Abstract
The regeneration of large bone defects is a common and significant clinical problem. Limitations associated with existing treatments such as autologous bone grafts and allografts have increased the need for synthetic bone graft substitutes. The objective of this study was to evaluate the capacity of novel hollow hydroxyapatite (HA) microspheres to serve as a carrier for controlled release of bone morphogenetic-2 (BMP2) in bone regeneration. Hollow HA microspheres (106–150 μm) with a high surface area (>100 m2/g) and a mesoporous shell wall (pore size 10–20 nm) were created using a glass conversion technique. The release of BMP2 from the microspheres into a medium composed of diluted fetal bovine serum in vitro was slow, but it occurred continuously for over 2 weeks. When implanted in rat calvarial defects for 3 or 6 weeks, the microspheres loaded with BMP2 (1 μg/defect) showed a significantly better capacity to regenerate bone than those without BMP2. The amount of new bone in the defects implanted with the BMP2-loaded microspheres was 40% and 43%, respectively, at 3 and 6 weeks, compared to 13% and 17%, respectively, for the microspheres without BMP2. Coating the BMP2-loaded microspheres with a biodegradable polymer, poly(lactic-co-glycolic acid), reduced the amount of BMP2 released in vitro and, above a certain coating thickness, significantly reduced bone regeneration in vivo. The results indicate that these hollow HA microspheres could provide a bioactive and osteoconductive carrier for growth factors in bone regeneration.
Keywords: bone regeneration, hollow hydroxyapatite microspheres, bone morphogenetic protein-2, rat calvarial defect model
1. Introduction
The regeneration of large bone defects resulting from trauma, malignancy, and congenital diseases represents a common and significant clinical problem [1]. Autologous bone grafts are the gold standard for treatment because they possess all the ideal characteristics for bone growth: osteoconductivity, osteoinductivity, and osteogenicity [1-3]. However, autografts suffer from problems such as donor site morbidity and limited supply [4-6]. Bone allografts are alternatives, but they are expensive and carry the risk of disease transmission and adverse host immune reaction. These problems associated with autografts and allografts have increased the need for synthetic bone graft substitutes.
A variety of synthetic bone graft substitutes have been developed over the last 30 years, including hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP), biphasic calcium phosphate (BCP), calcium phosphate cements, bioactive glass, and biodegradable polymers [1-3]. However, synthetic bone graft substitutes currently see limited use clinically because of their inferior in vivo performance when compared to autogeneous bone grafts [3]. Most synthetic bone grafts have a limited capacity to reconstitute bone in large defects because they lack the osteoinductivity and osteogenicity of autologous bone grafts,
The osteoinductive properties of bone morphogenetic proteins (BMPs) have been evaluated extensively both in vitro and in vivo [7,8]. Among the recombinant proteins, BMP2 and BMP7 have been tested in a number of orthopedic and dental applications, and they are now used clinically. Because direct injection of a growth factor in soluble form into a defect site is generally not effective for bone regeneration due to rapid diffusion away from the site, delivery is often provided through a carrier material. A wide range of materials in various forms such as particles, granules, sponges and porous three-dimensional scaffolds have been tested as carriers for BMPs. They include biodegradable polymers, natural and synthetic, such as collagen and poly(lactic-co-glycolic acid) (PLGA), hydrogels, and calcium phosphates such as HA and beta-tricalcium phosphate (β-TCP), as well as composites of these materials [8,9].
In the last few years, we have investigated the development of a hollow HA microsphere technology to meet the need for bone graft substitutes that could approach the combined osteoconductive, osteoinductive, and osteointegrative properties of autologous bone grafts. Hollow HA microspheres prepared by a novel glass conversion technique [10,11] have a high surface area (>100 m2/g) and a mesoporous shell wall (pores size = 10–20 nm) composed of nanocrystalline HA particles [12]. The hollow core and the mesopores can provide reservoirs for loading growth factors into the microspheres, while the shell wall can provide methods for controlled release by desorption and migration of the protein through the mesopores.
Our previous work showed that the hollow HA microspheres can be loaded with bovine serum albumin (BSA), used as a model protein, and that the microspheres could function as a carrier for controlled delivery of BSA into phosphate-buffered saline (PBS) or a poly(ethylene glycol) (PEG) hydrogel in vitro [13,14]. Our more recent work showed that loading the hollow HA microspheres with transforming growth factor beta 1 (TGFβ1) (5 μg/defect) can enhance bone regeneration in rat calvarial defects at 6 weeks but not at 12 weeks [15]. However, the amount of new bone formed in the defects implanted with the TGF-loaded microspheres was only ~20% of the total defect area after 6 or 12 weeks.
The objective of this study was to evaluate the capacity of the hollow HA microspheres to serve as a carrier for BMP2 in the regeneration of bone in an osseous defect model. BMP2 was used in the present study because of its more potent ability to stimulate bone formation when compared to TGFβ1 [16,17]. The use of a biodegradable polymer coating to modify the release of BMP2 from the microspheres was studied in vitro. Bone regeneration in rat calvarial defects implanted with the BMP2-loaded hollow HA microspheres was evaluated using histomorphometric techniques. The rat calvarial defect model was used because it is a standard inexpensive assay for evaluating new bone formation in an osseous defect [18].
2. Materials and methods
2.1 Preparation and characterization of hollow HA microspheres
Hollow HA microspheres were prepared by reacting solid glass microspheres in an aqueous phosphate solution as described in detail in our previous study [12]. Briefly, borate glass with the composition 15CaO, 11Li2O, 74B2O3 (wt%), designated CaLB3-15, was prepared by melting Reagent grade CaCO3, Li2CO3 and H3BO3 (Alfa Aesar, Haverhill, MA, USA) in a Pt crucible at 1200 °C for 45 min, and quenching the melt between cold stainless steel plates. Particles of size 106–150 μm were obtained by grinding the glass in a hardened steel mortar and pestle, and sieving through 100 and 140 mesh sieves. Glass microspheres were obtained by dropping the crushed particles down a vertical tube furnace at 1000 °C [19]. Hollow HA microspheres were obtained by reacting the solid glass microspheres for 2 days in 0.02 M K2HPO4 solution at 37 °C and a starting pH = 9.0. In the conversion process, 1 g of glass microspheres was placed in 200 ml solution, and the system was gently stirred continuously. The converted microspheres were washed 3 times with distilled water, soaked in anhydrous ethanol to displace residual water, and dried for at least 12 h at room temperature, then for at least 12 h at 90 °C.
