunstall, 2011).
The primary goal of the present experiment was to test whether footshock-induced reinstatement would reverse rats’ preference for a food lever over a cocaine lever. This involved first establishing a baseline preference by allowing rats to choose between food and cocaine alternatives. Then, food seeking and cocaine seeking was extinguished. After responding for both reinforcers was essentially eliminated, a test for footshock-induced reinstatement was administered. Based on the findings of Ahmed and Koob (1997), it would be expected that footshock exposure would result in reinstatement of cocaine seeking but no increase in food seeking (or perhaps even a suppression of responding on the food lever). It is important to note, however, that a major difference between the present design and that of Ahmed and Koob (1997) is that in the present study, subjects were trained to choose between food and cocaine within the same session. In contrast, Ahmed & Koob (1997) used separate groups of rats that responded for either cocaine or for food, but not for both. Thus, the present study serves as the first investigation of stress-induced reinstatement within a choice situation.
Exposure to a stressor is one of the most effective reinstaters of drug seeking in animals (Bossert et al., 2005; Erb, Shaham, & Stewart, 1996; Shaham, Erb, & Stewart, 2000; Shalev, Grimm, & Shaham, 2002). This parallels findings in humans that stress is one of the main causes of drug relapse (e.g. Epstein et al., 2006; Sinha, 2008). However, it is possible to observe a comparable reappearance of extinguished drug-seeking behavior following exposure to priming injections of the drug itself (for a review, see de Wit, 1996). Thus, a second goal of the present experiment was to investigate the effects of re-exposure to each reinforcer following extinction (cocaine-primed and food-primed reinstatement) within a choice situation. Following the footshock-induced reinstatement test, rats were given two further tests, each preceded by non-contingently delivered cocaine infusions or food pellets. Comparing the results of these priming tests with those of the footshock-induced reinstatement test could provide information on the commonality of the mechanisms underlying these different forms of reinstatement. Furthermore, these tests could identify potential situations in which cocaine seeking prevails over food seeking. Evidence of such a switch in preference between response alternatives should be relevant to understanding the contribution of drug relapse to drug abuse and addiction.
Materials and Methods
Subjects
Twelve adult male Long-Evans rats began the experiment. Four rats were dropped from the experiment due to issues related to catheter patency which prevented their acquisition of stable self-administration. One rat met the criterion for stable self-administration but was dropped from the experiment as catheter patency could not be confirmed. Rats were individually housed in plastic cages with wood-chip bedding and metal wire tops. They were maintained at 85% of their free-feeding weights (approximately 300–400 g) throughout the experiment by feeding them approximately 15–20 g of rat chow following training sessions. Rats had unlimited access to water in their home cages. The colony room where the rats were housed had a 12-h light:dark cycle with lights on at 08:00 h. Training sessions were conducted 5–7 days per week during the light phase of the light:dark cycle. Throughout the experiment, rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and all procedures were approved by American University’s Institutional Animal Care and Use Committee (IACUC).
Apparatus
Training took place in 6 Coulbourn Instruments (Whitehall Township, PA) modular test cages (30 × 25.5 × 29 cm) enclosed in sound attenuation chests. Each cage had aluminum front and rear walls and ceiling, with a grid floor and two clear plexiglass side walls. Footshock was delivered by a Coulbourn Instruments shocker-scrambler or Med-Associates (St. Albans, VT) model # ENV-414 shocker-scrambler connected to the operant chamber floor. Two Med-Associates retractable levers were positioned 5 cm from the floor and located on the front wall of the chamber, equidistant from the center where a food trough was located. Tone (4000 Hz and 70 dB) and white noise (65 dB) stimuli were delivered through a speaker mounted on top of the chamber. A shielded 100-mA light bulb mounted to the ceiling at the front of the chamber was used as a houselight to signal the start and end of sessions. Two 100-mA cue lights were also mounted to the front wall, located approximately 10 cm above the floor and directly above each lever. Experimental events were controlled by a Med-Associates computer system located in a room adjacent to the training chambers.
Cocaine (provided by the National Institute on Drug Abuse) in a saline solution at a concentration of 2.56 mg/ml was infused at a rate of 3.19 ml/min by 10-ml syringes driven by Med-Associates syringe pumps located outside of the sound attenuation chests. Tygon tubing extended from the 10-ml syringes to a 22-gauge rodent single-channel fluid swivel and tether apparatus (Alice King Chatham Medical Arts, Hawthorne, CA) that descended through the ceiling of the chamber. Cocaine was delivered to the subject through Tygon tubing that passed through the metal spring of the tether apparatus. This metal spring was attached to a plastic screw cemented to the rat’s head to reduce tension on the catheter.
Procedure
Phase 1: Food-pellet-reinforced Operant Response Training
Rats were trained to lever press for 45-mg food pellets on a fixed-ratio (FR) 1 schedule of reinforcement. Sessions began with the illumination of the houselight and with only the food lever (either the right or left retractable lever, counterbalanced across subjects) inserted into the operant chamber. Each lever press was followed by delivery of a 45-mg food pellet and presentation of a 10-s compound auditory-visual stimulus consisting of illumination of the stimulus light above the food lever and a noise or tone (counterbalanced across subjects) auditory stimulus. Lever presses during this 10-s post-reinforcement period were recorded, but had no consequences. The presence of a time-out (TO) period with food-paired cues in this condition was necessary to equate food training with cocaine self-administration, in which TO cues are commonly presented simultaneously with the infusion (e.g., Ahmed & Koob, 1997). Rats were trained in sessions lasting two hours or until 50 pellets were earned. Rats were trained until they completed a minimum of three sessions and at least two sessions in which 50 food pellets were earned.
