Abstract
The identification and surveillance of patients with preneoplastic lesions at high risk of progressing to gastric cancer (GC) represents the most effective way of reducing the burden of GC. The incomplete type of intestinal metaplasia (IM) could be considered as the best candidate for surveillance. However, the usefulness of subtyping of IM has been considered by some authors as limited and inconsistent. A search was carried out to identify all cross-sectional (n=14) and follow-up (n=10) studies that assessed the risk of GC among subjects with different types of IM. Out of the 14 cross-sectional studies, 13 reported that the prevalence of incomplete IM was statistically significantly higher in GC than in other gastric lesions. Out of the ten follow-up studies, six found a statistically significant association between incomplete IM and subsequent GC risk. The relative risks of GC were from 4- to 11-fold higher for the presence of incomplete type in comparison to complete type or in comparison to the absence of incomplete type, among the studies that reported the magnitude of the risk. According to this comprehensive review, most of the scientific evidence supports the utility of subtyping IM as a predictor of GC risk. Recognizing its usefulness by gastroenterologists should encourage pathologists to subtype IM.
Keywords: review, subtypes, gastric cancer, intestinal metaplasia
Gastric cancer (GC) is a major health burden. It is the second most frequent cause of cancer deaths worldwide.1 Most cases are diagnosed when the tumor cells have invaded the muscularis propria and have a dismal prognosis.2 The most promising hope to control the disease is through early diagnosis and prevention. GC is the result of a decades-long multistep and multifactorial process, presenting an opportunity for intervention to prevent the disease.3 The process starts as an inflammatory process in the antrum, usually associated with Helicobacter pylori infection, which may progress toward a multifocal chronic atrophic gastritis, followed by intestinal metaplasia (IM), dysplasia and finally invasive carcinoma.4
In countries with a relatively low risk of GC, the surveillance of all these GC precursor lesions is not cost-effective. On the other hand, in high or moderate risk populations the identification of patients with preneoplastic lesions at high risk of progressing to GC, and their periodic surveillance, represents the most effective way for reducing the burden of GC.5
It has long been recognized that IM is a heterogeneous lesion. Teglbjaer and Nielsen in 19786 reported that in gastrectomy specimens with gastric carcinoma, the nontumor mucosa harbored abundant foci of IM of the “small intestinal type” but that foci of IM of “colonic type” were most frequently associated with carcinoma of the intestinal types. Japanese investigators7 reported that some IM specimens expressed the full (complete) set of digestive enzymes, whereas in others such enzymes were either absent or incompletely expressed. Jass and Filipe8 described several variants of IM, according to the histology and types of mucin secreted. Although there are several classifications of IM types, currently they are generally subclassified as “complete” (small intestinal type) or “incomplete” (colonic type, which is thought to be the most advanced stage of IM9 and with the highest risk to progress to GC). Therefore, incomplete type of IM could be considered as the best candidate for surveillance.
However, the need to re-evaluate premalignant gastric lesions is controversial.10–12 Even so, an algorithm was recently proposed for management of these lesions5 taking into account the status of H. pylori infection and the type and extension of IM. Nevertheless, a very recently published Guideline13 recommended endoscopic surveillance every 3 years for patients with extensive atrophy and/or IM, but concluded that subtyping of IM was not advised for clinical practice, because evidence on its usefulness was thought to be “limited and inconsistent,” and “its evaluation requires the use of immunohistochemistry techniques that are not widespread in routine diagnostics.”
The aim of this systematic review is to describe all the published scientific studies that evaluated the usefulness of subtyping IM as marker of GC risk and to present the histological stains needed for diagnosis and classification of types of IM in clinical practice.
Methods
A comprehensive search in MEDLINE, LILACS, EMBASE and ScienceDirect was carried out up to January 2012 to identify studies on subtyping IM and GC using the terms “types of IM” AND “gastric cancer” (944, 76, 21 and 68 publications found respectively) as key words. The search was narrowed to include only articles examining the prevalence or effect of type of IM, through morphology and or histochemical staining, in relation with GC risk. Studies were included if the authors described the type of IM in relation with GC and at least one comparison group (other preneoplastic lesion, gastric ulcer or other gastric non-neoplastic lesions). Titles and abstracts of the articles selected in each of these multiple searches were reviewed, and those meeting our inclusion criteria were selected for the evaluation of the original article. The studies were classified according to the study type: (i) cross-sectional studies, as those that assessed the prevalence of different types of IM in the surrounding mucosa of the gastric tumor from surgical specimens and comparing it with the prevalence in biopsy specimens from patients with other gastric pathology and (ii) follow-up studies of prospective, retrospective or prospective-retrospective design that assessed the risk of GC (GC occurrence during a specific time) among different types of IM, comparing with other GC precursor lesion or other benign gastric lesion.