Characterization of the converted microspheres was performed using the methods described in our previous studies [12-15]. Briefly, the microstructure of the surface and cross section of the microspheres was examined using scanning electron microscopy (SEM) (S4700; Hitachi, Tokyo, Japan). The phase composition of the converted microspheres (ground into a powder) was checked using X-ray diffraction, XRD (D/mas 2550 v; Rigaku; The Woodlands, TX, USA) and Fourier transform infrared (FTIR) spectroscopy (NEXUS 670; Thermo Nicolet; Madison, WI, USA). XRD was performed using Cu Kα radiation (λ = 0.15406 nm) at a scan rate of 1.8°/min in the 2θ range 20–70°, while FTIR was performed in the wavenumber range of 400–4000 cm−1, (resolution = 8 cm−1) on pellets pressed from a mixture of 2 mg powder and 198 mg KBr. The carbon content of the microspheres was measured by a combustion technique at a commercial laboratory (LECO Corp., St. Joseph, MI, USA) and used to determine the carbonate content of the microspheres.
The specific surface area of the microspheres and the pore size distribution of the shell wall were measured using nitrogen adsorption (Autosorb-1; Quantachrome, Boynton Beach, FL, USA). A mass of 300–500 mg of microspheres was weighed and evacuated for 15 h at 120 °C to remove adsorbed moisture; then the volume of nitrogen adsorbed and desorbed at different relative gas pressures was measured and used to construct adsorption–desorption isotherms. The first five points of the adsorption isotherm, which initially followed a linear trend implying monolayer formation of the adsorbate, were fitted to the Brunauer–Emmett–Teller (BET) equation to determine the specific surface area. The pore size distribution was calculated using the Barrett-Joiner-Halenda (BJH) method applied to the desorption isotherm [20]. After they were loaded with BMP2 and after the conclusion of the BMP2 release experiments in vitro (described later), the dried microspheres were also characterized by SEM and the BET method using the procedures described above.
2.2 Loading the hollow HA microspheres with BMP2
After sterilization by soaking in anhydrous ethanol and drying in an incubator at 120 °C, some of the hollow HA microspheres were loaded with BMP2 (Shenandoah Biotechnology Inc., Warwick, PA, USA) and used to study the release profile of the BMP2 in vitro. A method described previously was used to load the microspheres with BMP2 [21,22]. Briefly, 10 mg of hollow HA microspheres were placed in a 1 ml micro-centrifuge tube, and 10 μl of BMP2 solution, formed by dissolving 10 μg BMP2 in 100 μl sterile citric acid (pH = 3.0), was pipetted on to the microspheres. A small vacuum was applied to the system to replace the air in the hollow HA microspheres with the BMP2 solution. The BMP2-loaded microspheres were dried overnight in a refrigerator at 4 °C and used immediately without further sterilization.
2.3 Coating the BMP2-loaded microspheres with a biodegradable polymer
The ability to modify the release profile of BMP2 from the hollow HA microspheres was studied by coating some of the BMP2-loaded microspheres with a biodegradable polymer, poly(DL-lactic-co-glycolic acid), PLGA (50/50; inherent viscosity = 0.17 dL/g; Birmingham Polymers, Inc., Birmingham, AL, USA). In the coating process, 20 μl of a PLGA solution in chloroform was added to 10 mg hollow HA microspheres in a centrifuge tube, and the system was dried at 4 °C for 24 h. Two different concentrations of the PLGA solution (50 mg/ml and 200 mg/ml) were used to form coatings with different thicknesses.
2.4 Measurement of BMP2 release profile in vitro
In measuring the release of BMP2 from the microspheres, 500 μl of a sterile solution (pH = 7.4) composed of equal volumes of fetal bovine serum (FBS) and phosphate-buffered saline (PBS) was added to 10 mg microspheres (coated with PLGA or uncoated) in a micro-centrifuge tube, and the samples were incubated at 37 °C. At selected times, the solution was removed as completely as possible for testing, and replaced with fresh solution. Control samples containing known amounts of BMP2 in the solution composed of FBS and PBS were also incubated at 37 °C. The amount of BMP2 present in the control samples was measured using an enzyme-linked immunosorbent assay (ELISA) kit (PeproTech, Rocky Hill, NJ, USA). The concentrations of the unknown samples were quantified relative to a BMP2 standard curve run on the same plate. Three samples of each group were tested at each time point, and the amount of BMP2 released was expressed as an average ± standard deviation (SD).
2.5 Animals and surgery
All animal experimental procedures were approved by the Missouri University of Science and Technology Animal Care and Use Committee, in compliance with the NIH Guide for Care and Housing of Laboratory Animals (1985). Twenty-five Sprague Dawley rats (3 months old; 350 ± 30 g) were housed in the animal care facility and acclimated to diet, water, and housing under a 12 hour/12hour light/dark cycle. The rats were anesthetized with an intramuscularly injected mixture of ketamine and xylazine (0.15 μl per 100 g). The surgical area was shaved, scrubbed with 70% ethanol, and then draped. With sterile instruments and aseptic technique, a cranial skin incision was sharply made in an anterior to posterior direction along the midline. The subcutaneous tissue, musculature and periosteum were dissected and reflected to expose the calvarium. Bilateral full-thickness defects 4.6 mm in diameter were created in the central area of each parietal bone using a trephine (4.6 mm outer diameter) attached to an electric drill. The sites were constantly irrigated with sterile PBS to prevent overheating of the bone margins and to remove the bone debris.
The calvarial defects were implanted with 4 groups of implants composed of hollow HA microspheres:
Hollow HA microspheres (positive control);
Hollow microspheres loaded with BMP-2 (1 μg/defect);
Hollow microspheres loaded with BMP-2 (1 μg/defect) and coated with PLGA (50 mg/ml);
Hollow microspheres loaded with BMP-2 (1 μg/defect) and coated with PLGA (200 mg/ml).
The defects were randomly implanted with 5 implants per group, but mixing of the implants with and without BMP2 was avoided in the same animal. Defects left empty served as the negative control group. The hollow HA microspheres without BMP2 and the unfilled defects were randomly assigned to 10 animals, while the 3 groups of microspheres loaded with BMP2 were randomly assigned to 15 animals. Each animal received an intramuscular injection of ~200 μl penicillin and ~200 μl buprenorphine post-surgery. The animals were monitored daily for condition of the surgical wound, food intake, activity and clinical signs of infection. After 3 or 6 weeks, the animals were sacrificed by CO2 inhalation, and the calvarial defect sites with surrounding bone and soft tissue were harvested.