Surgery
Following food training, rats were surgically prepared with chronic indwelling jugular vein catheters, using a modification of the procedure originally developed by Weeks (1962). In brief, under ketamine (60 mg/kg) and xylazine (10 mg/kg) anesthesia, approximately 3 cm of Silastic tubing (0.044mm i.d., 0.814mm o.d.) was inserted into the right jugular vein. This Silastic tubing was connected to 8 cm of vinyl tubing (Dural Plastics; 0.5mm i.d., 1.0mm o.d.) that was passed under the skin around the shoulder and exited the back at the level of the shoulder blades. The vinyl tubing was threaded through a section of Tygon tubing (10 mm long, 4 mm diameter) that served as a subcutaneous anchor. Six stainless steel jeweler’s screws were implanted in the skull, to which a 20-mm plastic screw was cemented with dental acrylic. Catheters were flushed daily with 0.1 ml of a saline solution containing 1.25 µg/ml heparin and 0.08 mg/ml gentamycin.
Phase 2: Cocaine self-administration
After 5–7 days of recovery from surgery, cocaine self-administration training began. Sessions began with illumination of the house light and with insertion of the cocaine lever (the lever not previously associated with food). Rats were trained to lever press for 1 mg/kg infusions of cocaine on a FR-1 schedule. Each infusion was followed by a 10-s TO period, during which the cocaine lever was inactive. This TO period was signaled by a compound auditory-visual stimulus consisting of illumination of the stimulus light above the cocaine lever and presentation of the auditory stimuli (noise or tone) not previously paired with food. The dose used throughout this experiment was 1 mg/kg, as used in previous studies that compared the essential reinforcing value of cocaine and a 45-mg food pellet (Christensen et al., 2008a, Christensen et al., 2008b, Christensen et al., 2009). Rats were trained in sessions lasting three hours or until 40 infusions were earned. Self-administration training continued until a minimum of ten self-administration sessions were completed and stable rates of responding were observed, defined as a change in number of infusions no greater than 15% over 3 consecutive sessions.
Phase 3: Establishing a Choice Baseline
In this phase, for the first time, both the food lever and the cocaine lever were available to the animal within the same session. Each choice session began with four forced-choice trials, in which either the food or cocaine lever was inserted. There were two trials of each type, with the order of presentation randomized within blocks of two. A lever press would result in delivery of the designated reinforcer (either a 1 mg/kg infusion of cocaine or a 45-mg food pellet), a 10-s presentation of the cues associated with the pressed lever, and retraction of the lever. A 10-min inter-trial interval (ITI) was implemented after all trials in order to minimize any potential interaction between the effects of one reinforcer with the other (e.g., to reduce the impact of cocaine’s anorectic properties on the motivation for food). Following the completion of forced-choice trials, 14 free-choice trials were presented. Each free choice trial began with the simultaneous insertion of the food and cocaine levers. A lever press on either lever resulted in the delivery of the designated reinforcer and 10-s presentation of the associated cues and the immediate retraction of both levers. Again, 10-min ITIs separated trials. Sessions lasted approximately 3 hours and this phase consisted of 5 such sessions.
Phase 4: Extinction
Both levers were inserted at the beginning of each 2-h extinction session and remained available to the animal for the entire session. This was done so that the final day of extinction could be used as a comparison baseline for reinstatement tests, where levers are available continuously to the animal (e.g. Erb et al., 1996; Ahmed & Koob, 1997). No cocaine infusions or food pellets were presented at any time. However, responding on either lever resulted in presentation of the 10-s audio-visual TO cue paired with that lever. Extinction sessions were continued until the rat made 15 or fewer responses on each lever in the same session.
Phase 5: Reinstatement Testing
After meeting the extinction criterion, rats received a footshock-induced reinstatement test on the following day. Following the footshock test, rats then received cocaine-primed and pellet-primed reinstatement tests on the following two days, with the order of the two types of priming test counterbalanced across animals.
Footshock-induced Reinstatement
Rats were exposed to a stressor in the form of intermittent footshock (0.30–0.55-mA; 0.5-s) administered according to a variable-time schedule (mean interval: 40-s; range: 10–70-s) delivered over a 15-min pre-test period. During the pre-test period, all stimuli remained off and both levers were retracted. In order to avoid fear-induced freezing behavior which may occur and interrupt with testing, the intensity of the footshock was determined on an individual-subject basis (e.g. Wang, You, & Wise, 2009). Immediately prior to footshock treatment, each rat was put into the chamber with the shock intensity set to 0 initially. Then, 0.5-s shocks were delivered in increasing 0.05-mA increments until a startle reaction was observed. A constant value of 0.15 mA was added to each subject’s “startle threshold” value, resulting in a range of footshock intensities used for testing between 0.30 mA and 0.55 mA. Following footshock treatment, an extinction session just like those administered during the extinction phase began immediately and served as a test for reinstatement of food- and cocaine-seeking behavior.