The variables extracted for cross-sectional studies were as follows: Author/Journal/Year, Country, Number of patients with GC, Type of controls and Matching, Method of IM typing, Number and site of biopsies and Main results. For follow-up studies, in addition were extracted: Years of follow-up, Setting (population or hospital based), Level of GC in the population, Design, Validation of diagnosis and Adjustment in the analysis. Type of lesions, was included as a variable, in replacement of Number of patients with GC and other pathology, Number and site of biopsies at baseline and at the end of follow-up, were also extracted. The outcome of interest for cross-sectional studies was the prevalence of subtypes of IM in GC cases and in the control group. For follow-up studies, the outcome of interest was the incidence of GC according to different IM types at the end of the follow-up.
Results
Characteristics of studies
A total of 14 cross-sectional14–27 and ten follow-up studies28–37 met all the search criteria. Three cross-sectional studies were excluded because of the lack of a comparison group.38–40 The cross-sectional studies, summarized in Table 1, were published between 1980 and 2008 (mostly before 1991) and were carried out in countries from Asia, Europe and Latin America. There was considerable variation in the sample size: GC cases range from 16 to 360 patients, whereas controls from 67 to 1326. Several types of controls were used, with the most common being: normal mucosa, chronic gastritis, gastric ulcer and duodenal ulcer.
Table 1.
Cross-sectional studies subtyping intestinal metaplasia
| Study/Author/Year (Reference) | Number of patients |
||||
|---|---|---|---|---|---|
| Country | Gastric cancer | Other pathology | N and site of biopsies | Results | |
| Kang et al. J Gast Hepatol 2009 (14) | Korea | 360 | 247 Control 156 Gastric Ulcer 98 Dysplasia | 2 Antrum 2 Body | Prevalence of IM type II significantly higher in Disp + GC than in controls, in samples from the body and Hp+ but not in samples from antrum. |
| Vukobrat-Bijedic Bosnian J of Basic Medic Sciences 2006 (15) | Bosnia-Herzegovina | 50 | 50 Chronic atrophic Gastritis Hp + | 1.2 cm from tumor and Corpus Antrum Pylori | IM was found in 41 (82%) out of 50 patients with GC 17 (34%) had complete IM 24 (48%) had incomplete IM No IM type III was found in the control group. Type I in 13 (26%) and type II in 7 (14%) |
| Cassaro et al Am J Gast 2000 (16) | Colombia | 68 | 67 Nonulcer Dyspepsia | 5 Antrum 6 Corpus 1 Cardia | Incomplete IM was found in 40 (59%) out of 68 with GC Incomplete IM was found in 4 (6%) out of 67 controls Complete IM was found in 22 (33%) out of 67 controls Incomplete IM was more extensive and multifocal in GC Incomplete IM significantly associated, with the presence of GC. |
| Wu Br J Cancer 1998 (17) | Taiwan | 135 (57 diffuse 78 intestinal) | 135 Matched controls Receiving Endoscopy Without ulcer or GC | 2 Antrum 2 Corpus 1 Incisura | Prevalence type I : GC 6 (4 %); controls 17 (13 %) Type II: GC 22 (16.%) ; controls 25 (18 %) Type III: GC 62 (46%) ; controls 7 (5 %) OR Type III = 39.8 (14–110) OR Type I = 0.82 (0.3–2.4) regarding those GC without IM |
| Craanen et al. Dig Dis Sci 1991 (18) | The Netherlands | 16 GC (8 diffuse 8 intestinal) | 126 Normal 298 Gastritis 67 Gastric ulcer 26 Erosion | Adjacent mucosa plus about 3 from antrum | No significant differences of type I or II among diseases Type III was strongly associated with intestinal-type GC as compared either benign lesions (p<0.01) or diffuse Type |
| Matsukuma et al. Cancer 1990 (19) | Japan | 42 (21 intestinal 21 diffuse) | 13 Gastric ulcer 7 Duodenal ulcer 1 Erosive gastritis | Swiss-roll slices Gastrectomy Specimens | No significant differences in frequency of type IIB IM between intestinal type GC and non-neoplastic lesions. |
| Kini et al. Indian J Pathol Microbiol 1990 (20) | India | ? GC | Normal Chronic gastritis | NS | Type III found only in gastric carcinoma, non in benign lesions Prevalence of sulfomucins producing IM higher in GC |
| Sirigu et al. Riv Eur Sci Med Farmacol 1991 (21) | Italy | 28 GC | 64 Chronic atrophic gast 42 Gastric ulcer | Mucosa bordering carcinoma and at least three sites | Complete IM most common in benign condition Incomplete IM associated, with the presence of GC |