2.6 Histology
The calvarial samples consisting of the defect sites with surrounding bone and soft tissue were washed with PBS and fixed in 10% formalin solution for 5 days. The fixed tissue samples were each cut transversely in half; half of each sample was for paraffin embedding and the other half for methyl methacrylate embedding. The samples for paraffin sections were decalcified for 4 weeks in EDTA (14 wt%) under mild agitation on a rocking plate. After the samples were dehydrated in ethanol and embedded in paraffin using standard histological techniques, 5 μm thick sections were cut and stained with hematoxylin and eosin (H&E) [23]. The undecalcified samples were dehydrated through a graded series of ethanol solutions, and embedded in methyl methacrylate. Sections were ground to a thickness of 30–40 μm using a micro grinding system (EXAKT 400CS, Norderstedt, Germany), and stained using the von Kossa technique to observe mineralization [24].
2.7 Histomorphometric analysis
Stained sections were examined in a transmitted light microscope (Model BX51; Olympus America, Center Valley, PA, USA) fitted with a digital color camera (Model DP71; Olympus). Images were analyzed on a computer using the ImageJ software (National Institutes of Health, USA). Sections stained with H&E were used to analyze the percent new bone formed within the defect. The newly formed bone was identified by outlining the edge of the defect, with the presence of original and new bone being identified by lamellar and woven bone, respectively. The total defect area was measured from one edge of the old calvarial bone, including the entire implant and tissue within it, to the other edge of the old bone. The newly formed bone within this area was then outlined and measured; the amount of new bone was expressed as a percent of the total defect area.
2.8 Statistical analysis
Measurements (n = 5) of percent new bone were expressed as a mean ± SD. Analysis for differences between groups was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test; differences were considered significant for p < 0.05.
3. Results
3.1 Characteristics of the as-prepared and PLGA-coated HA microspheres
SEM images of the surface of the as-prepared microspheres are shown in Fig. 1a, b. Examination of the cross section of the microspheres in the SEM confirmed that they were hollow (Fig. 1a, inset). As prepared, the hollow microspheres (external diameter = 106–150 μm) had a surface area of 102 ± 5 m2/g, a hollow core equal to 0.6 the microsphere diameter, and a mesoporous shell wall (pore size = 10–20 nm). SEM examination and the BET method did not show any measurable differences in the microstructure and surface area of the microspheres after loading with BMP2 or after the conclusion of the BMP2 release experiments in vitro at 14 days.
Fig. 1.
Low and high magnification SEM images of the surface of hollow HA microspheres as prepared (a, b), and after coating with solutions containing 50 mg/ml PLGA (c, d), and 200 mg/ml PLGA (e, f), followed by drying. The inset in (a) shows a cross section of a hollow HA microsphere.
The X-ray and FTIR patterns of the as-prepared microspheres were similar to those described in our previous work [12], confirming that the microspheres were composed of HA. The patterns are omitted for the sake of brevity. Briefly, the XRD patterns showed broad peaks at 2θ values which corresponded to those of a reference HA (JCPDS 72-1243). The broad peaks indicated that the HA was composed of nanometer-sized crystals, which is consistent with the fine, needle-like particles seen in the SEM image (Fig. 1b).
The most dominant resonances in the FTIR spectrum were the phosphate ν3 resonance, centered at ~1040 cm−1, and the phosphate ν4 resonance, with peaks at ~605 and 560 cm−1, which are associated with crystalline HA. The FTIR spectrum also showed a weak C–O resonance at 1420–1460 cm−1 corresponding to the (CO3)2− group substituting for (PO4)3− in HA [25], which resulted presumably from CO2 dissolved in the aqueous medium used in the glass conversion process. The carbonate content of the HA microspheres determined using a combustion technique described earlier was 2.1 wt%.
SEM images of the hollow HA microspheres after they were coated with a solution composed of 50 mg/ml or 200 mg/ml PLGA are shown in Fig. 1c–f. The microspheres coated with the 50 mg/ml PLGA solution consisted of aggregates bonded at their contact areas by the PLGA (Fig. 1c). The distribution of the PLGA coating on the surface of the microspheres was inhomogeneous, with no observable coating on some parts of the surface (Fig. 1d). When coated with the more concentrated PLGA solution (200 mg/ml), a larger amount of PLGA was apparent at the necks between the particles in the aggregates, and higher magnification SEM images showed that a larger surface area of microspheres was coated with a thicker layer (Fig. 1e, f).
3.2 BMP2 release profile in vitro
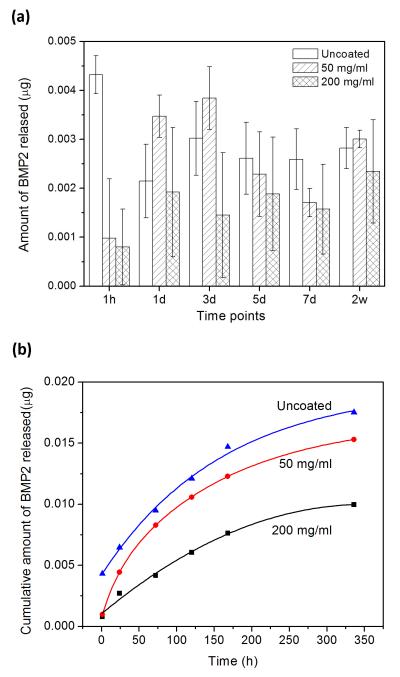
The amount of BMP2 released at selected time points from the as-prepared and PLGA-coated HA microspheres into a medium composed of FBS and PBS is shown in Figure 2a. As described earlier, at each time point, the medium was removed as completely as possible and fresh medium was added. The data were used to determine the cumulative amount of BMP2 released into the medium as a function of time (Fig. 2b). The uncoated microspheres showed an initial “burst” release at 1 h, followed by a more continuous release at longer times. In comparison, there appeared to be a delay in the release of BMP2 from the microspheres coated with the 50 mg/ml PLGA solution. The average amount of BMP released from the coated microspheres at 1 h was significantly smaller than that for the uncoated microspheres, but it became higher at day 1 and day 3 (Fig. 2a). Thereafter, the average amount of BMP2 released was lower or comparable to that for the uncoated microspheres. The average amount of BMP released from the microspheres coated with the thicker PLGA solution (200 mg/ml) at 1 h was significantly lower than that for the uncoated microspheres, and the amount released into the medium remained low thereafter.
Fig. 2.
(a) Amount of BMP2 released from hollow HA microspheres into a medium composed of equal volumes of FBS and PBS at selected time periods; (b) average cumulative amount of BMP2 released from the microspheres into the medium as a function of time. Data for the as-prepared microspheres (uncoated), and microspheres coated with 50 mg/ml PLGA solution (50 mg/ml), and with 200 mg/ml PLGA solution (200 mg/ml).
The average cumulative amount of BMP-2 released into the medium at any time decreased with the concentration of the PLGA coating solution (Fig. 2b). When the release experiments were terminated after 14 days, the cumulative amount of BMP-2 released from the three groups of microspheres into the medium, determined as a fraction of the amount initially loaded into the microspheres, was 1.8%, 1.5%, and 1.0%, respectively, for the as-prepared microspheres, the microspheres coated with the 50 mg/ml solution, and the microspheres coated with the 200 mg/ml solution.
