Abstract
Recent experimental and clinical data suggest that there is a link between dry eye disease (DED) and T cell-mediated immunity. However, whether these immune responses are a consequence or cause of ocular surface inflammation remains to be determined. Thus far, only models of acute DED have been used to derive experimental data. This is in contrast to clinical DED which usually presents as a chronic disease. In the present study, using a murine model of chronic DED, it was established that the chronic phase of the disease is accompanied by Th17 responses at the ocular surface, and that a significant memory T cell population can be recovered from chronic DED. This memory response is predominantly mediated by Th17 cells. Moreover, adoptive transfer of this memory T cell population was shown to induce more severe and rapidly progressing DED than did the adoptive transfer of its effector or naïve counterparts. Not only do these results clearly demonstrate that effector memory Th17 cells are primarily responsible for maintaining the chronic and relapsing course of DED, but they also highlight a potentially novel therapeutic strategy for targeting memory immune responses in patients with DED.
INTRODUCTION
Dry eye disease (DED) is an extremely common ocular disorder affecting tens of millions of people worldwide1,2 and is associated with a diverse array of etiopathogenic factors.3 Despite the advent of several therapeutics, notably topical cyclosporine A for DED, there remains a significant unmet medical need. It was only during the last decade that ocular surface inflammation was recognized as a hallmark of DED3 and T cell infiltration of the ocular surface was identified in a wide spectrum of patients with DED;4,5 however, the immunopathogenic mechanisms that mediate chronic DED have yet to be fully described.
The cellular and molecular mechanisms that underlie inflammation in DED have been investigated with a variety of experimental models. It has been demonstrated that CD4+ T cells mediate DED induction in mice;6,7 furthermore, CD4+ T cell subsets, including Th1 and Th17 cells, are now thought to be the major effector cells in DED.8,9,10 Interestingly, regulatory T cells (Tregs), which normally suppress immune responses, have also been found to be dysfunctional in DED.10 However, it is unclear to what extent these pathogenic findings seen in an acute care setting can be directly related to those found in a clinical setting, where DED is generally encountered as a chronic disorder.
In the present study, we provide, for the first time, a useful animal model of chronic DED and test the hypothesis that the chronic inflammation in DED is mediated by memory T cells. We examine the immunoinflammatory responses on the ocular surface in chronic DED, and characterize the phenotypes of the involved T cells. Furthermore, we determine whether or not the T cells from chronic DED can adoptively transfer the disease to immunocompetent naïve hosts.
RESULTS
Chronic DED is characterized by persistent ocular surface inflammation
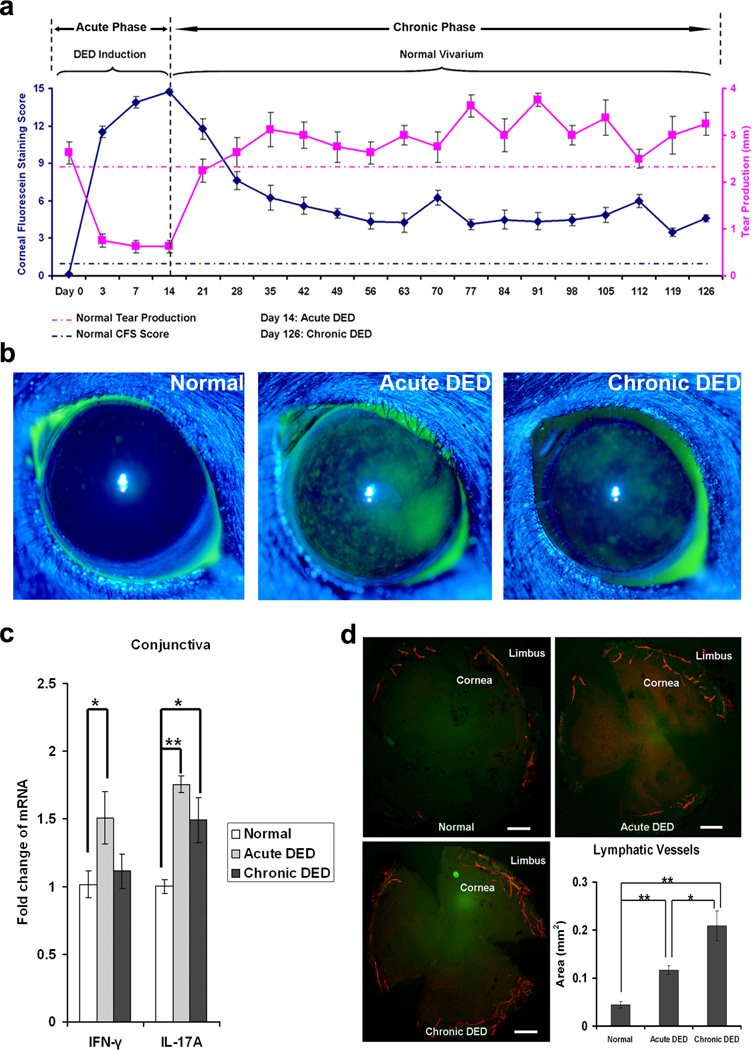
A widely used murine model of DED11,12 was modified to mirror the long-term, fluctuating course of human DED.13 Following the initial 14 d of desiccating stress, mice were housed in a standard, non-desiccated vivarium for 4 mo without any pharmacologic manipulations such as use of scopolamine or other anti-cholinergics (Figure 1a). Clinical disease severity peaked at the end of the desiccating challenges (day 14), as demonstrated by elevated corneal fluorescein staining scores (a common clinical readout for the severity of corneal epitheliopathy),11,14 and decreased aqueous tear secretion. Upon removal from the desiccating environment, corneal epitheliopathy gradually regressed to