Abstract
When exposed to the sights, sounds, smells and/or places that have been associated with rewards, such as food or drugs, some individuals have difficulty resisting the temptation to seek out and consume them. Others have less difficulty restraining themselves. Thus, Pavlovian reward cues may motivate maladaptive patterns of behavior to a greater extent in some individuals than in others. We are just beginning to understand the factors underlying individual differences in the extent to which reward cues acquire powerful motivational properties, and therefore, the ability to act as incentive stimuli. Here we review converging evidence from studies in both human and non-human animals suggesting that a subset of individuals are more “cue reactive”, in that certain reward cues are more likely to attract these individuals to them and motivate actions to get them. We suggest that those individuals for whom Pavlovian reward cues become especially powerful incentives may be more vulnerable to impulse control disorders, such as binge eating and addiction.
Keywords: rat, human, sign-tracking, goal-tracking, addiction, binge eating, obesity, motivation, dopamine, individual differences, learning, accumbens, Pavlovian, relapse
1. Introduction
“I couldn’t help it. I can resist everything except temptation.” (Oscar Wilde, Lady Windermere’s Fan, 1892)
To survive animals must navigate a complex, ever-changing environment. Stimuli associated with different behavioral outcomes help organisms do this, in part by coordinating approach towards desirable stimuli and avoidance of potentially harmful stimuli (Hebb, 1955; Ikemoto, 2010; Moltz, 1965; Schneirla, 1959). Thus, from worms to humans, environmental cues play an important role in guiding individuals to successfully seek out what is critical for survival, by signaling the current or future availability, location, quality, and/or quantity of rewards. The sensory systems of different animal species have evolved specifically to enable efficient processing of particular types of reward cues important for their survival. Color vision, for example, is thought to have evolved in many species, including insects and primates, due to selection pressures favoring the ability to visualize colorful flowers and fruits, which facilitates successful foraging. Thus, environmental cues serve a phylogenetically ancient purpose: to increase the probability of acquiring rewarding stimuli and avoiding aversive stimuli.
Cues, while serving this vital role in directing adaptive reward-seeking behavior, under certain conditions, may also serve as powerful temptations that can promote maladaptive patterns of behavior (Nesse and Berridge, 1997). This is best illustrated in many types of human psychopathology, where cues can instigate pathological reward seeking in disorders such as compulsive eating, gambling, hypersexuality, and drug abuse. Here, we review evidence from human and nonhuman animal studies demonstrating the role reward-associated cues play in controlling behavior, with a special emphasis on food and drug-seeking behavior. Furthermore, we emphasize that there is considerable individual variation in the extent to which reward-related cues, including drug-associated cues, gain motivational control over behavior. That is, we address why some individuals (such as Oscar Wilde’s character above), have much more difficulty resisting temptation than others.
2. The role of Pavlovian cues in reward seeking
In now classic studies, Pavlov (1927) demonstrated that if a previously neutral stimulus (conditional stimulus, CS) reliably predicts the delivery of a reward (unconditional stimulus, US), over time the CS will come to elicit a conditional response (CR). Pavlov found that in hungry dogs if the ticking of a metronome were paired with food delivery the sound of the metronome itself (the CS) came to elicit salivation (the CR). Given that the dogs initially salivated unconditionally when presented with the US, Pavlov referred to the CS-elicited CR as a conditional reflex (Pavlov, 1927). For many years after these experiments, researchers described Pavlovian conditioned behavior largely in terms of stimulus – response (S-R) habits (Berridge, 2001). That is, as a consequence of learning, a Pavlovian CS comes to evoke a rigid, inflexible behavioral response. Researchers have long known, however, that beyond eliciting simple, reflexive CRs, CSs may also be attributed with incentive motivational properties (“incentive salience”), becoming incentive stimuli, and thus acquire the ability to activate complex emotional and motivational states (Berridge, 2001; Bindra, 1978; Bolles, 1972; Cardinal et al., 2002; Konorski, 1967; Rescorla, 1988; Toates, 1986; Trowill et al., 1969; Young, 1959; Young, 1966). Incentive salience refers specifically to the acquired perceptual and motivational properties of a stimulus that render it attention grabbing and “wanted” (Berridge and Robinson, 1998). Thus, Pavlovian CSs not only have predictive or associative value, signaling upcoming rewards, but they can also acquire powerful motivational properties, acting as incentive stimuli. Importantly, the motivational properties of a reward or reward cue are not simply a fixed characteristic of the stimulus itself, but are modulated by the physiological state of the individual (Cabanac, 1979; Toates, 1986; Young, 1959). For example, when one is hungry, the incentive value of rewards and their cues is potentiated, when sated, their value is relatively diminished. Various circumstances, therefore, such as hunger, thirst, or even drug-induced states can modulate the motivational value of learned reward cues (Berridge, 2001; Richard et al., 2013). The complexity of these psychological responses to rewards and cues – well beyond simple S-R habits – can have the effect of greatly increasing the flexibility and diversity of an individual’s behavioral repertoire, allowing for adaptive reward seeking (Toates, 1986).