3.3 Bone regeneration in rat calvarial defects implanted with hollow HA microspheres
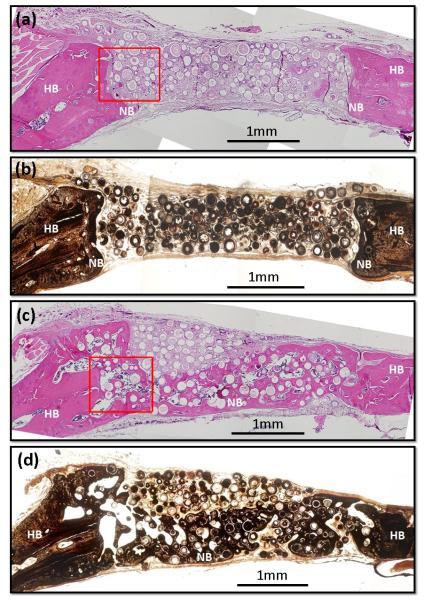
Figure 3 shows H&E and von Kossa stained sections of rat calvarial defects implanted for 6 weeks with the hollow HA microspheres without and with BMP2. New bone growth in the defects implanted with the microspheres without BMP2 was limited (17 ± 10%), and occurred mainly at the periphery with the host (old) bone (Fig. 3a, b). In comparison, new bone formation in the defects implanted with the BMP2-loaded microspheres was markedly greater (43 ± 6%), and the new bone bridged the defect within the 6 week implantation period (Fig. 3c, d).
Fig. 3.
(a, c) H&E and (b, d) von Kossa stained sections of rat calvarial defects implanted for 6 weeks with (a, b) as-prepared hollow HA microspheres (without BMP2), and (c, d) hollow HA microspheres loaded with BMP2 (1 μg/defect). HB = host (old) bone; NB = new bone.
Higher magnification images of the boxed areas in the H&E stained sections in Fig. 3 showed limited new bone formation in the pore space between the microspheres without BMP2 (Fig. 4a). Instead, most of the pore space was filled with fibrous tissue that contained some blood vessels (red arrow). It appeared that fibrous tissue also infiltrated the hollow cores of the microspheres. In comparison, along with the considerable amount of new bone in the pore space between the BMP2-loaded microspheres (Fig. 4b), blood vessels (red arrow) and bone marrow-like tissue (yellow arrow) were also present in the new bone.
Fig. 4.
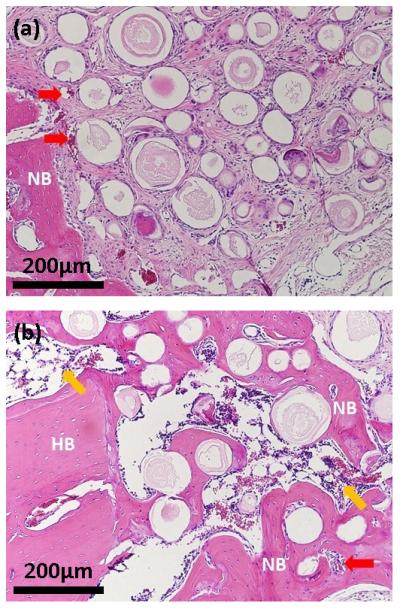
High magnification images of H&E stained sections of rat calvarial defects implanted for 6 weeks with (a) as-prepared hollow HA microspheres (without BMP2) and (b) hollow HA microspheres loaded with BMP2 (1 μg/defect). The images shown in (a) and (b) correspond to the boxed area outlined in Fig. 4a and b, respectively. HB = host (old) bone; NB = new bone; red arrows indicate blood vessels; yellow arrows indicate bone marrow-like tissue.
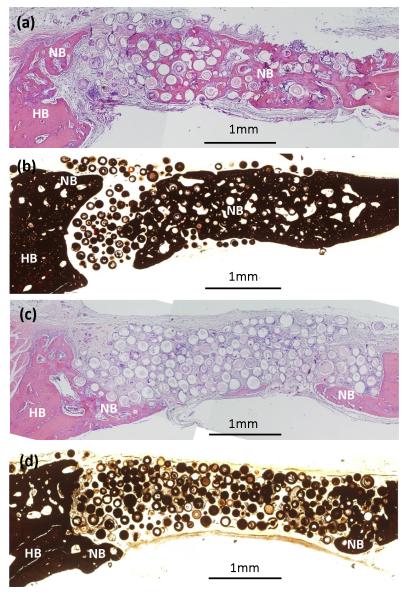
Von Kossa and H&E stained sections of the defects implanted for 6 weeks with BMP2-loaded hollow HA microspheres that were coated with PLGA are shown in Fig. 5. For the microspheres coated with the less concentrated solution (50 mg/ml PLGA), considerable new bone (46 ± 8%) was formed in the defects, and it almost completely bridged the defect. In comparison, for the microspheres coated with the more concentrated solution (200 mg/ml PLGA), the amount of new bone formed in the defects was significantly lower (19 ± 13%). New bone infiltrated the periphery (edge) of the implants and also formed on the dural (bottom) size.
Fig. 5.
(a, c) H&E and (b, d) von Kossa stained section of rat calvarial defects implanted for 6 weeks with hollow HA microspheres loaded with BMP2 and coated with different amounts of PLGA formed from solutions containing 50 mg/ml PLGA (a, b) and 200 mg/ml PLGA (c, d). HB = host (old) bone; NB = new bone.
Since considerable new bone formation was observed at 6 weeks in the defects implanted with the BMP2-loaded microspheres, uncoated or coated with the 50 mg/ml PLGA solution, an implantation time of 3 weeks was also used to examine the time-dependence of the new bone formation. The von Kossa and H&E stained sections are omitted for the sake of brevity. In general, for the same group of implants, the stained sections at 3 weeks did not show a marked difference from those at 6 weeks.