| Silva and Filipe Human Pathol 1986 (22) | Portugal | from biopsy: 110 GC from resected material: 99 (60 intest) | 478 Chronic gastritis 170 Gastric ulcer 283 Normal plus 19 Peptic ulcer from gastrectomy | tumour and gastrectomy plus antrum Body Incisura | Type III IM in 36% of GC, but only in 2% of benign lesions (p=<0.0001) Type III was significantly associated with intestinal but not diffuse GC. |
| Huang Cancer 1986 (23) | China | 54 | 61 Atrophic gastritis And Superficial | 4 quadrant for tumor and 6 sites (antrum, corpus, fundus, cardia) | 24 (73%) of 99 GC were sulfomucin + (colonic type) 11 (33%) of gastritis were sulfomucin + (colonic type) prevalence significantly higher in GC (p<0.01) |
| Rothery J Clin Pathol 1985 (24) | UK | 135 | 182 Normal 618 Chronic gastritis 441 Gastric ulcer 30 Dysplasia | Antrum Transition bodyCardia (x=3.5 per case) | Type I was most common in all kind of patients Type III only in GC and dysplasia (67%). Prevalence significantly higher (p = < 0.001) in GC than benign lesions |
| Filipe et al. Gut 1985 (25) | London Paris Reims | 24 | 480 normal 642 gastritis 204 gastric ulcer | Several from GC lesions antrum and Fundus (x=3.7 per case) | Prevalence: Type III in 7% of benign lesions Type III in of 35% carcinoma. Significantly higher (p<0.0001) in GC than in benign lesions |
| Sipponem Pathol Acta Pathol Microbiol Scand 1980 (26) | Finland | 125 | 62 pernicious anemia 301 first degree relatives of GC 183 first degree relatives of anemia 406 out patients 358 population controls | 2–4 from antrum 6–10 from body | Prevalence of colonic type (sulfomucins +), significantly higher in GC patients than in the other series |
| Matsukura et al. JNCI 1980 (27) | Japan | 84 | 27 gastric ulcer 16 duodenal ulcer | Surrounding lesions from gastrectomy | IM was found in 95% of minute GC of the intestinal thype 80% of them surrounding by incomplete IM |
The follow-up studies, summarized in Table 2, were published between 1986 and 2010 (mostly after 1991) and were carried out in countries from Asia, Europe and USA with diverse risk of GC. The number of participant patients range from 69 to 1,525, and some studies followed-up only patients with different types of IM, while others also included other gastric lesions. The length of follow-up ranged from 10 months to 19 years, and in only three studies28,33,36 the length of follow-up was more than 10 years. Two of the studies28,33 were population-based, whereas the others were hospital-based. Three studies were prospective,28,29,32 five studies were retrospective30,31,33–35 and two studies were retrospective and prospective.35 In three of the retrospective studies,33,36,37 the outcome was identified through record-linkage with cancer registries. There was considerable variation in the site and number of biopsies taken at baseline, while in several studies this information was missing30,32,34,36,37. In only three studies,28–30 the intra and or interobserver agreement in the pathological diagnoses was assessed. Prospective studies assessed the end-point, through new endoscopies and biopsies, whereas in retrospective studies it was defined by record linkage with Cancer Registries or review of clinical, pathological records and pathological material.
Table 2.
Follow-up studios subtyping intestinal metaplasia and gastric cancer risk
| Study Author Year (Ref) | Place | Level of GC risk | No Patients | Design | Adjusted by | Follow up (Years) | Settings | Type of lesions | No Biopsia and site baseline | No Biopsia and site End | Main results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonzalez et al. Int J Cancer 2010 (28) | Spain | Moderate/High | 478 | Prospective | Sex, age, family history of GC, NSAID use | 12.8 | Province-based | normal: 8 (2%) NAG: 55(12%) MAG: 223 (47%) CIM: 104(21%) IIM : 88(18%) | 3 to 4 Minimal 2 antral 1 corpus | 5 (Sidney) | Incidence rate of GC: 16.5 (10.1–26.9)/1000 person year for IIM 1.73 (0.7–4.2)/1000 person year for MAG HR 11.3 (95 %CI 3.8–33.9) IIM present vs. absent |
| Tava et al. . Human Pathol. 2006 (29) | Italy | Moderate | 471 | Prospective | Sex, age, immuturity atypia, H. pylori status | 4.3 | Hospital (patients Dyspeptic symptoms) | 100% IM covering at least 10% of mucosal area | 3 antral 2 angulus 2 corpus | 3 antral 2 angulus 2 corpus | Comparing IIM≥10% vs.<10% extension HR=3.1 (1.3–7.3) in univariate analysis but in multivariate, type or extension were not a prognostic factor (yes age, Hp, atipia) results comparing IIM vs. CIM not shown |
| Ribeiro et al. J Clin Pathol 2004 (30) | Portugal | High | 144 (239 pairs of biopsies) | Retrospective | Sex, age, H.pylori prevalence | up to 17 months | Hospital | CAG: 58 (24%) Type I : 62 (26%) Type II/III:57 (24%) LGD: 62 (26%) |