lower levels, but never normalized, through the end of observation (day 126) (Figure 1a,b). Meanwhile, aqueous tear secretion returned to normal, or even supra-normal levels, indicating that DED with mild corneal epitheliopathy persisted despite the resolution of aqueous tear deficiency or even with an enhanced compensatory tear secretion by the lacrimal apparatus. This demonstrated, for the first time, that following the induction of acute DED, ocular surface inflammation, as characterized by corneal epitheliopathy, persisted into a long-term chronic phase, even without continued exposure to desiccating stress. Since corneal epitheliopathy is the most evaluated clinical sign of DED,14,15 and absence of tear deficiency is very commonly seen in clinical DED patients,16 the persistent ocular surface inflammation indicates the development of a chronic DED state.
Figure 1.
Chronic dry eye disease (DED) involves immunoinflammatory responses at the ocular surface. (a) Development of chronic DED. Acute DED was induced in mice by desiccating stress for 14 d. Subsequently, mice were transferred to the normal vivarium without anti-cholinergic challenge and observed until day 126. Low-level corneal epitheliopathy persisted as evidenced by a slight elevation in corneal fluorescein staining scores (solid blue line) which were evaluated in a masked fashion. Meanwhile, aqueous tear production, as assessed with the cotton thread test, returned to normal or even supranormal levels (solid pink line). Age and sex-matched mice maintained in the standard environment were used as normal controls with mean levels of corneal fluorescein staining scores and tear production shown in dash blue and pink lines, respectively. Data shown represent the mean ± SEM of a single trial (n = 10 eyes) out of two performed. (b) Representative corneal fluorescein staining images of normal, acute DED (day 14) and chronic DED (day 126). (c) Relative quantification of IFN-γ and IL-17 mRNA levels in the conjunctiva. Data represent the mean ± SEM of six eyes per group from a single experiment that was reproduced in a similar independent experiment. *, p < 0.05; **, p < 0.01. (d) Representative whole-mount corneal immunofluorescence micrographs demonstrating lymphangiogenesis in DED. Corneas were stained with CD31 (green) and LYVE-1 (red) to evaluate blood (CD31hiLYVE-1−) and lymphatic (CD31loLYVE-1+) vessels. Scale bars, 50 µm. The corneal area covered by lymphatic vessels were quantified using Fiji software, and presented in a bar graph. *, p < 0.05; **, p < 0.01.
Ocular surface inflammation in chronic DED is associated with Th17, but not Th1, immune responses
We next sought to determine the immunoinflammatory factors that could be responsible for the corneal epitheliopathy observed in chronic DED. Our results in acute DED (day 14) (Figure 1c) were consistent with previous findings indicating that there is increased Th1-associated IFN-γ8 and Th17-associated IL-17 in the conjunctiva.8,10 However, in chronic DED (day 126), conjunctival IFN-γ returned to near-normal levels, while IL-17 levels remained elevated at a level comparable to those found in acute DED (Figure 1c). These findings suggest that chronic DED is associated with a Th17 response. We further examined whether chronic DED is accompanied by corneal angiogenesis. Previous studies from our lab have demonstrated that there is significant and exclusive growth of lymphatic (not blood) vessels in acute DED corneas,17 and that the selective lymphangiogenesis in cornea is due to IL-17-driven expression of pro-lymphangiogenic factors.12 In the present study, chronic DED corneas did not demonstrate regression, but displayed progressive ingrowth of these selectively formed lymphatics toward the center of corneas (Figure 1d).