Cocaine-primed reinstatement
This session was similar to footshock-induced reinstatement tests but in place of footshock exposure, two non-contingent infusions of cocaine (1.0 mg/kg, I.V.) were administered, evenly spaced over the 15-min pre-test period. These two non-contingent infusions served to re-expose animals to the same reinforcer which previously maintained cocaine-lever responding. The extinction session following immediately thereafter served as a measure of food and cocaine seeking reinstated by cocaine priming. The total amount of cocaine delivered in this period (2 mg/kg) matches the priming dose used by Erb et al. (1996) in their study which also compared footshock-induced and cocaine-primed reinstatement within subjects, across consecutive test days.
Food-primed reinstatement
This session was similar to the footshock-induced and cocaine-primed reinstatement tests but with 6 non-contingent pellets delivered during the 15-min pre-test period (3 pellets at 5 minutes, 3 pellets at 10 minutes) instead of footshocks or cocaine infusions. An extinction session followed and served as a measure of food- and cocaine-seeking behavior reinstated by re-exposure to the reinforcer which previously maintained food-lever responding.
Statistical Analyses
For all statistical tests, significance was set at α = 0.05. Baseline preference was analyzed using a one-sample t-test to compare percentage of cocaine choices made during the final two choice sessions to a test value of 50% (i.e., the expected percentage observed with indifferent choice behavior). Responding during extinction was analyzed with a 2 × 4 (lever by session) repeated measures ANOVA performed on responses made during the first extinction session and the final 3 sessions before meeting the extinction criterion. The efficacy of footshock, cocaine-prime and food-prime treatments in reinstating lever pressing was each assessed with the use of a 2 × 2 (lever by session) repeated measures ANOVA performed on responses made during the test and the last extinction session. Paired-samples t-tests were used to assess the significance of shifts in cocaine lever preference from baseline on each of the reinstatement tests. To assess lever preference during tests for reinstatement, one-sample t-tests were used to compare percentage of cocaine lever responses to a test value of 50%.
Results
Food-pellet-reinforced Operant Response Training
Rats required a mean of 6.1 (SEM = 1.0) sessions to meet the food lever pressing criterion. Over the last three sessions, subjects made an average of 106.5 (SEM = 7.6) total responses (active and TO responses) and earned an average of 47.3 (SEM = 2.2) food pellets per session.
Cocaine Self-administration
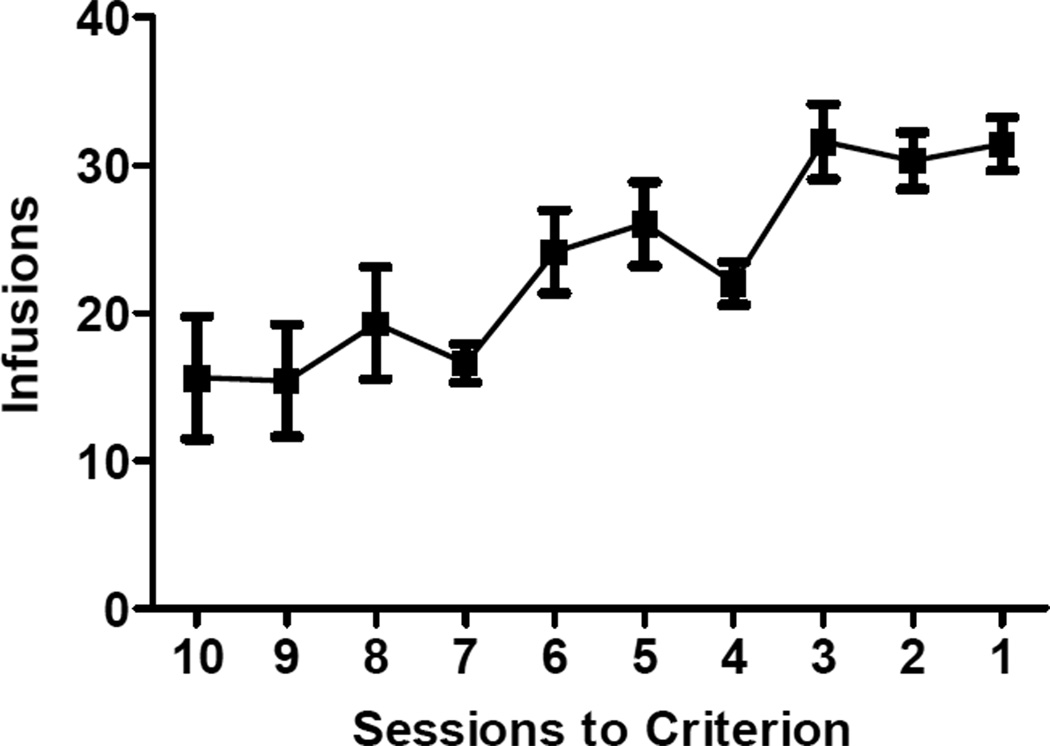
Rats required a mean of 13.0 (SEM = 1.1) sessions to reach criterion for stable cocaine self-administration. Figure 1 shows the mean number of infusions taken during the last 10 self-administration sessions prior to each rat reaching criterion. Over the last three sessions, subjects made an average of 34.0 (SEM = 3.3) total responses (active and TO responses) and administered an average of 31.1 (SEM = 1.9) infusions per session.
Figure 1. Self-administration.
Mean number (±SEM) of cocaine infusions self-administered over the last 10 self-administration sessions. Sessions lasted three hours or until 40 infusions were earned. The criterion for stable self-administration was a minimum of ten self-administration sessions and a change in number of infusions no greater than 15% over 3 consecutive sessions.