Here, we will focus specifically on the Pavlovian incentive motivational properties that stimuli can acquire, but it should be noted that stimuli can also develop what Dickinson and colleagues have termed instrumental incentive value (Berridge and Robinson, 2003; Dickinson and Balleine, 1994; Dickinson et al., 2000). The latter refers to an explicit cognitive expectation of a reward, and we will not focus on this psychological process here (see Berridge and Robinson, 2003; Dickinson et al., 2000 for a discussion of the difference between so-called Pavlovian versus instrumental incentives). We should also note that Pavlovian reward cues are broadly defined, and can be discrete and localizable, or diffuse and contextual, and can exist in any sensory modality. Depending of the physiology of the sensory systems of a given species, and the evolutionary niche it occupies, certain stimulus modalities may be more or less important for behavior (Timberlake, 1984), which is an important consideration for experiments. Additionally, reward cues do not have to be external to the individual, and may include reward-associated interoceptive states. Finally, in experimental settings, a cue is often a relatively simple stimulus, such as a light, tone, or image, but in reality, reward cues are often complex compound stimuli.
Barry Everitt and colleagues (e.g., Cardinal et al., 2002; Everitt et al., 2001; Milton and Everitt, 2010) have developed a useful conceptualization of Pavlovian incentive stimuli that defines their three fundamental properties. An incentive stimulus 1) is attractive and attention grabbing, drawing individuals into close proximity with it. 2) It is itself desirable, in the sense that it can reinforce novel actions to obtain it. 3) Its presence can evoke a conditioned motivational state capable of both instigating reward-seeking behavior, and invigorating ongoing behavior. Collectively, these properties define an incentive stimulus but, importantly, they are psychologically dissociable, and rely on overlapping but different neural systems (Cardinal et al., 2002). Taken together, if a reward-associated cue acquires these properties it is, in effect, transformed from a predictive but motivationally “cold” CS into a “hot” incentive stimulus, which can exert motivational control over behavior (Cardinal et al., 2002; Meyer et al., 2012a).