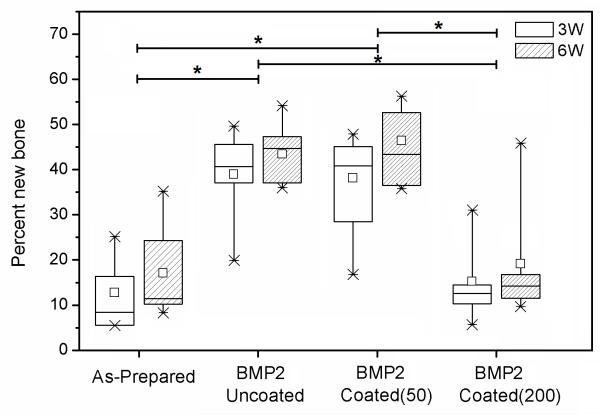
A box plot of the percent new bone formed in the defects implanted with the four groups of HA microspheres at 3 and 6 weeks is shown in Fig. 6; the data for the mean ± SD are summarized in Table I. For each group, the average percent new bone increased from 3 weeks to 6 weeks, but the difference was not significant. The results also showed that new bone formation in the defects implanted with the BMP2-loaded implants, either uncoated or coated with the 50 mg/ml PLGA solution, was significantly higher than in the implants without BMP2 or the BMP2-loaded implants coated with the 200 mg/ml PLGA solution. The amount of new bone formed in BMP2-loaded implants coated with the 200 mg/ml PLGA solution was not significantly different from that for the implants without BMP2.
Fig. 6.
Box plot comparing the percent new bone formed in rat calvarial defects implanted for 3 weeks (3W) and 6 weeks (6W) with four groups of implants composed of hollow HA microspheres: (a) as prepared; (b) loaded with BMP2 (denoted BMP2/Uncoated); (c) loaded with BMP2 and coated using a 50 mg/ml PLGA solution (denoted BMP2/Coated(50)); (d) loaded with BMP2 and coated using a 200 mg/ml PLGA solution (denoted BMP2/Coated(200)). The median and mean values, interquartile range, and the high and low values for each group are shown. (n = 5; *significant difference between groups; p < 0.05).
Table I.
Percent new bone (mean ± SD) formed in rat calvarial defects implanted for 3 weeks and 6 weeks with four groups of hollow HA microspheres used in this study: microspheres without BMP2; microspheres with BMP2; BMP2-loaded microspheres coated with 50 mg/ml PLGA solution; BMP2-loaded microspheres coated with 200 mg/ml PLGA solution.
| HA microspheres | Implantation time | |
|---|---|---|
|
| ||
| 3 weeks | 6 weeks | |
| Without BMP2 | 13 ± 5 | 17 ± 10 |
| With BMP2 | 39 ± 10 | 43 ± 6 |
| With BMP2; PLGA coating (50 mg/ml) | 38 ± 11 | 46 ± 8 |
| With BMP2; PLGA coating (200 mg/ml) | 15 ± 8 | 19 ± 13 |
4. Discussion
With a hollow core, high surface area, and a mesoporous shell wall, the HA microspheres used in this study could provide a novel growth factor carrier that is bioactive and osteoconductive. BMP2 is a potent growth factor for stimulating bone formation, but it is limited by high cost and increasing concerns about adverse biological effects associated with the use high doses [26,27]. Consequently, the ability to control the sustained release of BMP-2 at clinically desirable rates is relevant in bone regeneration. The feasibility of combining the hollow HA microspheres with a biodegradable polymer coating to control the release profile of BMP2 was shown. This study also showed that when loaded with BMP2, the hollow HA microspheres had a considerable capacity to regenerate bone in vivo, despite a slow and limited capacity to release BMP2 in vitro.
4.1 Hollow HA microspheres as a carrier for BMP2
The results showed that BMP2 was released continuously but slowly from the hollow HA microspheres into the medium in vitro (Fig. 2b). BMP2 has a high affinity for HA and, consequently, its release rate from HA in vitro is often low. The adsorbed BMP2 is reported to be strongly immobilized on the surface of the HA by electrostatic and other interactions, such as hydrogen bonding [28]. This strong interaction makes it difficult for the BMP2 to be displaced from the HA surface by competitive adsorption from other proteins [29]. In one study, 23% of the BMP2 initially adsorbed on a nanostructured HA coating was released at 21 days, but the release rate was almost doubled from a coarse-grained HA coating [30]. In another study, after an initial release of 22% within the first 24 h, subsequent release of BMP2 from a porous calcium phosphate cement was limited [29]; the addition of a carrier protein, bovine serum albumin, had little effect on the BMP2 release profile.
While there is a tendency for low release of BMP2 from calcium phosphate bioceramics in vitro, the amount released from the hollow HA microspheres used in this study was still lower than those observed from more conventional HA materials [29,30]. A few factors might have contributed to the much lower release rate, but the unique structural features of the hollow HA microspheres, such as the high-surface-area mesoporous shell, might be the most important. The theoretical amount of BMP2 that can be adsorbed onto the surface of HA has been estimated to be ~800 μg/m2 [30]. The specific surface area of the hollow HA microspheres used in this study was ~100 m2/g. Consequently, ~800 μg of BMP-2 could theoretically be adsorbed on the surface of the 10 mg of HA microspheres used in these experiments. Since the total amount of BMP2 initially loaded into the hollow HA microspheres was 1 μg, therefore a large amount of the HA surface area would remain available for adsorption. Presumably the desorbed BMP2 could be readily re-adsorbed on the largely free HA surface, which would markedly reduce the amount of BMP2 released into the medium. Furthermore, the tortuous mesopores of the shell wall could make migration of BMP2 from the hollow core or from the pores to the external surface of the microspheres difficult because of the large surface area available for adsorption.
Other factors that could also contribute to the limited amount BMP2 released in vitro are the limited flow of the liquid media used in the release experiments, and the tendency of the microspheres to aggregate. However, a higher release rate of BMP2 from the microspheres can be expected in vivo because of a higher degradation rate of the HA due to cell-mediated degradation in addition to dissolution-mediated degradation [31,32] and the higher solubility of proteins in vivo. If required, the release of BMP2 from the as-prepared microspheres can be improved by increasing the dissolution or degradation rate of the HA [28-30] which is dependent on the composition and structure of the HA [33,34]. The HA microspheres used in the present study had a nearly stoichiometric composition and, consequently, a low degradation rate. As described earlier, no measurable degradation of the microspheres was observed after the two-week BMP2 release experiments in vitro. Our previous experiments showed no measurable degradation of the microspheres at 12 weeks in rat calvarial defects [15]. In our ongoing experiments, hollow HA microspheres with varying amounts of carbonate substitution are being prepared by a similar glass conversion process and they are being evaluated in vitro and in vivo.
The rate at which the BMP2 is released with time is also relevant in the use of these hollow HA microspheres as a carrier in bone regeneration. In particular, a high initial burst release of BMP2 has often been observed from HA carrier materials [35]. A burst release of BMP2 can induce inflammatory reactions which are believed to be beneficial for promoting bone formation, but it can also cause significant adverse biological effects. While a reduction in the BMP2 dose delivered in vivo could reduce those side effects, a high dose is often required to induce significant bone formation [26,36].