Baseline Preference
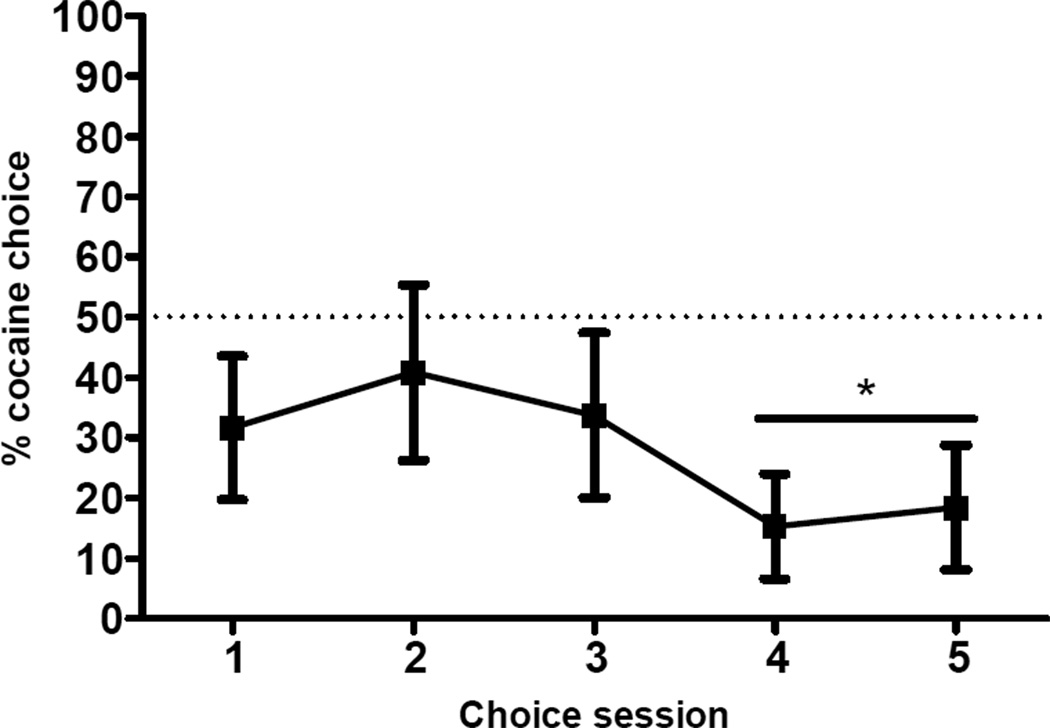
Figure 2 shows average percentage of free choice trials on which cocaine was selected. There was little variability in preference over the last two sessions, with an average change in preference of only 5%. Over these last two sessions, rats on average chose food on 83% (SEM = 9.4) of free-choice trials. A one-sample t-test indicated that this was significantly higher than the percentage of food choices expected (50%) if rats had no preference (t[6] = 3.5, p < 0.05). Considering individual subjects, 6 rats out of 7 (86%) chose food more frequently than cocaine during the final two sessions. Although rats had more food training sessions than cocaine self-administration sessions, they had approximately equal numbers of food- and cocaine-reinforced lever presses at the end of the choice phase (i.e., prior to the extinction and reinstatement phases). On average, rats made 230.4 (SEM =19.7) food-reinforced lever presses and 296.7 (SEM =33.9) cocaine-reinforced lever presses.
Figure 2. Baseline Preference.
Mean percentage (±SEM) of free choice trials (14 per session) on which cocaine was chosen, across the 5 sessions of the choice phase. * p < 0.05 different from 50% (i.e. no preference).
Extinction
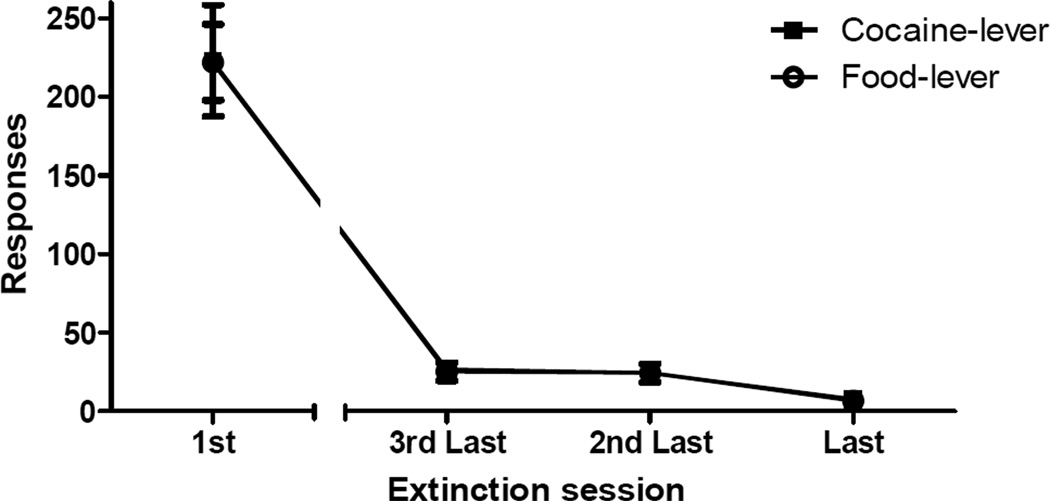
Rats required a mean of 6.6 (SEM = 0.6) extinction sessions before meeting the criterion of making 15 or fewer responses on each lever. Figure 3 shows mean lever presses on both the cocaine- and food-lever during the first extinction session as well as the last three extinction sessions approaching criterion. Rats demonstrated robust and remarkably comparable efforts to acquire both cocaine and food on the first day of extinction, indicated by a large number of total responses (M = 445.1, SEM = 31.7), distributed evenly between the cocaine lever (M = 223.1, SEM = 38.3) and food lever (M = 222.0, SEM = 26.2). Responding on both levers declined at approximately even rates. These observations were confirmed by a 2 × 4 (lever × session) repeated-measures ANOVA which revealed a significant main effect of session (F[3,18] = 178.9, p < 0.001), but no significant main effect of either lever or the session-by-lever interaction (Fs < 1).