3. Incentive stimuli
3.1. Conditioned Approach
An important feature of an incentive stimulus is its ability to grab one’s attention and attract, which has the effect of drawing individuals into close physical proximity with it, and thus usually with the reward itself. Experimentally, this phenomenon is measured as Pavlovian conditioned approach behavior. It was demonstrated several decades ago that if a localizable Pavlovian CS reliably predicts the presentation of a reward, some animals will learn to approach the CS itself, even though no response is necessary to obtain the reward (Brown and Jenkins, 1968; Zener, 1937). This CS-directed approach behavior was called “sign-tracking” (Hearst and Jenkins, 1974), the word “sign” referring to the cue, and often includes vigorous engagement with the cue that mimics the consummatory response associated with the type of reward delivered (Davey and Cleland, 1982; Jenkins and Moore, 1973; Pavlov, 1932). Originally, the term “autoshaping” was used to describe the procedure that produces this type of Pavlovian CR (Brown and Jenkins, 1968), but this is actually a misnomer, because during the Pavlovian procedure no responses are ever reinforced (i.e., shaped). Indeed, the development of conditioned approach is not due to accidental reinforcement or “superstitious” behavior (Skinner, 1948). This was neatly demonstrated in Pavlovian conditioning studies in which a negative contingency was implemented, whereby contact with the CS resulted in omission of the reward. Under these conditions, animals continue to approach and sometimes even contact the CS, despite no longer receiving reward (Killeen, 2003; Lajoie and Bindra, 1976; Schwartz and Williams, 1972; Timberlake and Lucas, 1985; Williams and Williams, 1969).
Many species of animals, including birds, fish, rats, mice, monkeys, and humans, are known to exhibit sign-tracking behavior (Breland and Breland, 1961; Brown and Jenkins, 1968; Burns and Domjan, 1996; Cole and Adamo, 2005; Gamzu and Schwam, 1974; Hearst and Jenkins, 1974; Nilsson et al., 2008; Pithers, 1985; Tomie et al., 2012; Wilcove and Miller, 1974; Williams and Williams, 1969). However, there is considerable individual variation in the extent to which CS-US pairing leads to the development of a strong sign-tracking (ST) CR (Tomie et al., 2000). Zener (1937) first described such variation in dogs, for which a bell was paired with the delivery of food. These studies were nearly identical to those done by Pavlov, but in his case Zener released the dogs from their harnesses, allowing them to move freely. Zener found that the type of CR the CS elicited varied across dogs. Some dogs exhibited “small but definite movement of approach toward the conditioned stimulus…followed by a backing up later to a position to eat”; similar to what was later called sign-tracking behavior (Hearst and Jenkins, 1974). Other dogs, however, exhibited “an initial glance at the bell” followed by “a constant fixation…to the food pan” (p. 391). Studies after this described similar individual variation in approach behavior, but Boakes (1977) was the first to systematically describe goal location-directed conditioned approach in the context of autoshaping experiments, which he termed “goal-tracking (GT)”. We will use this ST/GT terminology here in respect of historical precedence.
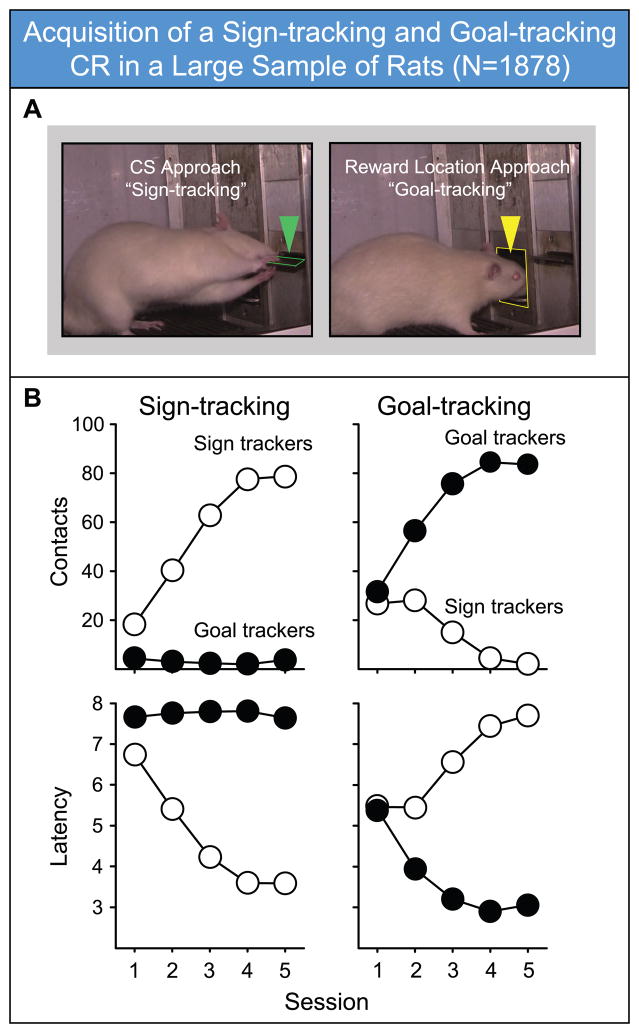