NS | NS | 4 GC+ 7 HGD = all preceded by IIM or LGD IIM vs. CIM or CAG: HR 4.64 (95% CI 1.8–11.8) |
| El Zimaity et al. J Clinic Pathol 2001 (31) | USA | Low | 79 | Retrospective | Risk was not estimated | 74: 1 y 28: 2 ys 36: 9 ys |
Hospital | IM 100% Type II: 33 (42%) Type III: 34(43%) |
3 to 12 minimal 2 antral 1 corpus | 3 to 12 minimal 2 antral1 corpus | During follow up no patient developed GC or dysplasia |
| Lin et al. Chin Med Journal 2001 (32) | Taiwan | Moderate/High | 69 | Prospective | None | mean: 10 months range: 1–23 endoscopy every 3 M | Hospital | Mild Dysp.:25; Moderat Dys:24; Severe Dysp.: 20 Type I:36; Type II:18 Type III:15 |
Several No: NSsite: NS | NS | 10 GC out of 15 type III and 11 severe dysplasia 36 IM type 1 and 2 severe dysplasia and 15 moderate dysplasia none with malignant changes |
| Filipe et al. Int J Cancer 1994 (33) | Slovenia | Moderate/High | 1525 900 had confirmed IM | Retrospective | Sex, age | 19 | Country-based | IM 100% Type I = 518(34%) Type II =197(13%) Type III = 275(18%) Not confirmed = 291 (19%) |
3 (2 antrum 1 corpus) | GC Diagnosis | HR 2.14 (95 % CI 0.7-7.0) Type II vs. Tipe I HR 4.58 (1.8-11.9)Type III vs. Type I |
| Rokkas et al. Gut 1991 (34) | UK | Low | 412 (cross sectional) (282 follow- up) 26 with IM | Retrospective and Prospective | Risk was not estimated | 6 | Hospital | 26 with IM | NS | NS | Type III was present in 16 of 18 GC diagnosed during period. 11 out of 16 GC cases detected by regular endoscopic surveillance during follow-up, most as early GC. |
| Silva et al. Gut 1990 (35) | Portugal | High | 58 CAG with IM, 66 GU (peptic) | Retrospective and Prospective | Risk was not estimated | up to 6 58 = 2ys 41 = 3ys | Hospital | Type I = 47(81%) Type II = 3 (5%) Type III = 8 (14%) |
Antrum and Body No: 4.1 | NS | 8 Type III IM → 4 developed Dysplasia → 1 developed GC 47 Type I IM → 45% reverted → 55% remained unchanged |
| Ramesar et al. J Clin Pathol 1987 (36) | Scotland | Low | 174 | Retrospective | Risk was not estimated | up to 11 | Hospital | no IM = 130(75%) Type I = 16 (9%) Type II =14 (8%) Type III = 14 (8%) |
Na: NS Site: NS | GC Diagnosis | 130 no IM → GC=0 16 type I IM → GC=0 14 type II IM → GC=1 (7.1%) 14 type III IM → GC=1 (7.1%) |
| Ectors and Dixon Histophatol 1986 (37) | UK | Low | 230 with CAG | Retrospective | Risk was not estimated | up to 9 | Hospital | no IM = 59 (26%) IMSM- = 79 (34%) IMSM+ =92 (40%) |
NS | GC Diagnosis | Sulfomucins + (all type III and part of type I and type II) vs. sulfomucins - 59 IM absent → 1 GC 79 SM– → 1 GC 92 SM + → 1 GC |
Abbreviations: NAG, non atrophic gastritis; MAG, multifocal atrophic gastritis; CIM, complete intestinal metaplasia; IIM, incomplete intestinal metaplasia; LGD, low grade dysplasia; HGD, high grade dysplasia; CAG, chronic atrophic gastritis; GU, gastric ulcer; IMSM–, intestinal metaplasia sulfomucins negative; IMSM+, intestinal metaplasia sulfomucins positive; NS, not specified.
Subtyping IM: Diagnosis and classification
There was considerable variation in the criteria used for diagnosis and classification of the type of IM in the reviewed studies. Subtyping of IM was done by a combination of morphology and mucins staining. Most of the studies used specimens stained with hematoxylin and eosin (H&E), alcian blue (AB)/periodic acid-Shiff (PAS) at pH 2.5, and high iron diamine (HID)/AB.14,17,26,27,31 One study27 used also marker enzymes as aminopeptidase and phosphatase. Most of the studies classified IM as type I, II or III, or as complete (type I) or incomplete (type II and III), whereas others15,19,21,24 classified the IM as type I, type IIa (sialomucin) and type IIb (sulfomucin positive). Sulfomucins may be found in type IIb and type III IM columnar cells. Types I and IIa display sialomucins and/or neutral mucins, but no sulfomucins, at least in the columnar cells.25
Association between type of IM and GC in cross-sectional studies
Of the 14 cross-sectional studies, 13 reported that the prevalence of type III IM or incomplete IM was statistically significantly higher in GC than in other gastric lesions.14–18,20–27 In most of the studies, type III IM was more frequently associated with intestinal-type GC than with diffuse type. One study,19 with only 21 GCs of intestinal type, was the only one without significant differences in the prevalence of incomplete IM between GC cases and controls.