Figure 3. Extinction.
Mean total responses (±SEM) recorded on the food and cocaine levers during 2-h extinction sessions. Responding on either lever resulted in presentation of the 10-s audio-visual timeout cue paired with that lever. Extinction sessions were continued until the rat made 15 or fewer responses on each lever in the same session.
Footshock-induced reinstatement
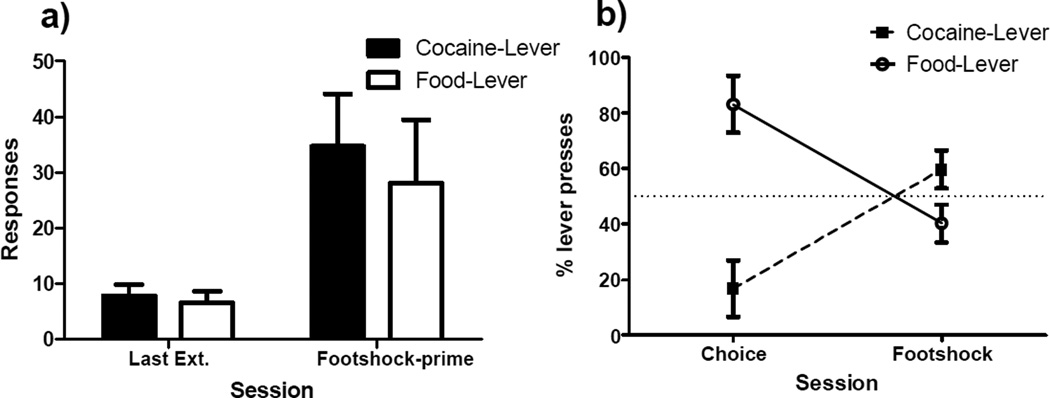
Figure 4 presents the results of footshock-induced reinstatement testing. On the final day of extinction (used as the baseline comparison for all tests of reinstatement), few responses were made on either the cocaine lever (M = 7.9, SEM = 2.2) or the food lever (M = 6.6, SEM = 2.1) lever. Panel A of Figure 4 shows that rats made approximately 30–35 responses on both the cocaine lever and on the food lever during this reinstatement test, a greater than quadrupling of the number of responses made on each lever during the final extinction session. Responding was raised similarly for both levers during the test and this was reflected in the results of a repeated measures ANOVA, which indicated a significant main effect of session (F[1,6] = 6.3, p < 0.05) but no evidence of a main effect of either lever or a session-by-lever interaction (Fs < 1).
Figure 4. Footshock-induced Reinstatement.
a) Mean total responses (±SEM) recorded on the food and cocaine levers during the last day of extinction (Last Ext.) and the 2-h test for footshock-induced reinstatement. b) Mean percentage (±SEM) of responses made on each lever during baseline preference and during the test for footshock-induced reinstatement.
Panel B of Figure 4 presents footshock-induced reinstatement test results from a different perspective. The first set of points represent the percentage of cocaine- and food-lever responses made during the final two free-choice sessions, when both reinforcers were available. The second set of points represents the percentage of total test responses made on each lever during the reinstatement test. While rats chose the cocaine lever on only 17% of trials at the end of free-choice training, they made 60% of their responses on this lever during the shock-induced reinstatement test. A paired samples t-test indicated this shift in percentage responding upon the cocaine lever was significant (t[6] = −5.5, p = 0.001). However, a one-sample t-test indicated that percentage of responses made on the cocaine lever during the test was not significantly greater than 50% (t[6] = 1.6, p = 0.17).
Cocaine-primed reinstatement
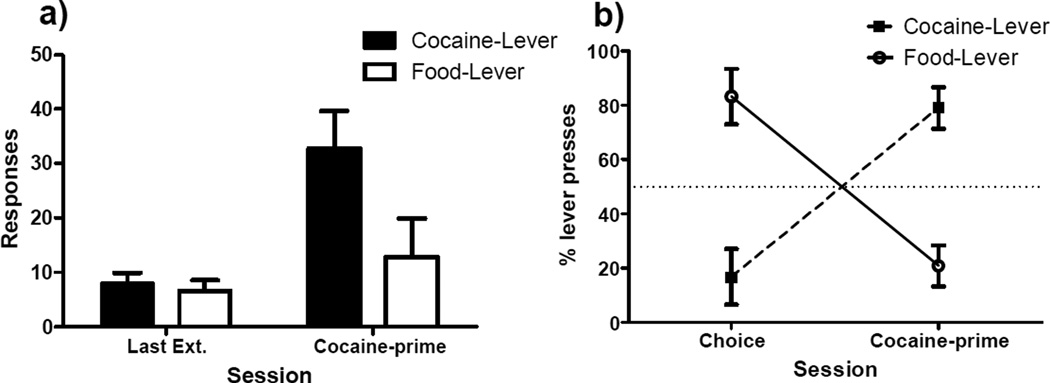
Figure 5 presents the results of cocaine-primed reinstatement testing. Panel A of Figure 5 shows that rats made on average more than 30 responses on the cocaine lever, which is greater than four times as many responses that they made on this lever during the final extinction session. In contrast, responding on the food-lever during the test only slightly increased compared to the final day of extinction. A repeated-measures ANOVA indicated a significant main effect of session (F[1,6] = 7.5, p < 0.05), as well as lever (F[1,6] = 10.0, p < 0.05), but no significant session-by-lever interaction (F[1,6] = 2.8, p = 0.14).