T cells from chronic DED are pathogenic and can induce the disease
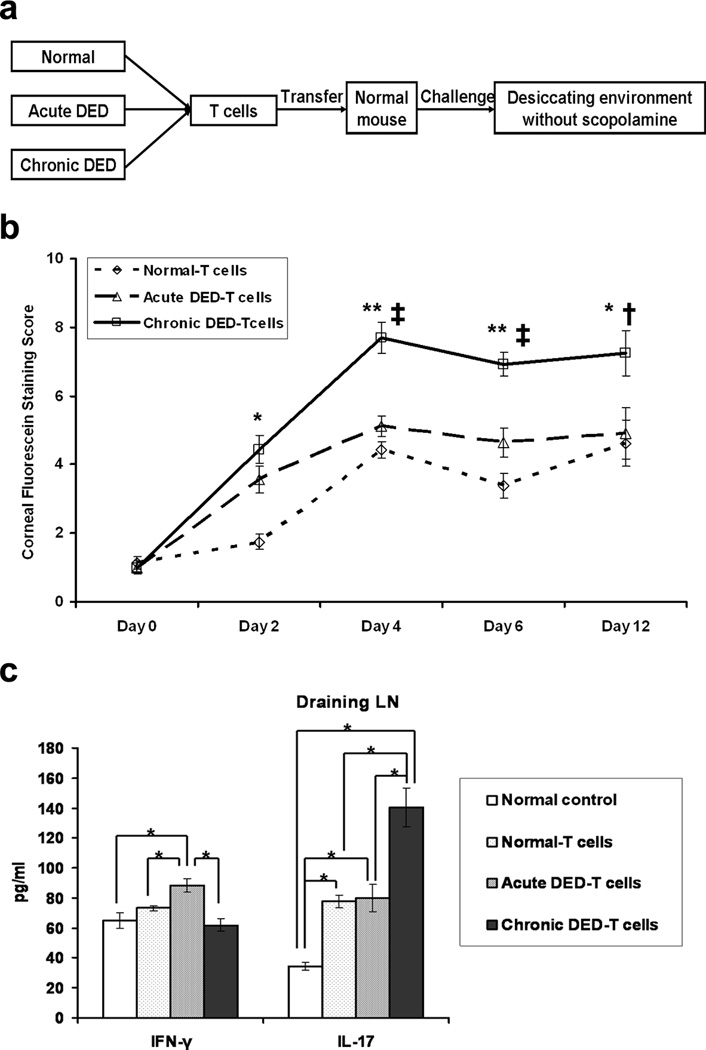
To determine if the T cells generated in DED are indeed pathogenic, we next investigated whether or not the T cells isolated from chronic DED can induce disease in naïve mice. T cells from the various groups (normal, acute DED, and chronic DED) were adoptively transferred to naïve mice, and these mice were subsequently challenged with desiccating environmental stress without scopolamine (Figure 2a). Chronic DED-T cell recipients developed clinical disease much more rapidly, and in a more severe form than did normal (non-DED) T cell-transferred or acute DED-T cell-transferred recipients (Figure 2b). The highest Th17 responses were observed in recipients of chronic DED-T cells on day 6 after challenge (Figure 2c), suggesting a close link between the Th17 response and DED severity. In contrast, there were no increased Th1 responses in chronic DED-T cell-transferred recipients (Figure 2c). Recipients of acute DED-T cells exhibited only a slightly higher disease score on day 6 after challenge than did recipients of normal T cells (Figure 2b), with comparable Th17 responses (Figure 2c). Although recipients of acute DED-T cells demonstrated the strongest Th1 responses (Figure 2c), this was not paralleled by simultaneous development of clinical disease severity, suggesting that the Th17 response was the dominant pathogenic factor.
Figure 2.
T cells from chronic dry eye disease (DED) mice induce the most severe disease in naïve recipients. (a) Schematic study design of adoptive transfer experiment. Total T cells were isolated from normal, acute and chronic DED (n = 3–5 mice/group). Each naïve recipient was then injected i.v. with 1 × 106 cells. Recipients were challenged in the controlled-environment chamber for 22 d without use of scopolamine. (b) Disease severity comparison among the recipients transferred with the different T cells. The mean corneal fluorescein staining score ± SEM for each group (n = 16 eyes) is shown. *, p < 0.05; **, p < 0.01 compared to normal-T cell recipients. †, p < 0.05; ‡, p < 0.01 compared to acute DED-T cell recipients. (c) T cell response at the recipient ocular surface. Conjunctival tissue was collected from the recipients at day 6, and relative mRNA expression of IFN-γ and IL-17 transcripts (n = 6 eyes per group) was quantified. Data shown are mean ± SEM. *, p < 0.05; **, p < 0.01. (d) T cell response in the recipient draining lymph nodes (LNs). Draining LNs were harvested at day 6, and analyzed for IFN-γ and IL-17 levels by ELISA (n = 3 mice per group). Data shown are mean ± SEM. *, p < 0.05. Groups are labeled as follows: Normal T-cells, recipients of T cells from normal mice; Acute DED-T cells, recipients of T cells from acute DED mice; Chronic DED-T cells, recipients of T cells from chronic DED mice.