Individual variation in conditioned approach behavior in rats has recently been explored in a series of studies utilizing a simple Pavlovian conditioning procedure, in which the extension of a lever (the CS) is paired with delivery of a food pellet (the US) into an adjacent food hopper. Under these conditions, in which a discrete localizable cue that can also be manipulated is presented (versus, for example, a tone), some rats come to preferentially approach and engage the lever-CS itself (sign-trackers; “STs”), as described above. However, upon lever-CS presentation other rats (goal-trackers; “GTs”), initially glance at the lever-CS, but then go immediately to the food hopper (Figure 1), and make head and mouth movements in the hopper while awaiting food delivery (Mahler and Berridge, 2009). Yet other rats are ambivalent, alternating responses (Flagel et al., 2007; Meyer et al., 2012a). Both STs and GTs learn their respective CRs at the same rate, indicating that the food cue is an equally effective CS – it evokes a reliable approach CR in both – the conditioned approach response is just directed to different locations in the environment (Robinson and Flagel, 2009). Critically, the different approach behaviors of STs and GTs are not a reflection of differential learning capabilities, as both groups learn a variety of tasks equally well (Morrow et al., 2011; Robinson and Flagel, 2009; Saunders and Robinson, 2010). Rather, we have suggested that variation in the topography of the CR reflects underlying variation in the propensity to attribute incentive salience to discrete Pavlovian CSs (Flagel et al., 2009; Flagel et al., 2007; Meyer et al., 2012a; Robinson and Flagel, 2009). Thus, only for STs does the CS acquire those incentive stimulus properties that make it attractive.
Figure 1.
Acquisition of a cue (conditional stimulus, CS)-directed conditional response (CR; (sign-tracking, ST) versus a CR directed toward the location of reward delivery (goal-tracking, GT) in a large sample of rats (N=1878). Rats were defined as STs and GTs as described in detail in Meyer et al. (2012a). Briefly, we utilize a composite index score that incorporates three measures of Pavlovian conditioned approach: (1) the probability of either deflecting the lever or entering the food cup during each CS period [P(lever) - P(food cup)]; (2) the response bias for contacting the lever or the food cup during each CS period [(#lever deflections - #food-cup entries) / (#lever deflections + #food-cup entries)]; and (3) the latency to contact the lever or the food cup during the CS period [(lever deflection latency - food-cup entry latency) / 8]. Thus, the Pavlovian conditioned approach index (PCA Index) score consisted of [(Probability difference score + Responses bias score + Latency difference score) / 3]. This formula produces values on a scale ranging from -1.0 to +1.0, where scores approaching -1.0 represent a strong food cup-directed bias and scores approaching +1.0 represent strong lever-directed bias. In this case, rats were designated STs if they obtained an index score of +0.5 or greater (which means they directed their behavior towards the lever at least twice as often as to the food cup), and as GTs if they obtained a score of -0.5 or less. Note that the specific index score cutoff used is somewhat arbitrary, and may change from experiment to experiment. A) Representative pictures of a rat engaged in a ST CR (left), directed at the CS (lever), and a rat engaged in a GT CR (right), directed at the food hopper, during the CS period (note the extended lever). B)Across training sessions (days), rats classified at STs (but not GTs) progressively increased the number of lever-CS contacts, and contacted the lever-CS faster and faster after its presentation. Rats classed as GTs also learned a Pavlovian approach CR, but it was characterized by approaching the food hopper more and more rapidly after CS presentation, and interacting with it more and more vigorously (as indicated by head entries). Symbols represent group means (SEM’s are smaller than the symbols, and therefore not visible). Data in B adapted from Meyer et al. (2012a), with permission. Please see that paper for a more detailed analysis.