Figure 5. Cocaine-primed Reinstatement.
a) Mean total responses (±SEM) recorded on the food and cocaine levers during the last day of extinction (Last Ext.) and the two hour test for cocaine-primed reinstatement. b) Mean percentage (±SEM) of responses made on each lever during baseline preference and during the test for cocaine-primed reinstatement.
Panel B of Figure 5 shows the shift in preference when comparing percentage of total responses made on each lever during the final two free-choice sessions and the cocaine-primed reinstatement test, where rats made on average 79% of total test responses on the cocaine lever. A paired samples t-test indicated that the shift in percentage of responses made upon the cocaine-lever was significant (t[6] = −6.6, p = 0.001). Furthermore, a one-sample t-test indicated that percentage of responses made on the cocaine lever was now significantly greater than 50% (t[6] = 4.1, p < 0.01), the first time in this experiment that rats made significantly more responses on the cocaine lever than the food lever.
Food-primed reinstatement
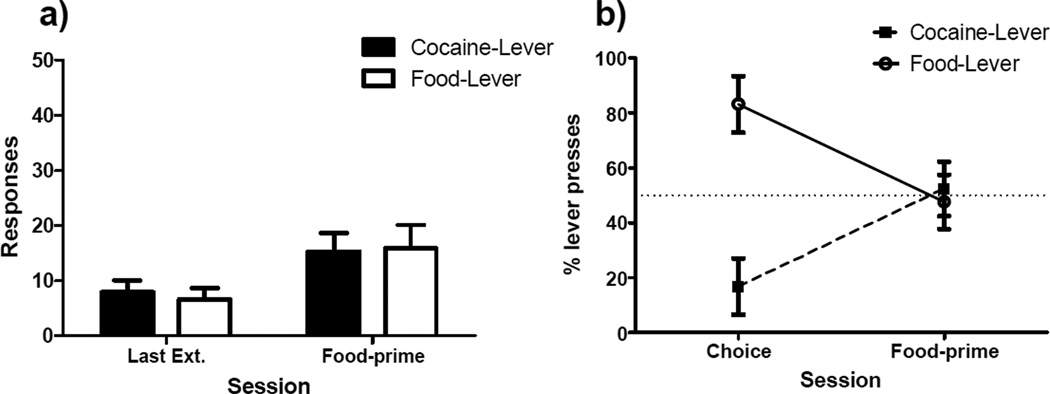
Figure 6 presents the results of the test for food-primed reinstatement. Panel A shows the mild increase in responding which occurred on both the cocaine (M = 15.3, SEM = 3.3) and food lever (M = 15.9, SEM = 4.3) during the reinstatement test. This general increase in lever pressing was confirmed by a repeated measures ANOVA, which indicated a significant main effect of session (F[1,6] = 8.6, p < 0.05). However, there was no main effect of either lever or a session-by-lever interaction (Fs < 1). Panel B of Figure 6 demonstrates the change in lever preference from the last two days of free-choice trials to the food-primed reinstatement test. A paired samples t-test indicated this increase in percentage of responding upon the cocaine lever was significant (t[6] = −3.4, p < 0.05). However, as was observed following footshock-induced reinstatement, a one sample t-test indicated cocaine lever preference was not significantly different from 50% (t[6] = 0.3, p = 0.8).
Figure 6. Food-primed Reinstatement.
a) Mean total responses (±SEM) recorded on the food and cocaine levers during the last day of extinction (Last Ext.) and the two hour test for food-primed reinstatement. b) Mean percentage (±SEM) of responses made on each lever during baseline preference and during the test for food-primed reinstatement.
Discussion
Rats in the present study demonstrated near exclusive preference for food over cocaine when both alternatives were available. This result is consistent with several recent studies that compared the reward value of cocaine with natural rewards in rats (Ahmed, 2010, 2012; Christensen et al., 2008a; Christensen et al., 2008b; Christensen et al., 2009; Kerstetter et al., 2012). However, the present study also found that following extinction of both food and cocaine seeking, a preference shift was observed during a test for reinstatement of lever pressing by a priming dose of cocaine. This shows that, despite a robust preference for food over cocaine when both are available, there are at least some situations in which cocaine seeking predominates over food seeking.