Chronic DED is characterized by increased effector memory Th17 cells
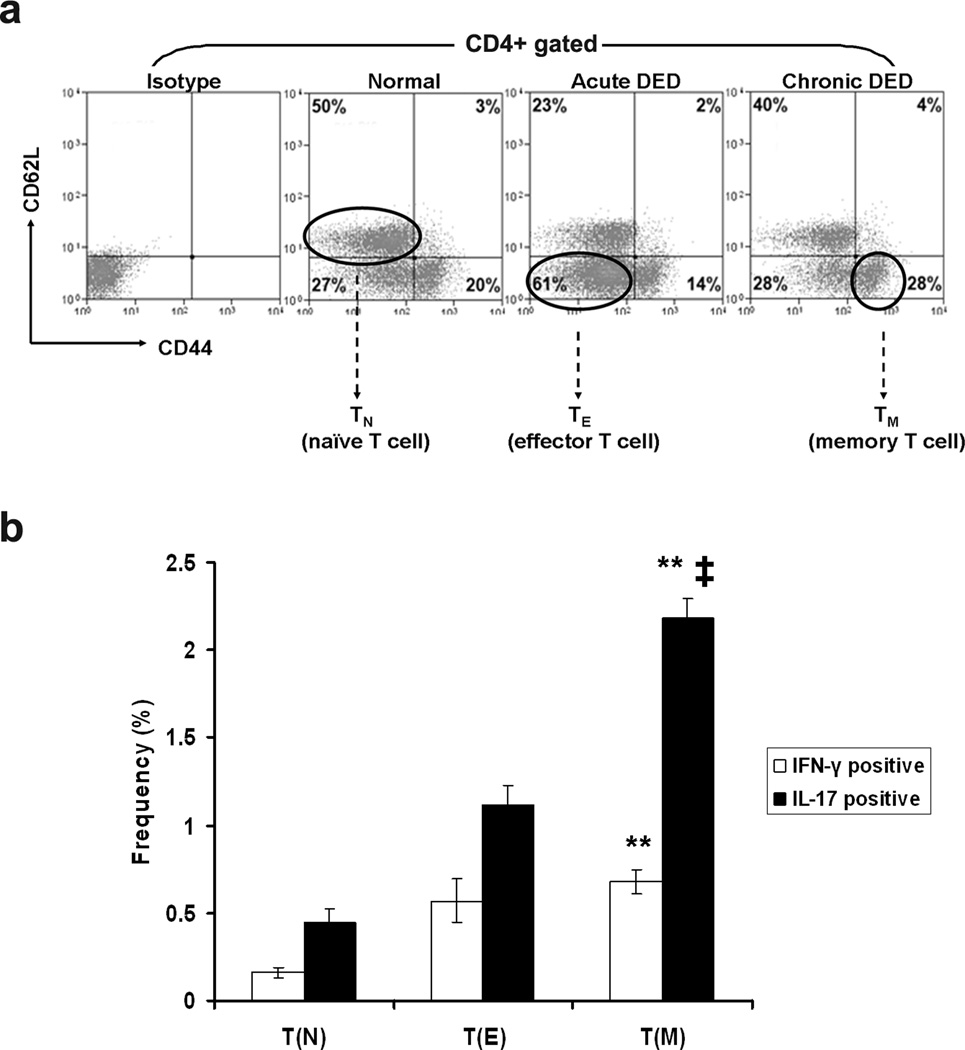
Phenotypic and functional assays were employed to further delineate the CD4+ T cell populations in normal, acute DED, and chronic DED mice. Draining lymph nodes and spleens were harvested, and their CD4+ T cell populations were analyzed for naïve, effector, and effector memory T cells, using flow cytometry analysis18 (Figure 3a). As expected, normal T cells contained the highest frequencies of CD62L+CD44lo naïve T cells (TN), accounting for 50% of the total CD4+ population. Compared to normal T cells, acute DED-T cells contained significantly more CD62L−CD44lo effector T cells (TE), while chronic DED-T cells contained the highest frequency of CD62L−CD44hi effector memory T cells (TM). The most prominent fraction in each group (i.e., TN from normal, TE from acute DED, and TM from chronic DED) were sorted and functionally characterized. TN (naïve) exhibited baseline levels of IFN-γ+ and IL-17+ cell frequencies. Both TE (effector) and TM (memory) demonstrated increased IFN-γ+ and IL-17+ cell frequencies. While TE and TM showed similar frequencies of IFN-γ+ cells, TM contained twice as many IL-17+ cells as TE. In addition, the frequencies of IL-17+ cells in TM were several-fold higher than those of IFN-γ+ cells (Figure 3b). No differences were noted in CD4+CD25+Foxp3+ Treg frequencies among TN, TE and TM groups (data not shown).
Figure 3.
T cells from chronic DED show increased effector memory Th17 population. Draining lymph node cells and spleen cells from normal, acute and chronic DED mice were analyzed by flow cytometry. Representative results from spleens are shown, with similar findings in draining lymph nodes. (a) CD62L versus CD44 expression on gated CD4+ cells is presented. Four T cell populations were differentiated16: CD62L+CD44lo naïve T cells (TN), CD62L−CD44lo effector T cells (TE), CD62L+CD44hi central memory T cells, and CD62L−CD44hi effector memory T cells (TM). Percentages indicate frequencies of each cell population. (b) Sorted TN from normal, TE from acute, and TM from chronic DED were further analyzed for IFN-γ and IL-17 expression. Frequencies of IFN-γ+ or IL-17+ cells in each individual populations are summarized as mean ± SEM of three independent experiments shown in the bar graph. **, p < 0.01 compared to TN. ‡, p < 0.01 compared to TE.