Association between type of IM and GC in follow-up studies
Of the 10 follow-up studies,28–37 six found a statistically significant association between type III IM or incomplete IM and subsequent GC risk.28,30,32–35 The risk and cumulative incidence of GC for type III IM was higher than for type II and type I, and higher than for other gastric lesions. The relative risks of GC were from 4- to 11-fold higher for type III in comparison to type I or in comparison to the absence of type III IM, among the studies that reported the magnitude of the risk. One of these studies34 showed that surveillance of patients with type III IM increased the probability of diagnosing dysplasia and early GC. In a second phase of this study, 26 patients identified as having type III IM were followed with endoscopy and biopsy at 6 to 12 month intervals. Out of these 26 patients, 11 (42%) were diagnosed with early GC during the follow-up, indicating a high positive predictive value.
Other two follow-up studies29,36 also found an association between the type of IM and GC risk. One of these studies29 found a significant association with type III IM in the univariate analysis, but not when age, H. pylori infection status and level of atypia were included in the multivariate model. The other study36 that followed-up a small number (n=44) of patients with IM, observed two GCs, one diagnosed at baseline as type III and the other diagnosed as type II, while no GC was observed among type I IM or in patients without IM. On the contrary, another study,31 that followed-up only 79 patients with diverse types of IM for 1 to 9 years, found that no patient developed GC or dysplasia. Most of patients were, however, lost to follow-up. Out of the 34 patients with type III IM at baseline, it was possible to obtain a new biopsy at the second year of follow-up in only 13 patients, and at the 9th year in only nine patients. Finally there is one more study,37 in which only three GC were identified after 8 years of follow-up, out of 171 patients with IM at baseline (92 classified as sulfomucin positive). Only one of these three GC cases was classified as sulfomucin positive IM at baseline.
Discussion
In the present comprehensive review of studies, utilizing IM subtyping, we have observed that out of the 14 cross-sectional studies, 13 reported that the prevalence of incomplete IM was statistically significantly associated with a higher GC risk than in other gastric lesions. Several of these studies, including one with negative result,19 used patients with gastric or duodenal ulcer or normal mucosa as control, that may not be appropriated to be compared with GC cases. However, there are at least four studies15–17,23 with more appropriate controls such as chronic atrophic gastritis or non ulcer dyspepsia, for which incomplete IM was significantly associated with GC. Only one study17 described that the controls were matched to the cases by sex, age, ethnic and residence; this means they were controlled for confounding factors. On the other hand, even though cross-sectional studies are not suitable to identify prognosis factors, they provide evidence that when GC is diagnosed, incomplete type IM is the most frequent lesion found in the gastric mucosa, suggesting that it is the most procarcinogenic phenotype and one of the last steps in the cascade of GC precursor lesions, that could fulfill the histological criteria for being classified as low-grade dysplasia.41
Follow-up studies with a relative large sample size and long follow-up are the best design to assess the usefulness of subtyping of IM as a marker of GC risk. Out of the ten follow-up studies, we found six with a statistically significant association between incomplete IM and subsequent GC risk28,30,32–35 and two29,36 with a nonsignificant positive association. These results have been observed in populations with relative high GC risk such as Portugal, China and Slovenia as well as in populations with moderate GC risk as Italy and Spain. This indicates that subtyping IM plays a major role in predicting GC risk. Three of the studies that found a significant association with incomplete IM28,33,34 were the largest studies and with the longest follow-up. Two of them28,33 were the only population-based studies, which tend to be less affected by selection bias. One of these studies34 also showed that surveillance of patients with type III IM increase the probability of diagnosing dysplasia and early GC.
Two very important potential limitations of follow-up studies are sample size and length of follow-up. The likelihood of observing a statistical significant association between type of IM and GC risk in studies with small sample size and/or short follow-up is very low. This could be the explanation of studies31,36,37 without significant association in which no GC or few cases of GC were identified at the end of follow-up. The limited statistical power of these studies could be also affected by the low level of GC risk of the population in which they were carried-out (USA, UK and Scotland). Among follow-up studies that have evaluated the incidence rate of GC, one study found an incidence rate of 16.5 per 1,000 patients with incomplete IM per year of follow-up in a population with a relative high risk of GC.28 In a nationwide study in The Netherlands,42 a country with a low risk of GC, the annual incidence of GC, within 5 years after diagnosis of IM, was 2.5 cases for every 1,000 patients with IM. This means that, depending of the level or risk, it is necessary to follow-up between 61 cases (with incomplete IM) to 400 cases (with IM) to report the occurrence of one case of GC in 1 year.