The primary purpose of the present study was to determine whether rats would continue to engage in preferential food seeking (as opposed to cocaine seeking) on a test for stress-induced reinstatement after extinction. In contrast to the results of previous studies showing that footshock selectively reinstates drug seeking (Ahmed & Koob, 1997; Buczek et al., 1999; Le et al., 1998; Mantsch & Goeders, 1999), footshock in the present experiment resulted in a non-selective increase in both drug-seeking and food-seeking responses. A major procedural difference between those previous studies and the present one is that rats here had experience responding for both food and cocaine within the same session, while the previous studies used separate groups of animals that experienced either food or cocaine, but not both.
It is worth noting that stress-induced reinstatement of food seeking has been demonstrated with the pharmacological stressor yohimbine instead of footshock (e.g. Ghitza et al., 2005; Nair, Gray & Ghitza, 2006). As Nair et al. (2009) suggest, the apparent differences between the effects of footshock and yohimbine stressors on the reinstatement of food seeking could be parameter-dependent. For example, the shock intensity used in most reinstatement studies may be sufficiently high to produce sympathetic nervous system responses that interfere with food-seeking behaviors, but doses of yohimbine that reinstate food seeking produce stress without producing the responses that interfere with food seeking. It is possible that the individual adjustment for footshock intensity conducted prior to the footshock test in the present study served to limit effects that disrupt food-seeking behavior. The average footshock intensity used here (.41 mA) was substantially lower than that (between .8 and .86 mA) used in those previous studies showing that footshock reinstates drug but not food seeking. (Ahmed & Koob, 1997; Buczek et al., 1999; Le et al., 1998; Mantsch & Goeders, 1999). Echoing Nair et al. (2009), more research is needed to determine the parameters under which footshock-induced reinstatement of food seeking does and does not occur.
The cocaine-priming test was the only situation in the current experiment where rats demonstrated significant preference for the cocaine lever. It may be that cocaine infusions – but not footshock – produced a relatively large increase in motivation specific to cocaine seeking. In agreement with this notion, Leri and colleagues (Leri & Stewart, 2001; Leri et al., 2004) demonstrated drug-primed, selective reinstatement of cocaine seeking or heroin seeking within rats previously trained to self-administer both drugs. In explaining these results, they noted that the discriminative properties of a drug can be dissociated from its incentive-motivational properties and suggested that drug-priming motivates drug-specific drug seeking by reactivating the incentive-motivational properties of the exteroceptive conditioned cues previously associated with the drug. According to this account, cocaine priming in the present experiment would have selectively reinstated cocaine seeking by reactivating the incentive-motivational properties of the audio-visual cues previously paired with cocaine (but not food) that were presented response-contingently during the test.
Food-priming resulted in a mild, non-selective increase in responding on both levers. Delamater (1997) also found a non-selective reinstating effect of food pellets on lever pressing previously maintained by either food pellets or drops of sucrose within the same rats. Interestingly, however, Delamater also found a selective reinstating effect of food pellets upon the consummatory, food-magazine response. He suggested that the selectivity of reinstatement could be a function of proximity of a particular response to reward, with selective reinstatement observed for responses proximal to reward (e.g., consummatory responses) and non-selective reinstatement for more distal responses (e.g., instrumental lever pressing). This account could help explain the selectivity of reinstatement observed for cocaine-primed reinstatement and the non-selectivity of reinstatement observed for food-primed reinstatement. As Wise (1987) has noted, a lever press for food is separate from the consummatory response (i.e., collecting and eating the magazine delivered pellet) for food. In drug self-administration however, the lever press serves as both the instrumental response and the consummatory response (Wise, 1987). This is an important, but often neglected difference between drug and food reinforcers.
In the present experiment, there were no intervening extinction sessions between the multiple reinstatement tests. (See Erb et al., 1996, 1998, 2000; Leri et al., 2002, 2004 for other examples of studies where multiple reinstatement tests were administered without intervening extinction sessions.) The effect of stress-induced reinstatement was our primary interest in this study and so we gave that test first to all rats. Previous studies suggest that subsequent reinstatement tests should not have been influenced by footshock carry-over effects. For example, Brown and Erb (2007) found that footshock acted as a reinstater only if the reinstatement test were given within 40 minutes of footshock delivery. Longer post-stress delays did not result in reinstatement. Similarly, de Vries et al. (1998) performed multiple cocaine-primed reinstatement tests separated by saline-primed (i.e. extinction) tests given on the intervening days between cocaine-primed tests. They found no evidence of the effects of cocaine priming carrying over and influencing responding on any of the saline-primed tests, where responding was no different from the final extinction session. Based on the results of these studies showing that the effects of reinstaters are fairly limited in duration, it seems unlikely that carryover effects would have influenced results in the present experiment.
The different patterns of results on the footshock-induced vs. cocaine-primed reinstatement tests suggest that these two reinstaters differentially affect the mechanisms underlying the reinstatement of cocaine and food seeking. It has been suggested that footshock and drug-priming, while working upon dissociable neurochemical and neuroanatomical substrates (for review see Shaham et al., 2000; Shaham et al., 2003; Stewart 2000), likely have effects that converge upon a final common pathway to reinstate drug-seeking (Bossert et al., 2005; Shaham et al., 2003). More research is needed to determine the extent to which the neurocircuitry underlying the reinstatement of drug- and food-seeking behavior overlaps (Nair et al., 2009). The procedure used here, where the reinstatement of both drug seeking and food seeking can be observed simultaneously in the same subjects, may be especially useful in this regard.