Adoptive transfer of effector memory T cells induces severe dry eye and dominant Th17 responses
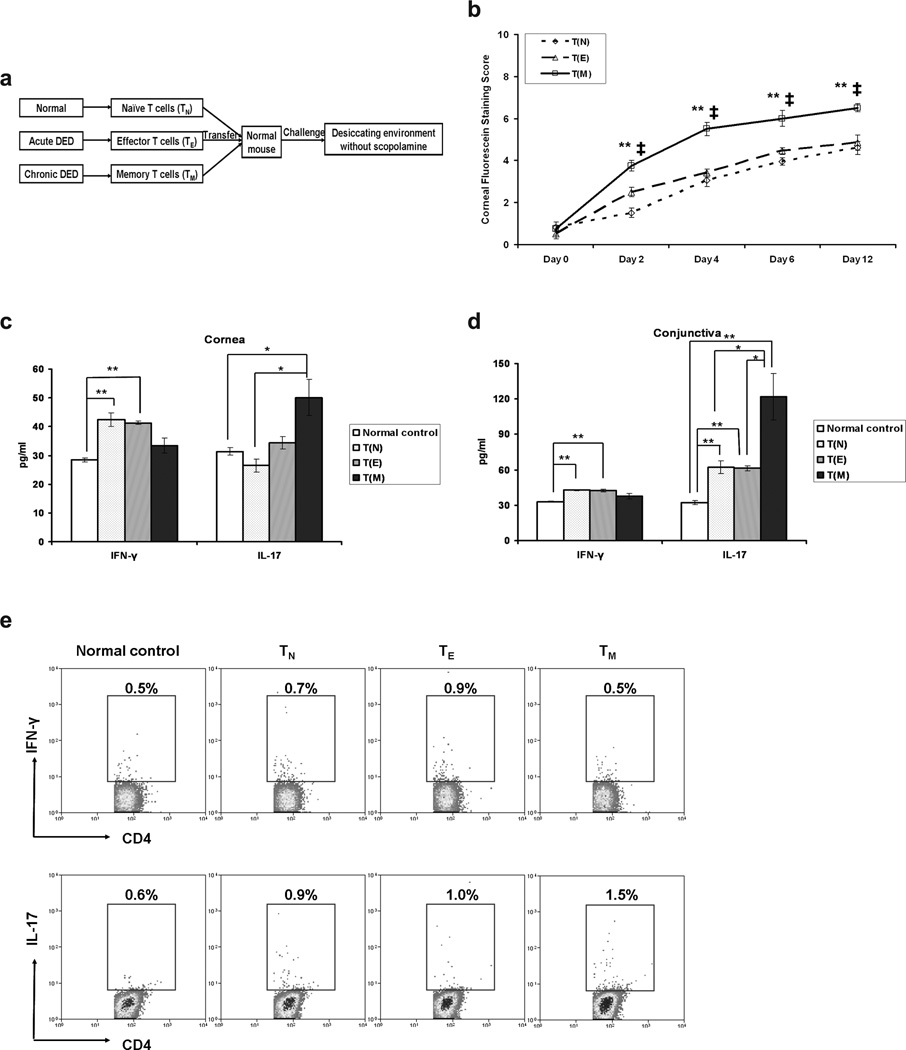
Next, to determine whether the CD4+CD62L−CD44hi TM fraction of chronic DED-T cells can mediate chronic DED, these TM were adoptively transferred to naïve mice, with TN and TE as controls, and recipient mice were subjected to desiccating stress (without use of scopolamine) (Figure 4a). TM recipients exhibited faster onset and significantly higher level of severity of the disease than did recipients of either TN or TE (Figure 4b). TE and TN recipients experienced similar courses of disease progression except for the timing of disease onset (day 2), when the TE recipients had a higher disease score. The fact that TM contained more IL-17-producing cells and comparable Treg cells at the time of transfer suggests that the higher clinical disease score observed in the recipients of TM was due primarily to the effector memory Th17-driven immune responses. To confirm this hypothesis, ocular surface and draining lymphoid tissues were harvested for examination of Th17/Th1 responses at day 12 after challenge. Both cornea (Figure 4c) and conjunctiva (Figure 4d) from the TM recipients demonstrated significantly increased IL-17 but not IFN-γ protein levels in comparison to those of the TN or TE recipients, or naïve mice, though the substantial increase of corneal IL-17 expression in the TM recipients compared to TE recipients did not reach statistic significance. In concordance with disease severity, TN and TE recipients showed similar increases in IFN-γ and IL-17. Consistent with the ocular surface findings, the draining lymph nodes of TM recipients exhibited the highest frequencies of Th17 cells (Figure 4e).