Humans also find reward cues “attractive”, such that they receive greater perceptual and attentional resources, even outside volitional awareness (Hickey et al., 2010a; Hickey and van Zoest, 2012; Raymond and O'Brien, 2009). This is often measured by their ability to bias attention relative to neutral cues (Field and Cox, 2008). Interestingly, studies in humans also demonstrate substantial individual variation in the degree to which reward cues are allocated with visual and attentional resources. For example, Hickey et al. (2010b) found that individuals with “reward-seeking” personality characteristics, as measured by the Behavioral Inhibition/Activation Scale (Carver and White, 1994), allocated more visual resources to reward-associated visual stimuli. A few studies have even attempted to examine individual differences in reward-cue approach tendencies in humans (Christiansen et al., 2012; Field et al., 2008; Field et al., 2005; Palfai, 2006; Thewissen et al., 2007; Van Gucht et al., 2008; Wiers et al., 2009). Direct measures of behavioral approach are difficult to examine in people, so investigators have developed experimental paradigms that allow for approach to be inferred. For example, Field et al. (2005) found that individuals with high levels of alcohol craving had more pronounced “approach” to alcohol-related pictures, as measured by the speed at which they moved a character on a computer screen toward the pictures. Wiers et al. (2009) found a similar relationship between alcohol drinking history and the tendency to use a computer joystick to “approach” alcohol-related images on a screen.
3.2. Conditioned Reinforcement
In addition to being attractive, incentive stimuli can also become desirable, in the sense that they will reinforce actions to obtain them. In experimental terms, incentive stimuli act as conditioned or secondary reinforcers. Conditioned reinforcers are capable of maintaining responding for long periods of time in the absence of the primary reward, and can support the learning of new and complex behavioral chains (Fantino, 1977; Hall, 1951; Hull, 1943; Kelleher and Gollub, 1962; Mackintosh, 1974); thereby greatly increasing the persistence and complexity of behavior. Pavlovian CSs are thought to produce conditioned reinforcement via two mechanisms: 1) by triggering a general motivational state, independent of particular outcomes, and/or 2) by evoking a representation of a specific rewarding outcome that reinforces further behavior (Burke et al., 2007, 2008; Parkinson et al., 2005).
The conditioned reinforcing properties of reward cues can become quite powerful. For example, they maintain behavior in the absence of rewards (Di Ciano and Everitt, 2004), they are also resistant to extinction (Arroyo et al., 1998; Di Ciano and Everitt, 2005; Panlilio et al., 2005), and they continue to reinforce responding even after US devaluation (Davis and Smith, 1976). The ability of cues to act as conditioned reinforcers is clearly illustrated by self-administration studies utilizing traditional extinction-reinstatement procedures to model relapse behavior (Nair et al., 2009; Shaham et al., 2003). In these studies, animals are trained to self administer a reward in the presence of an explicitly associated cue (often a light or tone), after which instrumental responding is extinguished in the absence of the cue. The cue’s ability to reinstate and maintain reward-seeking behavior is examined in a reinstatement test, wherein responses again produce the reward-paired cue, but under extinction conditions (that is, they do not receive the primary reward). Using this procedure, many studies have demonstrated that cues associated with a variety of rewards promote reward-seeking behavior (de Wit and Stewart, 1981; Kruzich et al., 2001; Milton and Everitt, 2010; Nair et al., 2009; Shaham et al., 2003). We should note that these studies typically refer to this effect as “cue-induced reinstatement”, but the way these procedures are usually applied (but see de Wit and Stewart, 1981; Deroche-Gamonet et al., 2002; Grimm et al., 2000) the cue does not “induce” an action, but the action produces the cue, and therefore it is presumably the conditioned reinforcing properties of the cue that primarily increases drug-seeking actions.