Another potential limitation of follow-up studies is sampling error associated with multifocal lesions. In half of the follow-up studies that found a significant association between IM and GC risk30,32,34 and in half of the studies that did not find such association,36,37 the information about the number and site of biopsies at baseline was missing. It is well known that the opportunity to identify lesions depends on the number and site of biopsy specimens taken, the Sydney system being the ideal design. With few samples, true lesions may be missed, resulting in false negative diagnoses, and this could lead to an underestimation of the true association between type of IM and GC risk, affecting mainly those studies with negative results and with a retrospective design.
The recent published Guideline for endoscopic management of precancerous conditions and lesions in the stomach13 concluded that subtyping of IM is not recommended for clinical practice “because the evidence about their usefulness is limited and inconsistent.” The same recommendation —for the same reason—has been given by the Royal College of Pathologists12; however, these guidelines were based only on the studies with negative results. If all studies are taken into account, as may be appreciated in the present review, there is so far overwhelming evidence about their utility, although more large and well designed follow-up studies are needed to reach a definitive conclusion.
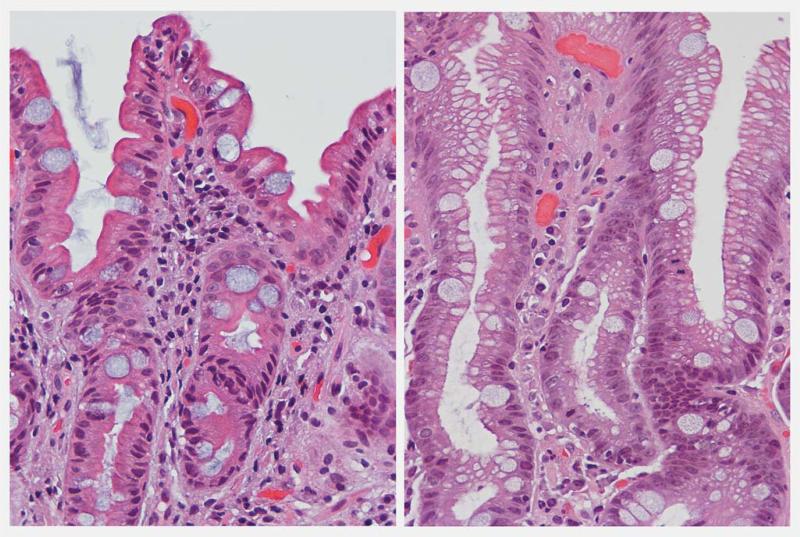
The other apparent limitation encountered by some authors is that “subtyping of IM requires the use of immunohistochemistry techniques that are not widespread in routine diagnostic,”13 which may limit its clinical application. Comparison studies about the sensibility and specificity of different methods and techniques for the diagnosis of the type of IM are lacking. However, according to our experience, subtyping of IM does not require the use of immunohistochemistry techniques; in fact, the classification of IM (complete or incomplete) was documented before the development of immunohistochemistry. Subtypes of IM can be easily recognized with H&E, which is the main routine histological stain widely used in clinical practice. Based on morphology (Fig. 1) in sections stained with H&E,5,41 “complete IM” (small intestinal type or type I) is characterized by an epithelium that resembles the small intestinal phenotype, with well-defined brush border cells (representing absorptive microvilli) and well-formed goblet cells. “Incomplete IM” (colonic type or type II/IIa and III/IIb) resembles a colonic epithelium phenotype, with columnar cells without brush border cells, showing multiple intracytoplasmic mucin droplets of different size and shape.
Figure 1.
Intestinal metaplastic glands with H&E. Complete IM with brush border and globet cells (left). Incomplete IM with slight architectural glandular distortion and presence of globet cells and multivacuolated hybrid columnar intermediate cells, without brush border cells (right). (Courtesy of Dr. Blanca Piazuelo, Vanderbilt University).
Distinguishing complete from incomplete IM is useful in clinical practice. The use of staining to assess the types of mucins expressed by each subtype should be restricted to research. In clinical practice, in cases of doubt because of insufficient or inadequate tissue, the pathologist could use the PAS-AB staining at pH 2.5, which is an easy, cheap and standardized mucin stain. The only reagent of concern is AB/HID because iron diamine is toxic. It could be argued that without the HID we would not be able to differentiate between sulfomucin positives IM (incomplete IM type IIb/III) and sulfomucin negatives IM (complete IM and IIa). However, we do not need to identify sulfomucins because the morphologic criteria from H&E stain is enough to properly classify the subtypes of IM. Incomplete IM frequently coexists with complete IM in the same sample. Moreover, the incomplete IM or the predominantly incomplete type correlates positively with cancer risk28
The extension of atrophic and metaplastic changes is also a determinant of cancer risk. Schemes to evaluate the extension of lesions are available.43–45 On the other hand, it is well known that there is a strong correlation between the type and extension of the IM. Using 12 biopsy specimens,16 it was found that extensive IM always display incomplete type of IM. In the updated Sydney System,46 IM was graded according to morphology in normal, mild, moderate and marked but the type and extension are not clearly specified. The key issue related with extension of IM and suptyping of IM was reported by Cassaro et al.,16 concluding that if extensive IM is observed (in four or more of 12 biopsy specimens) the determination of IM subtype is not needed, because this patient should be considered as being at risk for GC, irrespective of the IM subtype. On the other hand, when only a few biopsies are available for examination (as happens in clinical practice) the finding of incomplete IM foci may be used as a surrogate to indicate that extensive metaplastic changes have been taken place and, therefore, that the patient has an increased cancer risk.