In order to test the ability to modify the BMP2 release rate, the hollow HA microspheres were coated with PLGA after they were loaded with BMP2. PLGA solutions with two different concentrations (50 mg/ml and 200 mg/ml) were used to control the thickness or the amount of the PLGA deposited on the microspheres. It is expected that the PLGA could reduce the size of the mesopores in the shell wall of the microspheres or completely block the pores, depending on the concentration of the coating solution. Consequently, the initial release of any unadsorbed BMP-2 from the microspheres would be impeded.
The results showed that both coatings were effective in reducing the amount of BMP2 released during the first hour when compared to the uncoated microspheres (Fig. 2a); the amount of BMP2 released from the coated microspheres was 4 to 5 times lower than that for the uncoated microspheres. Subsequently, the amount of BMP2 released from the microspheres coated with the 50 mg/ml PLGA solution increased, and the cumulative amount of BMP2 released was not significantly different from that of the uncoated microspheres (Fig. 2b). In comparison, the amount of BMP2 released from the microspheres coated with the 200 mg/ml solution remained at a much lower value, presumably because the larger PLGA coverage provided a greater barrier to the release of BMP2.
The results showed the feasibility of combining the hollow HA microspheres with a biodegradable polymer coating to control the release profile of BMP2. However, it is expected that optimization of the coating will depend on the clinical application. For example, the use of a much higher BMP2 dose (1500 ug/ml), as in some clinical applications [37], will require the coating thickness to be adjusted accordingly in order to achieve optimum control on the release profile.
4.2 BMP2-loaded hollow HA microspheres as implants in bone regeneration
The in vivo experiments showed the effectiveness of using the hollow HA microspheres as a carrier to deliver BMP2 in the stimulation of bone regeneration. Loading the microspheres with BMP2 significantly enhanced new bone formation in the rat calvarial defects at 3 weeks and the amount of new bone did not change significantly at 6 weeks (Fig. 6). This indicates that the BMP2-induced new bone formation was almost completed as early as 3 weeks, although bone remodeling could continue thereafter. As the pore space between the microspheres was almost completely infiltrated with new bone at 3 weeks, there was little opportunity for a significant further increase at 6 weeks.
An interesting observation was the effect of the PLGA coating on the capacity of the BMP2-loaded microspheres to regenerate bone. New bone formation in the implants coated with the PLGA solution of lower concentration (50 mg/ml) was not significantly different from that in the uncoated implants whereas new bone formation in the implants coated with the PLGA solution of higher concentration (200 mg/ml) was significantly lower (Fig. 6). In fact, the percent new bone formed in the BMP2-loaded implants coated with the 200 mg/ml PLGA solution was comparable to that for the microspheres without BMP-2. The reason for the difference in bone generation among the uncoated and coated implants loaded with BMP is not clear at present and it is the subject of ongoing studies. However, a few factors that could contribute to the difference are briefly discussed at this stage.
The BMP2 release profiles in vitro showed that while the average BMP2 release rate (amount per period) was fairly similar among the three groups of microspheres after 3 days, there appeared to be more pronounced differences at earlier times (Fig. 2a). A possible explanation therefore is that differences in the initial release within the first 3 days could be responsible for the differences in new bone formation in the 3 implant groups loaded with BMP2. The cumulative amount of BMP2 released from the uncoated microspheres and the microspheres coated with the 50 mg/ml solution (8–10 μg) was more than twice the value (~4 μg) for microspheres coated with the 200 mg/ml solution. The higher dose of BMP2 in the defect site released in the initial time period from the uncoated microspheres and the microspheres coated with the 50 mg/ml PLGA solution might have reached the threshold value to trigger the repairing cascades. In comparison, the lower BMP2 dose released from the microspheres coated with the 200 mg/ml PLGA solution might have been below the threshold value. The time at which exogenous BMP2 is administered can be critical. In a previous study [38], the administration of BMP2 at day 0 or day 4 post-fracture was found to enhance periosteal callus formation, bone mineral content, and biomechanical properties when compared to later administration of BMP2 (day 8).
Another factor that could contribute to the difference in bone regeneration among the BMP2-loaded implants is the modification of the surface properties of the HA by the PLGA coating. As described earlier, the coating produced by the 50 mg/ml PLGA solution was inhomogeneous and mainly concentrated at the necks between the microspheres, leaving a large area of the microsphere surface uncoated (Fig. 2c, d). The microspheres could still provide a large area of osteoconductive surface for bone regeneration. This, combined with the release of BMP2, apparently did not reduce the capacity of the coated microspheres to regenerate bone. In comparison, a larger fraction of the microsphere surface was coated with the 200 mg/ml PLGA solution (Fig. 2 e, f), which markedly reduced the osteoconductive surface area. The slow release of BMP2 from the microspheres presumably compensated for the reduction in osteoconductive surface area, resulting a similar capacity to regenerate bone as the uncoated microspheres without BMP2 (Fig. 6).
It is also possible that the coating process could reduce the bioactivity of the BMP2 in the microspheres. At 14 days when the in vitro release experiments were concluded, the difference in the cumulative amount of BMP2 released from the microspheres coated with the 50 mg/ml and the 200 mg/ml solution was small (1.5% vs. 1.0%). A greater reduction in the bioactivity of the BMP2 released from the microspheres coated with the more concentrated solution could contribute to the significantly lower bone regeneration in those implants.
Another possible explanation for the difference in bone regeneration among the BMP2-loaded implants might be the critical role played by the immobilized BMP2 in bone repair (when compared to the released BMP2). It has been suggested that BMPs immobilized in extracellular matrices are essential for the osteoblastic differentiation of cells [39]. The BMP2 in the uncoated microspheres or the microspheres coated with 50 mg/ml PLGA solution was presumably able to interact with cells. In comparison, the thicker coating on the microspheres coated with the 200 mg/ml PLGA solution presumably made the BMP2 in those microspheres inaccessible to the cells. Further studies are required to elucidate the mechanism.
Table II gives a comparison of the amount of new bone formed in rat calvarial defects implanted with the hollow HA microspheres (with or without BMP-2) used in this study and with a variety of biomaterials implanted in the same animal model. This list is not meant to be exhaustive; instead it provides data for a few selected bioactive materials: hollow HA microspheres, similar to those used in this study, loaded with TGFβ1 (5 μg/defect) [15]; particles (150–250 μm) of silicate 45S5 bioactive glass [40]; three-dimensional (3D) scaffolds of a borate bioactive glass (designated 13-93B3) with a microstructure similar to dry human trabecular bone [41]; 3D scaffolds of a calcium-deficient HA (CDHA) without or with BMP2 (2 μg/defect) [42]; and 3D scaffolds of a commercial biphasic calcium phosphate (BCP) without or with BMP2 (2.5 μg/defect) [43]. Silicate 45S5 glass is included in this comparison because it is considered to be the gold standard for bioactive glasses, whereas the 13-93B3 bioactive glass scaffolds are receiving interest for bone repair applications. In common with the HA used in the present study, the CDHA and BCP are calcium phosphate biomaterials.