Reward cues also serve as conditioned reinforcers in humans. Indeed, in day-to-day life, most of human behavior produces no immediate primary reward, and thus cues must have the ability to maintain responding for prolonged periods of time. This has been formally demonstrated in several studies (Fantino and Case, 1983; Panlilio et al., 2005; Perone and Baron, 1980; Pithers, 1985; Wyckoff, 1952).
Interestingly, there is considerable individual variation in the extent to which reward-associated cues acquire conditioned reinforcing properties. For example, for STs, the same food CS that was attractive is also an effective conditioned reinforcer (i.e., these rats will learn a new instrumental response for presentations of just the CS). However, for GTs, who did not approach the CS but instead the food hopper, the CS is a less effective conditioned reinforcer (Lomanowska et al., 2011; Meyer et al., 2012a; Robinson and Flagel, 2009). Furthermore, Yager and Robinson (2010) found that a cue associated with food during an instrumental task was more effective in reinstating responding after extinction in STs than in GTs. These studies provide additional support for the hypothesis that STs and GTs differ in their propensity to attribute incentive salience to reward-associated cues (Meyer et al., 2012a).
3.3. Conditioned Motivation
Finally, incentive stimuli can arouse or evoke a conditioned motivational state that spurs reward-seeking behavior (Bindra, 1968; Cardinal et al., 2002; Milton and Everitt, 2010). This is an important mechanism by which cues produce craving, which may not only instigate new actions to procure the reward, but also invigorate ongoing actions. We should note here that craving, in the context of this review, refers to the conscious subjective state of desire for rewards, often directly measured in human studies, while “craving”, in quotations marks, refers to inferred implicit conditioned motivation. Importantly, conditioned motivational states need not reach conscious awareness to affect behavior or physiology (Childress et al., 2008). The ability of a Pavlovian CS to invigorate instrumental behavior has traditionally been examined using Pavlovian-to-instrumental transfer (PIT) procedures (Estes, 1943; Estes, 1948; Holmes et al., 2010; Lovibond, 1983; Ostlund and Maidment, 2012; Rescorla and Solomon, 1967). Typically, individuals first receive Pavlovian training, where a discrete CS is paired with reward delivery independent of any action. This is followed by an instrumental training phase, where the individual learns to make a response (e.g., lever press) for a reward. Subsequent noncontingent presentations of the Pavlovian CS (under extinction conditions) increase the rate, or “vigor”, of instrumental responding for reward. Similar to conditioned reinforcement, two varieties of this transfer effect have been described. First, PIT can occur in an outcome-independent manner (Dickinson and Dawson, 1987), where a CS enhances instrumental responding for any appetitive outcome, even those the CS was never paired with. For example, Balleine (1994) demonstrated that rats trained to self administer water responded at a higher rate when presented with either a water-associated CS, or a food-associated CS. Importantly, this general ability of CSs to invigorate instrumental behavior is tied to internal motivational states, such that transfer is greatest when the individual is highly motivated for the associated rewards. If rats have been stated on food, for example, a food-associated CS does not increase responding for water (Balleine, 1994). Second, an outcome-specific form of transfer can occur (Colwill and Rescorla, 1988; Kruse et al., 1983), where a CS biases instrumental actions to favor the one that produces the same outcome that was paired with that CS. This form of transfer appears to be somewhat less dependent on internal motivational states (Corbit et al., 2007). Therefore, Pavlovian CSs can directly modulate instrumental actions via multiple, dissociable processes.
As described above, most reinstatement studies typically use procedures in which the conditioned reinforcing properties of cues control behavior (that is, the cue is presented contingent upon an action). But noncontingent presentation of rewards can also produce a conditional motivational state that invigorates or reinstates extinguished reward seeking. Skinner (1938) demonstrated that, following extinction, non-contingent presentation of a food pellet to rats reinstated responding. Similarly, Rescorla and Skucy (1969) found that giving rats noncontingent food retarded the rate of extinction of food-seeking, and this occurred even if continued responding delayed the next availability of food. Many of these reward “priming” studies have now demonstrated that exposure to even small amounts of a variety of rewards can renew extinguished or long abstinent instrumental behavior (de Wit, 1996; Jaffe et al., 1989; Konorski, 1967; Skinner, 1938).