According to this comprehensive review, most of the scientific evidence supports the utility of subtyping IM as a predictor of GC risk, identifying the sub-group of patients with incomplete IM as possible candidates for endoscopic surveillance. IM subtypes are well defined in the updated Sydney System original paper,46 but in routine diagnostic practice, unfortunately, only a few pathologists specify IM subtypes in their reports. It is not because subtyping IM is a difficult or time consuming task, but merely because clinicians do not request IM subtyping diagnoses. However, that is not a reason to ignore that subtyping of IM is a relatively easy, cheap and valid procedure. Recognizing its usefulness by gastroenterologist should encourage pathologists to subtype IM.
Acknowledgement
The authors thank Eric Duell for his comments and help in editing the article.
Grant sponsor: The Health Research Funds; Grant number: FIS Exp 10=01089; Grant sponsor: RETICC of the Spanish Ministry of Health; Grant number: RD06/0020/0091; DOI: 10.1002/ijc.28003
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010. 127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Axon A. Symptoms and diagnosis of gastric cancer at early curable stage. Best Pract Res Clin Gastroenterol. 2006;20:697–708. doi: 10.1016/j.bpg.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37–S43. [PubMed] [Google Scholar]
- 4.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–8. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teglbjaerg PS, Nielsen HO. “Small intestinal type” and “colonic type” intestinal metaplasia of the human stomach, and their relationship to the histogenetic types of gastric adenocarcinoma. Acta Pathol Microbiol Scand A. 1978;86A:351–5. doi: 10.1111/j.1699-0463.1978.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsukura N, Kawachi T, Sugimura T, et al. Variety of phenotypical expression of intestinal marker enzymes and mucins in human stomach intestinal metaplasia. Acta Histochem Cytochem Jap. 1980;13:499–507. [Google Scholar]
- 8.Jass JR, Filipe MI. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology. 1979;3:191–9. doi: 10.1111/j.1365-2559.1979.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 9.Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193–201. doi: 10.1111/j.1440-1746.2008.05774.x. [DOI] [PubMed] [Google Scholar]
- 10.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1–15. doi: 10.1111/j.1523-5378.2007.00475.x. Review. [DOI] [PubMed] [Google Scholar]
- 11.Hirota WK, Zuckerman MJ, Adler DG, et al. Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.The Royal College of Pathologists . Histopathology and cytopathology of limited or no clinical value. 2nd edn. London, UK: 2005. [Google Scholar]
- 13.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang KP, Lee HS, Kim N, et al. Role of intestinal metaplasia subtyping in the risk of gastric cancer in Korea. J Gastroenterol Hepatol. 2009;24:140–8. doi: 10.1111/j.1440-1746.2008.05546.x. [DOI] [PubMed] [Google Scholar]
- 15.Vukobrat-Bijedić Z, Radović S, Husić-Selimović A, et al. Incomplete intestinal metaplasia as an indicator for early detection of gastric carcinoma in the events of Helicobacter pylori positive chronic atrophic gastritis. Bosn J Basic Med Sci. 2006;6:48–53. doi: 10.17305/bjbms.2006.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassaro M, Rugge M, Gutierrez O, et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431–38. doi: 10.1111/j.1572-0241.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu MS, Shun CT, Lee WC, et al. Gastric cancer risk in relation to Helicobacter pylori infection and subtypes of intestinal metaplasia. Br J Cancer. 1998;78:125–8. doi: 10.1038/bjc.1998.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craanen ME, Blok P, Dekker W, et al. Prevalence of subtypes of intestinal metaplasia in gastric antral mucosa. Dig Dis Sci. 1991;36:1529–36. doi: 10.1007/BF01296393. [DOI] [PubMed] [Google Scholar]
- 19.Matsukuma A, Mori M, Enjoji M. Sulphomucin-secreting intestinal metaplasia in the human gastric mucosa. An association with intestinal-type gastric carcinoma. Cancer. 1990;66:689–94. doi: 10.1002/1097-0142(19900815)66:4<689::aid-cncr2820660417>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Kini U, Nirmala V, Thomas JA. Intestinal metaplasia in benign and malignant gastric lesions. Indian J Pathol Microbiol. 1990;33:137–43. [PubMed] [Google Scholar]
- 21.Sirigu F, Dessì A, Tocco G, et al. Intestinal metaplasia of the gastric mucosa: a study of its incidence in various disease states. Riv Eur Sci Med Farmacol. 1991;13:25–8. [PubMed] [Google Scholar]
- 22.Silva S, Filipe MI. Intestinal metaplasia and its variants in the gastric mucosa of Portuguese subjects: a comparative analysis of biopsy and gastrectomy material. Hum Pathol. 1986;17:988–95. doi: 10.1016/s0046-8177(86)80082-x. [DOI] [PubMed] [Google Scholar]