Figure 4.
Effector memory T cells (TM) from chronic dry eye disease (DED) mice induce the most severe disease in naïve recipients. (a) Schematic study design of adoptive transfer experiment. Draining lymph nodes and spleens from normal, acute and chronic DED mice were harvested. CD62L+CD44lo naïve T cells (TN) from normal, CD62L−CD44lo effector T cells (TE) from acute, and CD62L−CD44hi effector memory T cells (TM) from chronic DED were sorted with CD4 beads (negative selection) and FACS. Each naïve recipient was then injected i.v. with 3 × 105 TN, TE, or TM cells. Recipients were challenged with the controlled-environment chamber for 12 d without use of scopolamine. (b) Clinical disease evaluation among the recipients transferred with different cells. The mean corneal fluorescein staining score ± SEM for TN (n = 10 eyes), TE (n = 12 eyes), or TM (n = 8 eyes) recipients is shown. *, p < 0.05; **, p < 0.01 compared to TN recipients. ‡, p < 0.01 compared to TE recipients. (c, d) T cell response at the recipient ocular surface. Cornea (c) and conjunctiva (d) were collected from the recipient mice in (b) at day 12, and analyzed for IFN-γ and IL-17 levels by ELISA. Data shown represent the mean ± SEM of a single experiment out of two performed (n = 5–9 eyes per group). *, p < 0.05; **, p < 0.01. (e) T cell response in the recipient draining lymph nodes. Single cell suspension was prepared from lymph nodes and analyzed by flow cytometry for IFN-γ+CD4+ Th1 and IL-17+CD4+ Th17 cells. The indicated percentages of Th1 or Th17 cells as a proportion of total CD4+ T cells in the representative flow cytometry dot plots were measured in different recipient groups. Groups labeled as: T(N), recipients of naïve T cells from normal mice; T(E), recipients of effector T cells from acute DED mice; T(M), recipients of effector memory T cells from chronic DED mice.
DISCUSSION
Herein, we have demonstrated that chronic DED involves persistent ocular surface inflammation associated with a Th17 response. A significant effector memory Th17 population can be recovered from chronic DED and shown to mediate the long-term course of the disease.
The current study reveals, for the first time, that the corneal epithelial damage mediated by ocular surface inflammation in DED persists long term. However, the underlying immune response changes with time. Although the acute phase of DED exhibits both Th1 and Th17 responses as indicated in previous reports8,10 and the present study, the chronic phase of DED only is mediated by a Th17 response alone, including increased IL-17 expression and lymphangiogenesis on the ocular surface. These lymphatic vessels are potential conduits for immune cell migration between the ocular surface and draining lymphoid tissues.19 We have noted that the selective lymphangiogenesis in cornea is due to IL-17-driven expression of pro-lymphangiogenic factors.12 Therefore, we speculate that the persistent and progressively ingrowing lymphatic vessels in chronic DED corneas are due, at least in part, to the elevated levels of IL-17. Together, these findings suggest that relentless Th17 response at the ocular surface, even in the absence of triggering factors, could be one of the critical inflammatory pathways operating in the vicious cycle of DED.3
Previously, Yoon et al.20 found that some parameters of experimental DED severity normalize following the removal of desiccating stress. In their study, acute DED was induced using a methodology similar to ours, for 10 d, followed by maintenance in the standard vivarium for 28 d. They found that tear production, corneal smoothness, conjunctival goblet cell density, and conjunctival CD4+ T cell density normalized by day 28 in the standard vivarium. However, that study had several limitations. Clinically, the authors did not perform the important corneal epithelial defect assessment using corneal fluorescein staining, as is recommended by the National Eye Institute/Industry workshop for DED evaluation.14 Furthermore, although the numbers of conjunctival CD4+ T cells were counted, their cytokine secretion function was not examined.