The notion that reward cues can produce conditioned motivation that invigorates instrumental behavior has also been demonstrated in humans (Bray et al., 2008; Hogarth et al., 2007; Holmes et al., 2010; Nadler et al., 2011; Paredes-Olay et al., 2002; Talmi et al., 2008). One of the first clear demonstrations of this came in a study by Talmi et al. (2008). They found that noncontingent presentation of a Pavlovian-conditioned money cue invigorated responding for money rewards. Another recent study by Bray et al. (2008) showed that Pavlovian CSs can also bias instrumental behavior in a outcome-specific way. Furthermore, reward “priming” also occurs in humans. As demonstrated by Cornell et al. (1989), people who were primed with a small amount of food subsequently ate significantly more food, relative to those who were not primed, even though these individuals had just eaten until satiation.
While several studies have characterized individual variation in the propensity to attribute incentive salience to reward cues by assessing their ability to motivate approach behavior, and act as conditioned reinforcers, few have assessed variation in the ability of cues to evoke a conditioned motivational state, as measured specifically with PIT procedures. To our knowledge, only one study has examined individual variation in PIT. Barker et al. (2012) recently found that mice vary in the degree that a food-associated CS invigorates food-seeking behavior. Interestingly, high PIT mice showed greater resistance to extinction of alcohol taking behavior and greater cue-induced reinstatement of alcohol seeking behavior than low PIT mice, suggesting that high PIT mice, like STs, attributed greater motivational value to both food and alcohol cues.
In summary, many studies in human and non-human animals, using a variety of procedures, indicate that Pavlovian stimuli, in addition to informing an individual about upcoming rewards, can acquire powerful incentive properties. While there is little disagreement about this general concept, it is important to point out that it is often assumed, either explicitly or implicitly, that a CS will also necessarily function as an incentive stimulus. We argue, however, that the individual differences in reward-cue responsivity described above demonstrate that this assumption is not valid. For both STs and GTs a discrete localizable Pavlovian cue serves as an effective CS, evoking CRs, but it is attributed with incentive salience to a much greater degree in STs than GTs. Thus, a reward cue acquires the ability to instigate conditioned approach towards it, to act as a powerful conditioned reinforcer, and to arouse a conditioned motivational state to a greater extent in STs than GTs. This leads us to conclude: the conditional, predictive relationship between a CS and a US is not sufficient to confer motivational properties to the CS. The fact that the motivational and predictive properties of reward cues are dissociable has considerable implications for thinking about the psychology and neurobiology of reward, as in most situations these properties are confounded, and tend to change together.
4. Individual variation in drug-cue responsivity
The transformation of a predictive CS into a motivationally significant incentive stimulus is important for normal reward-seeking behaviors, as described above, but may become especially relevant to the persistence of maladaptive reward seeking, characteristic of disorders such as compulsive drug use, overeating, and gambling. Several theories of addiction have emphasized the importance of drug cues (Di Chiara, 1998; Robinson and Berridge, 1993; Stewart et al., 1984; Tomie, 1996), as it is known that encounters with drug-associated cues can instigate craving and relapse behavior (DeJong, 1994; Hser et al., 2001; O'Brien et al., 1998; Shaham et al., 2003). We will now shift our discussion to the role that drug-associated stimuli play in drug-seeking behavior, and individual variation in the extent to which drug cues acquire motivational control over behavior.