Table II.
Comparison of percent new bone (mean ± SD) formed in rat calvarial defects implanted with the hollow HA microspheres (106-150 μm) used in this study, and with selected bioactive ceramic and glass implants.
| Implant | Growth factor | Implantation time (weeks) |
% new bone |
Reference |
|---|---|---|---|---|
| Hollow HA microspheres | – | 6 | 17 ± 10 | This study |
| Hollow HA microspheres | BMP2 (1 μg/defect) |
6 | 43 ± 6 | This study |
| Hollow HA microspheres | TGFβ1 (5 μg/defect) |
12 | 19 ± 4 | 15 |
| 45S5 silicate glass particles | – | 12 | 19 ± 3 | 40 |
| (150-250 μm) | ||||
| 13-93B3 borate glass (porosity = 77%; pore size = 200-400 μm) |
– | 12 | 15 ± 3 | 41 |
| Calcium-deficient HA (porosity = 75%; pore size = 400-500 μm) |
– | 8 | 10 ± 3 | 42 |
| Calcium-deficient HA (porosity = 75%; pore size = 400-500 μm) |
BMP2 (2 μg/defect) |
8 | 27 ± 4 | 42 |
| BCP (porosity = 70%; pore size = 300-600 μm and <10 μm)1 |
– | 8 | 23 ± 5 | 43 |
| BCP (porosity = 70%; pore size = 300-600 μm and <10 μm)1 |
BMP2 (2.5 μg/defect) |
8 | 55 ± 8 | 43 |
1BCP: biphasic calcium phosphate (HA:βTCP = 20:80); two-thirds of the porosity in the range 300-600 μm; one-third of the porosity <10 μm
For implants composed of the hollow HA microspheres used in this study, the data show that BMP2 is considerably more effective in stimulating new bone formation than TGFβ1. The data also indicate that the hollow HA microspheres loaded with BMP2 have a greater capacity to regenerate bone when compared to silicate 45S5 bioactive glass particles and porous scaffolds of borate 13-93B3 bioactive glass. The hollow HA microspheres also showed a better capacity to regenerate bone at 6 weeks when compared to highly porous scaffolds of the CDHA implanted for 8 weeks; when loaded with BMP2, the microspheres also showed a better capacity to regenerate bone than the CDHA scaffolds loaded with twice the amount of BMP2. New bone formation in the defects implanted with the hollow HA microspheres (without BMP2) for 6 weeks was comparable to that in highly porous commercial BCP scaffolds at 8 weeks. When loaded with BMP2, new bone formation in the BCP scaffolds was higher than that in the BMP2-loaded hollow HA microspheres. However, the implantation time was longer and the BMP2 loading was 2.5 times the amount used in the present work.
5. Conclusions
Hollow hydroxyapatite (HA) microspheres with a high-surface-area mesoporous shell wall can provide a potential novel carrier for BMP2 in bone regeneration. The capacity of the microspheres to deliver BMP2 in vitro was shown, although the amount of BMP2 released into the medium was small (1.8% after 2 weeks). The release of BMP2 from the hollow HA microspheres, particularly at early times, can be reduced and controlled by coating the microspheres with different amounts of a biodegradable polymer, poly(lactic-co-glycolic acid) (PLGA), indicating the feasibility of controlling the initial burst release of BMP2 typically observed from HA implants. When loaded with BMP2 (1 μg/defect), the hollow HA microspheres, uncoated or coated with a 50 mg/ml PLGA solution significantly enhanced bone regeneration in rat calvarial defects within 3 to 6 weeks. Bone regeneration in those two groups of BMP2-loaded HA microspheres was considerably higher than in 45S5 glass particles (150–250 μm), the gold standard of bioactive glasses.
Acknowledgement
This work was supported by the National Institute of Dental and Craniofacial Research, National Institutes of Health, Grant # 1R15DE018251-01, and the Center for Bone and Tissue Repair and Regeneration, Missouri S&T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36S:S20–S37. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- [2].Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354–61. [PubMed] [Google Scholar]
- [3].Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- [4].Fernyhough JC, Schimandle JJ, Weigel MC. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–80. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- [5].Perry CR. Bone repair techniques, bone graft and bone graft substitutes. Clin Orthop Rel Res. 1999;360:71–86. doi: 10.1097/00003086-199903000-00010. [DOI] [PubMed] [Google Scholar]
- [6].Fleming JE, Cornell CN, Muschler GE. Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am. 2000;31:357–74. doi: 10.1016/s0030-5898(05)70156-5. [DOI] [PubMed] [Google Scholar]
- [7].Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92:161–8. doi: 10.1007/s12306-008-0064-1. [DOI] [PubMed] [Google Scholar]
- [8].Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Del Rev. 2000;43:65–92. doi: 10.1016/s0169-409x(00)00078-8. [DOI] [PubMed] [Google Scholar]
- [9].Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- [10].Day DE, Conzone SA. Method for preparing porous shells or gels from glass particles. US Patent No. 6,358,531. 2002 Mar 19;
- [11].Conzone SD, Day DE. Preparation and properties of porous microspheres made form borate glass. J Biomed Mater Res A. 2009;88:531–42. doi: 10.1002/jbm.a.31883. [DOI] [PubMed] [Google Scholar]
- [12].Fu H, Rahaman MN, Day DE, Fu Q. Effect of process variables on the microstructure of hollow hydroxyapatite microspheres prepared by a glass conversion method. J Am Ceram Soc. 2010;93:3116–23. doi: 10.1111/j.1551-2916.2010.03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fu H, Rahaman MN, Day DE, Brown RF. Hollow Hydroxyapatite microspheres as a device for controlled delivery of proteins. J Mater Sci Mater Med. 2011;22:579–91. doi: 10.1007/s10856-011-4250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fu H, Rahaman MN, Brown RF, Day DE. Evaluation of BSA protein release from hollow HA microspheres into PEG hydrogel. Mater Sci Eng C. 2013 doi: 10.1016/j.msec.2013.01.048. DOI: 10.1016/j.msec. 2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fu H, Rahaman MN, Brown RF, Day DE. Evaluation of bone regeneration in implants composed of hollow HA microspheres loaded with transforming growth factor β1 in a rat calvarial defect model. Acta Biomater. 2013;9:5718–27. doi: 10.1016/j.actbio.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. J Bone Jt Surg Am. 2002;84:1032–44. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- [17].Ruhé P, Kroese-Deutman H, Wolke J, Spauwen P, Jansen J. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits. Biomaterials. 2004;25:2123–32. doi: 10.1016/j.biomaterials.2003.09.007. [DOI] [PubMed] [Google Scholar]