In addition to the experimental data provided herein, several other clinical reports have indicated that DED is associated with a T cell-mediated immune response, as evidenced by increased infiltration of T cells in the conjunctiva4 and elevated levels of T cell cytokines, including IFN-γ21 and IL-1722 in the tears. However, it is not known whether this immune response is a cause or consequence of DED. In the present study, we demonstrate that T cells from the chronic DED mice can efficiently induce the disease in the naïve host, indicating that these T cells are pathogenic during the course of the disease. Further phenotypic analysis of the T cells shows a significant effector memory population, where secretion of IL-17 predominates over that of IFN-γ. This finding is consistent with the most recent recognition on the nature of Th17 cells as long-lived effector memory cells.23,24 Previously, it was assumed that both human and mouse Th17 cells are short-lived effector T cells. However, a recent study on human Th17 cells reveals that these cells have a high capacity for proliferative self-renewal, potent persistence, and apoptotic resistance.23 Subsequently, mouse Th17 cells have been found not only to be long lived but also to be empowered with an enhanced ability to enter the memory pool.24 These novel discoveries support the association of Th17 cells with various autoimmune diseases such as multiple sclerosis, Crohn’s disease, uveitis, and graft-versus-host disease,25,26,27,28 and support their proposed long-term anti-tumor activity.29,30
Next, we examined whether or not the effector memory T cell population contributes to the protracted damage to the ocular surface identified in chronic DED. Compared to the transfer of effector T cells to naïve recipients, effector memory T cells elicited a more severe DED which was similar to total T cell transfer, indicating that effector memory T cells is the principal subpopulation mediating the disease. However, effector T cells also induced a higher disease score than did naïve T cells only at disease onset, suggesting that effector T cells act for a short time, possibly because of their susceptibility to activation-induced cell death.31 In contrast, effector memory T cells demonstrated a strong and lasting effect on the disease, which can be attributed to their rapid expansion and resistance to apoptosis.32,33
In summary, the current work demonstrates that the persistent immunoinflammatory responses in chronic DED exhibit hallmarks of IL-17-mediated pathology, and implicates effector memory Th17 cells as the principal effector cells in maintaining chronic DED. These findings suggest a pathogenic role of autoimmunity in DED and expand our understandings of memory T cell response in chronic disease states. Accordingly, immunomodulatory therapies that target effector memory Th17 cells may effectively treat DED.
METHODS
Animals
Female C57BL/6 mice aged 6 to 8 wk (Charles River Laboratories, Wilmington, MA) were used for this study. All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
DED models
Acute DED was induced in mice as previously described, with some modification.11 Mice were placed in a controlled-environment chamber with a relative humidity below 20%, airflow of 15 L/min, and a constant temperature of 21 to 23°C, for 14 consecutive d. To maximize ocular surface dryness, mice received s.c. 0.1 mL injections of 5 mg/mL scopolamine hydrobromide (Sigma-Aldrich Corp, St. Louis, MO). Chronic DED was developed by transferring acute DED mice to a standard vivarium (temperature: 21 ~ 23°C, relative humidity: 40% ~ 60%) and maintained for an additional 4 mo without any environmental or anti-cholinergic (scopolamine) desiccating challenge.
Clinical evaluation
Corneal fluorescein staining and the National Eye Institute grading system (NEI, Bethesda, MD) was used to evaluate corneal epithelial damage caused by DED.14 Briefly, 1 µl of 2.5% fluorescein (Sigma-Aldrich Corp) was applied into the lateral conjunctival sac of the mice and after 3 minutes corneas were examined with a slit lamp biomicroscope under cobalt blue light. Punctate staining was recorded in a masked fashion with the standard National Eye Institute grading system of 0 to 3 for each of the five areas of the cornea – central, superior, inferior, nasal and temporal. The cotton thread test was used to evaluate aqueous tear production as previously described.11 Briefly, a phenol red thread (Zone-Quick; Lacrimedics, Eastsound, WA) was placed in the lateral canthus of the conjunctival fornix of the right eye for 30 seconds after excess tears had been removed for a standard time of 4 seconds and tear distance (in millimeters) was read under a microscope.
Immunohistochemical staining
Freshly excised corneas were washed in PBS, fixed in acetone for 15 min, and double stained with CD31 and LYVE-1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, the corneas were mounted using VECTASHIELD® mounting medium (Vector Laboratories, Burlingame, CA) and visualized under an epifluorescence microscope (Model E800; Nikon, Melville, NY) with a 2× objective lens at 20× magnification, and then the digitial micrographs were captured. Contrast of these acquired micrographs were linearly adjusted using Photoshop CS2 (Adobe, San Jose, CA) before the lymphatic area was calculated using Fiji software.34,35
Real-time PCR
Corneas and conjunctivae from mice were harvested, frozen in TRIzol® Reagent (Invitrogen Corp, Carlsbad, CA) and stored at −80°C until use. Total RNA was isolated with an RNeasy® Micro kit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations and reverse transcribed using a SuperScriptTM III kit (Invitrogen Corp). Real-time PCR was performed using TaqMan® Universal PCR Master Mix (Roche, Branchburg, NJ) and predesigned primers for IL-1β (Mm00434228_m1), IL-23 (Mm00518984_m1), IFN-γ (Mm00801778_m1), IL-17 (Mm00439619_m1) and GAPDH (Mm99999915_g1) (Applied Biosystems, Foster City, CA) in a LightCycler® 480 II System (Roche Applied Science, Indianapolis, IN). The GAPDH gene was used as an endogenous control for each reaction. The results of quantitative PCR were analyzed by the comparative CT method in which the target change = 2−ΔΔCT. The results were normalized by the CT value of GAPDH, and the mean CT of relative mRNA levels in the normal group was used as the calibrator.