- 23.Huang CB, Xu J, Huang JF, et al. Sulphomucin colonic type intestinal metaplasia and carcinoma in the stomach. A histochemical study of 115 cases obtained by biopsy. Cancer. 1986;57:1370–5. doi: 10.1002/1097-0142(19860401)57:7<1370::aid-cncr2820570721>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Rothery GA, Day DW. Intestinal metaplasia in endoscopic biopsy specimens of gastric mucosa. J Clin Pathol. 1985;38:613–21. doi: 10.1136/jcp.38.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipe MI, Potet F, Bogomoletz WV, et al. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut. 1985;26:1319–26. doi: 10.1136/gut.26.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sipponen P, Seppälä K, Varis K, et al. Intestinal metaplasia with colonic-type sulphomucins in the gastric mucosa; its association with gastric carcinoma. Acta Pathol Microbiol Scand A. 1980;88:217–24. doi: 10.1111/j.1699-0463.1980.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 27.Matsukura N, Suzuki K, Kawachi T, et al. Distribution of marker enzymes and mucin in intestinal metaplasia in human stomach and relation to complete and incomplete types of intestinal metaplasia to minute gastric carcinomas. J Natl Cancer Inst. 1980;65:231–40. [PubMed] [Google Scholar]
- 28.González CA, Pardo ML, Liso JM, et al. Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer. 2010;127:2654–60. doi: 10.1002/ijc.25273. [DOI] [PubMed] [Google Scholar]
- 29.Tava F, Luinetti O, Ghigna MR, et al. Type or extension of intestinal metaplasia and immature/atypical “indefinite-for-dysplasia” lesions as predictors of gastric neoplasia. Hum Pathol. 2006;37:1489–97. doi: 10.1016/j.humpath.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, et al. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol. 2004;57:177–82. doi: 10.1136/jcp.2003.11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Zimaity HM, Ramchatesingh J, Saeed MA, et al. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol. 2001;54:679–83. doi: 10.1136/jcp.54.9.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CK, Lai KH, Lo GH, et al. Cathepsin E and subtypes of intestinal metaplasia in carcinogenesis of the human stomach. Zhonghua Yi Xue Za Zhi. 2001;64:331–6. [PubMed] [Google Scholar]
- 33.Filipe MI, Muñoz N, Matko I, et al. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer. 1994;57:324–9. doi: 10.1002/ijc.2910570306. [DOI] [PubMed] [Google Scholar]
- 34.Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110–3. doi: 10.1136/gut.32.10.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva S, Filipe MI, Pinho A. Variants of intestinal metaplasia in the evolution of chronic atrophic gastritis and gastric ulcer. A follow up study. Gut. 1990;31:1097–104. doi: 10.1136/gut.31.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramesar KC, Sanders DS, Hopwood D. Limited value of type III intestinal metaplasia in predicting risk of gastric carcinoma. J Clin Pathol. 1987;40:1287–90. doi: 10.1136/jcp.40.11.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ectors N, Dixon MF. The prognostic value of sulphomucin positive intestinal metaplasia in the development of gastric cancer. Histopathology. 1986;10:1271–7. doi: 10.1111/j.1365-2559.1986.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 38.Conchillo JM, Houben G, de Bruïne A, Stockbrügger R. Is type III intestinal metaplasia an obligatory precancerous lesion in intestinal-type gastric carcinoma? Eur J Cancer Prev. 2001;10:307–12. doi: 10.1097/00008469-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Turani H, Lurie B, Chaimoff C, et al. The diagnostic significance of sulfated acid mucin content in gastric intestinal metaplasia with early gastric cancer. Am J Gastroenterol. 1986;81:343–5. [PubMed] [Google Scholar]
- 40.Lida F, Kusama J. Gastric carcinoma and intestinal metaplasia. Significance of types of intestinal metaplasia upon development of gastric carcinoma. Cancer. 1982;50:2854–8. doi: 10.1002/1097-0142(19821215)50:12<2854::aid-cncr2820501227>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Tosi P, Filipe MI, Luzi P, et al. Gastric intestinal metaplasia type III cases are classified as low-grade dysplasia on the basis of morphometry. J Pathol. 1993;169:73–8. doi: 10.1002/path.1711690112. [DOI] [PubMed] [Google Scholar]
- 42.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–52. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–6. doi: 10.1136/gut.2006.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rugge M, de Boni M, Pennelli G, et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104–11. doi: 10.1111/j.1365-2036.2010.04277.x. [DOI] [PubMed] [Google Scholar]
- 45.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–8. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]