- [18].Wang J, Glimcher MJ, Mah J, Zhou HY, Salih E. Expression of bone microsomal casein kinase ii, bone sialoprotein, and osteopontin during the repair of calvarial defects. Bone. 1998;22:621–8. doi: 10.1016/s8756-3282(98)00057-x. [DOI] [PubMed] [Google Scholar]
- [19].Day DE, White JE, Brown RF, McMenamin KD. Transformation of borate glasses into biologically useful materials. Glass Technol. 2003;44:75–81. [Google Scholar]
- [20].Barrett EP, Joyney LG, Halenda PP. The determination of pore volume and area distributions in porous substances I: computations from nitrogen isotherms. J Am Chem Soc. 1951;73:373–80. [Google Scholar]
- [21].Nandi SK, Kundu B, Basu D. Protein growth factors loaded highly porous chitosan scaffold: A comparison of bone healing properties. Mater Sci Eng C. 2013;33:1267–75. doi: 10.1016/j.msec.2012.12.025. [DOI] [PubMed] [Google Scholar]
- [22].Van de Watering FCJ, Molkenboer-Kuenen JDM, Boerman OC, van den Beucken JJJP, Jansen JA. Differential loading methods for BMP-2 within injectable calcium phosphate cement. J Control Release. 2012;164:283–90. doi: 10.1016/j.jconrel.2012.07.007. [DOI] [PubMed] [Google Scholar]
- [23].Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002;17:1822–31. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- [24].Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–47. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- [25].Vignoles M, Bonel G, Holcomb DW, Young RA. Influence of preparation conditions on the composition of type B carbonated hydroxyapatite and on the localization of the carbonate ions. Calcif Tissue Int. 1988;43:33–40. doi: 10.1007/BF02555165. [DOI] [PubMed] [Google Scholar]
- [26].Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- [27].Woo EJ. Does bone morphogenetic protein increase the incidence of perioperative complications in spinal fusion? A comparison of 55,862 cases of spinal fusion with and without bone morphogenetic protein. Spine. 2012;37:259. doi: 10.1097/BRS.0b013e31823fcf42. [DOI] [PubMed] [Google Scholar]
- [28].Boix T, Gomez-Morales J, Torrent-Burgues J, Monfort A, Puigdomenech P, Rodriguez-Clemente R. Adsorption of recombinant human bone morphogenetic protein rhBMP-2 onto hydroxyapatite. J Inorg Biochem. 2005;99:1043–50. doi: 10.1016/j.jinorgbio.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [29].Ruhe PQ, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J Mater Sci Mater Med. 2006;17:919–27. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- [30].Autefage H, Briand-Mesange F, Cazalbou S, Drouet C, Fourmy D, Goncalves S, Salles JP, Combes C, Swider P, Rey C. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J Biomed Mater Res B Appl Biomater. 2009;91:706–15. doi: 10.1002/jbm.b.31447. [DOI] [PubMed] [Google Scholar]
- [31].Doi Y, Iwanaga H, Shibutani T, Moriwaki Y, Iwayama Y. Osteoclastic responses to various calcium phosphates in cell cultures. J Biomed Mater Res. 1999;47:424–33. doi: 10.1002/(sici)1097-4636(19991205)47:3<424::aid-jbm19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [32].Leeuwenburgh S, Layrolle P, Barrère F, de Bruijn J, Schoonman J, van Blitterswijk CA, de Groot K. Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro. J Biomed Mater Res. 2001;56:208–15. doi: 10.1002/1097-4636(200108)56:2<208::aid-jbm1085>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [33].LeGeros RZ. Biodegradation/bioresorption of CaP materials. Clin Mater. 1993;14:65–88. doi: 10.1016/0267-6605(93)90049-d. [DOI] [PubMed] [Google Scholar]
- [34].Dorozhkin SV. Calcium orthophosphates. Occurrence, properties, biomineralization, pathological calcification, and biomimetic applications. Biomatter. 2011;1:121–64. doi: 10.4161/biom.18790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rohanizadeh R, Chung K. Hydroxyapatite as a carrier for bone morphogenetic protein. J Oral Implantol. 2011;37:659–72. doi: 10.1563/AAID-JOI-D-10-00005. [DOI] [PubMed] [Google Scholar]
- [36].Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235–9. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [37].Boden SD. Bioactive factors for bone tissue engineering. Clin Orthop Relat Res. 1999:S84–94. doi: 10.1097/00003086-199910001-00009. [DOI] [PubMed] [Google Scholar]
- [38].Murnaghan M, McIlmurray L, Mushipe MT, Li G. Time for treating bone fracture using rhBMP-2: a randomised placebo controlled mouse fracture trial. J Orthop Res. 2005;23:625–31. doi: 10.1016/j.orthres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- [39].Suzawa M, Takeuchi Y, Fukumoto S, Kato S, Ueno N, Miyazono K, Matsumoto T, Fujita T. Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology. 1999;140:2125–33. doi: 10.1210/endo.140.5.6704. [DOI] [PubMed] [Google Scholar]
- [40].Bi L, Jung SB, Day DE, Neidig K, Dusevich V, Eick JD, Bonewald LF. Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical-sized rat calvarial defects implanted with bioactive glass scaffolds. J Biomed Mater Res A. 2012;100:3267–75. doi: 10.1002/jbm.a.34272. [DOI] [PubMed] [Google Scholar]
- [41].Bi L, Rahaman MN, Day DE, Brown Z, Samujh C, Liu X, Mohammadkhah A, Dusevich V, Eick JD, Bonewald LF. Effect of borate bioactive glass microstructure on bone regeneration, angiogenesis, and hydroxyapatite conversion in a rat calvarial defect model. Acta Biomater. 2013 doi: 10.1016/j.actbio.2013.04.043. http://dx.doi.org/10.1016/j.actbio.2013.04.043. [DOI] [PubMed] [Google Scholar]
- [42].Zhao J, Shen G, Liu C, Wang S, Zhang W, Zhang X, Zhang, Ye D, Wei J, Zhang Z, Jiang X. Enhanced healing of rat calvarial defects with sulfated chitosan-coated calcium-deficient hydroxyapatite/bone morphogenetic protein 2 scaffolds. Tissue Eng A. 2012;18:185–97. doi: 10.1089/ten.TEA.2011.0297. [DOI] [PubMed] [Google Scholar]
- [43].Jang J-W, Yun JH, Lee K-I, Jang J-W, Jung U-W, Kim C-S, Choi S-H, Cho K-S. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral Surg Oral Med O. 2011;113:480–7. doi: 10.1016/j.tripleo.2011.04.013. [DOI] [PubMed] [Google Scholar]