During the extinction phase, rats demonstrated robust and remarkably comparable efforts to acquire both cocaine and food, as indicated by a large number of total responses distributed evenly between the response alternatives. Such responding was well beyond the number of responses that rats made during any previous session of the experiment. In fact, prior to the extinction phase, the response requirement for reinforcement was kept at FR-1 and the most reinforcers that could be earned were 50 during food training and 40 during cocaine self-administration training. Most impressive was the increase in lever pressing on the cocaine lever, where rats went from making just a few cocaine choices on the last baseline session (see Figure 2) to over 200 cocaine-seeking responses on the first extinction session (see Figure 3). This very clearly illustrates the role that alternative reinforcers can play in reducing drug-seeking behavior (Ahmed, 2005).
While the response rates during the first session of extinction were higher than is customarily observed in reinstatement studies, 100+ responses on the first extinction session are not unprecedented (e.g., Mantsch & Goeders, 1999; Shelton & Beardsley, 2005; Sorge & Stewart, 2005). The relatively high rate of lever pressing seen here may have been partially due to the fact that rats were trained to lever press for food as well as cocaine. Lever pressing for food often engenders much higher rates than lever pressing for cocaine. For example, in the present study, rats made about three times the number of responses during terminal food training sessions than during terminal cocaine training sessions. It is possible that this practice pressing levers at high rates acted to enhance response rates in extinction.
Future research that compares the reinstatement behavior of the minority of rats that prefer cocaine over food with the majority that do not could prove very interesting. This would require large starting numbers of subjects because only 10–15% of rats prefer cocaine over natural reinforcers (e.g., present study; Ahmed, 2010; Cantin et al., 2010; Lenoir et al., 2007). Thus, approximately 80 rats would have to be trained on the choice procedure used here to identify a subset of 8–12 rats that prefer cocaine. Though time- and labor-intensive, determining whether these cocaine-preferring rats are especially susceptible to the different forms of reinstatement would be very informative and could have clinical implications for understanding risk of relapse in human drug addicts.
In conclusion, even though rats generally prefer food over cocaine when presented with a mutually exclusive choice, the present results suggest there can be instances where the rate of cocaine-seeking behavior surpasses that of food-seeking behavior. This may model those times when an abstinent human drug addict experiences increased motivation to use drugs and therefore faces an increased risk of relapse. Identifying other situations or environmental factors which contribute to a preference for drug rewards over non-drug rewards may help to explain the maladaptive choice for drug rewards that characterizes addiction.
Acknowledgements
This research was supported by Award Number R01DA008651 from the National Institute on Drug Abuse. The National Institute on Drug Abuse had no role other than financial support and as such the content is solely the responsibility of the authors.
Footnotes
Author Contributions
BT and DK were responsible for the study concept and design, the acquisition of animal data as well as the data analysis and interpretation of findings. BT drafted the manuscript. BT and DK critically reviewed content and approved final version for publication.
References
- Ahmed SH. Imbalance between drug and non-drug reward availability: A major risk factor for addiction. Eur J Pharmacol. 2005;526:9–20. doi: 10.1016/j.ejphar.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Ahmed SH. Validation crisis in animal models of drug addiction: Beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Ahmed SH. The science of making drug addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Lê AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine Is Low on the Value Ladder of Rats: Possible Evidence for Resilience to Addiction. PLoS ONE. 2010;5(7):e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Huntsberry ME, Riley AL. Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology. 2008a;198:221–229. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Roma PG, Riley AL. Demand for cocaine and food over time. Pharmacol Biochem Behav. 2008b;91:209–216. doi: 10.1016/j.pbb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CJ, Kohut SJ, Handler S, Silberberg A, Riley AL. Demand for food and cocaine in Fischer and Lewis rats. Behav Neurosci. 2009;123:165–171. doi: 10.1037/a0013736. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- Delamater AR. Selective reinstatement of stimulus-outcome associations. Anim Learn Behav. 1997;25:400–412. [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacol. 2005;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Gomez-Serrano MA, Tunstall BJ. A review of preclinical research demonstrating that drug and non-drug reinforcers differentially affect behavior. Curr Drug Abuse Rev. 2011;4:261–269. doi: 10.2174/1874473711104040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacol. 2012;37:2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense Sweetness Surpasses Cocaine Reward. PLoS ONE. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Stewart J. Drug-induced reinstatement to heroin and cocaine seeking: a rodent model of relapse in polydrug use. Exp Clin Psychopharmacol. 2001;9:297–306. doi: 10.1037//1064-1297.9.3.297. [DOI] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE, Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacol. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology. 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Mihindou C, Vouillac C, Koob GF, Ahmed SH. Preclinical validation of a novel cocaine exposure therapy for relapse prevention. Biol Psychiat. 2011;70:593–598. doi: 10.1016/j.biopsych.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Gray SM, Ghitza UE. Role of food-type in yohimbine- and pellet-priming-induced reinstatement of food-seeking. Physiol Behav. 2006;88:559–566. doi: 10.1016/j.physbeh.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89(1):18. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. International journal of comparative psychology. 2005;18:154–166. [Google Scholar]
- Sinha R. Chronic stress, drug use and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology. 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatr Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Wang B, You Z, Wise RA. Reinstatement of cocaine-seeking by hypocretin (orexin) in the ventral tegmental area: Independence from the local CRF network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Intravenous drug self-administration: A special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 117–141. [Google Scholar]
- Weeks JR. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]