ELISA
For protein extraction, corneas, conjunctivae and draining lymph nodes were harvested and stored in cold sterile PBS containing protease inhibitors (Sigma-Aldrich Corp) at −80°C until use. The samples were homogenized on ice and centrifuged. The supernatant was assayed for levels of IFN-γ and IL-17 using commercial ELISA kits (eBioscience, San Diego, CA).
Flow cytometry analysis
Cells were triple stained with the following antibodies: FITC-conjugated anti-CD4, APC-conjugated anti-CD44, and PE-conjugated anti-CD62L (BioLegend, San Diego, CA). For intracellular IFN-γ and IL-17 staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin (Sigma-Aldrich Corp) for 6 h at 37°C and 5% CO2 in the presence of GolgiStopTM (4 µl per 6 mL cell culture), (BD Biosciences, San Jose, CA) to inhibit cytokine secretion. The cells were then stained for FITC-conjugated anti-CD4, APC-conjugated anti-INF-γ, and PE-conjugated anti-IL-17; or PE-Cy5-conjugated anti-CD4, FITC-conjugated anti-CD44, PE-conjugated anti-CD62L, Alexa Fluor® 700-conjugated anti-INF-γ, and PE-Cy7-conjugated anti-IL-17 (BioLegend). Control samples were stained with appropriate isotype-matched control antibodies. Stained cells were examined with an LSR II Flow Cytometer™ (BD Biosciences), and the results were analyzed using Summit v4.3 software (Dako Colorado Inc, Fort Collins, CO).
T cell adoptive transfer
Draining lymph node and spleen cells from normal, acute DED, and chronic DED mice were pooled separately and enriched for T cells with CD90.2 magnetic cell isolation kit (Miltenyi Biotec Inc, Cambridge, MA). 1 × 106 cells were then adoptively transferred i.v. into each naïve recipient. For fractional T cell adoptive transfer, draining lymph node and spleen cells from normal, acute DED, and chronic DED mice were pooled separately and enriched for CD4+ T cells with a negative magnetic cell isolation kit (Miltenyi Biotec Inc). The CD4+ T cells were then quantified, stained with FITC-conjugated anti-CD44 and PE-conjugated anti-CD62L, and sorted for naïve (TN), effector (TE), and effector memory (TM) T cells using a MoFlo® Fluorescence Activated Cell Sorter (FACS) (Dako Cytomation, Carpinteria, CA). 5 × 105 cells were adoptively transferred i.v. into each naïve recipient.
Statistical analyses
An unpaired, two-tailed Student’s t test was used, and differences were considered significant at p < 0.05.
ACKNOWLEDGMENTS
We thank Dr. William Stevenson, and Gale Unger, Schepens Eye Research Institute, Boston, MA, for critical reading of this manuscript, and Dr. Sang-Mok Lee, Schepens Eye Research Institute, Boston, MA, for quantitative analysis of corneal lymphatic vessels. This work was supported by NIH grant EY20889.
Footnotes
Conflict of interest: None.
DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch. Ophthalmol. 2009;127:763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subcommittee of the international Dry Eye Workshop. The definition and classification of dry eye disease: report of the Definition and Classification. Ocul. Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 4.Stern ME, et al. Conjunctival T cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 5.Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000;118:1489–1496. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY, et al. Desiccating stress induces T cell-mediated Sjögren's Syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 7.Schaumburg CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 8.De Paiva CS, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest. Ophthalmol. Vis. Sci. 2009;50:3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan SK, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest. Ophthalmol. Vis. Sci. 2005;46:2766–2771. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan SK, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr. Opin. Ophthalmol. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 14.Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO. J. 1995;21:221–232. [PubMed] [Google Scholar]
- 15.Mokhtarzadeh M, Casey R, Glasgow BJ. Fluorescein punctate staining traced to superficial corneal epithelial cells by impression cytology and confocal microscopy. Invest. Ophthalmol. Vis. Sci. 2011;52:2127–2135. doi: 10.1167/iovs.10-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan BD, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2012 doi: 10.1111/aos.12012. [DOI] [PubMed] [Google Scholar]
- 17.Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch. Ophthalmol. 2010;128:819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 19.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch. Ophthalmol. 2012;130:84–89. doi: 10.1001/archophthalmol.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon KC, et al. Tear production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Invest. Ophthalmol. Vis. Sci. 2011;52:7267–7273. doi: 10.1167/iovs.11-7231. [DOI] [PubMed] [Google Scholar]
- 21.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. . Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 22.Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J. Korean Med. Sci. 2011;26:938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kryczek I, et al. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 2011;12:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muranski P, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amadi-Obi A, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 28.Kappel LW, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;22:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elyaman W, et al. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am. J. Pathol. 2008;173:411–422. doi: 10.2353/ajpath.2008.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor JJ, Jenkins MK. CD4+ memory T cell survival. Curr. Opin. Immunol. 2011;23:319–323. doi: 10.1016/j.coi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 33.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr. Opin. Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung ES, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am. J. Pathol. 2009;175:1984–1992. doi: 10.2353/ajpath.2009.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]