4.1. Nonhuman Animal Studies
For a large part of the second half of the twentieth century, the predominantly held psychological explanation for why addicts continue to self administer drugs despite many adverse consequences was because doing so alleviated an aversive state associated with withdrawal symptoms (Koob and Le Moal, 2001; Lindesmith, 1968; Solomon and Corbit, 1974; Wikler, 1973). This was partly due to the prevalence of drive-reduction theories at the time (Hull, 1943), but also because the majority of early drug self-administration studies utilized opiates, which produce physical dependence that leads to marked withdrawal symptoms upon abstinence. Many studies (e.g., Deneau et al., 1969; Stewart et al., 1984; Woods and Schuster, 1971) eventually demonstrated, however, that opiate use – and that of other drugs – develops and progresses in the absence of physical dependence and withdrawal symptoms. Furthermore, studies began to demonstrate that relapse of drug seeking could be instigated through presentation of drug-associated cues or contexts, or by “priming” individuals with small amounts of drug, even in users who had been long abstinent (de Wit and Stewart, 1981; Hodgson et al., 1979; Stretch and Gerber, 1973). Based on studies like this, consensus began to shift to the view that drug use, similar to consumption of biologically relevant rewards such as food and water (Bindra, 1978), is usually – though not always – mediated by the positive incentive motivational properties of drugs and associated cues, rather than an internal drive to reduce a negative withdrawal state. In regard to drug use, this conceptual change was summarized by Stewart et al. (1984), who noted, “Need and drive views of motivation are gradually being replaced by a view … that ascribes a primary role to incentive stimuli as the generators of motivational states and elicitors of actions”. It is, “the drug itself, or the presentation of a stimulus previously paired with the drug, that acts to create a motivational state that facilitates drug-seeking behavior” (p. 251, 256), a view that currently has broad support (Milton and Everitt, 2010; Robinson and Berridge, 1993). Indeed, we now know that if drug cues act as incentive stimuli, they may become especially critical for the development and persistence of addiction, in part because they facilitate three “routes to relapse” (see Figure 4 in Milton and Everitt, 2010). They may 1) bias attention, eliciting approach to drug-associated places and paraphernalia; 2) reinforce actions that lead to obtaining drugs; and 3) spur intense drug seeking by evoking a conditioned motivational state (e.g., implicit “craving” or explicit craving). Though dissociable, these incentive motivational properties of drug cues in addicts may work in concert to promote relapse, such that maintaining abstinence becomes overwhelmingly difficult in addicts.
4.1.1. Conditioned Approach: Drug Cues
Until recently, there was no clear evidence that discrete drug cues would support conditioned approach behavior directed towards the drug cue itself (i.e., sign tracking) in non-human animals, as is readily demonstrated with food cues (see above). Indeed, as late as 2005, Everitt and Robbins (2005) speculated with regard to Pavlovian drug cue approach, “it may…be that the behavioral influence of CSs associated with drugs and natural reinforcers differ fundamentally in this regard” (p. 1482). Nevertheless, several studies using rats have now demonstrated that drugs delivered in a variety of fashions do in fact support conditioned approach behavior (Cunningham and Patel, 2007; Krank et al., 2008; Tomie et al., 2008; Uslaner et al., 2006). Tomie (1996) was amongst the first to suggest that the ability of drug cues to instigate approach and engagement is important in the development of maladaptive drug use, given that many drug-associated stimuli (e.g., drinking containers, needles, and pipes, etc) are embedded within drug-delivery apparatuses. If these cues become attractive and facilitate approach and engagement, the likelihood of continued drug use will be high.
Recent studies have demonstrated that variation in the extent to which individuals assign incentive salience to food cues predicts the degree to which drug-associated cues motivate approach. For example, in a study using selectively bred rats, STs, who developed robust approach behavior directed at a discrete food, also readily approached a discrete visual cue that had been paired with noncontingent intravenous cocaine infusions, while GTs did not (Flagel et al., 2010), and similar results have been found in outbred rats (Yager and Robinson, 2012).
4.1.2. Conditioned Reinforcement: Drug Cues
The ability of drug cues to act as conditioned reinforcers is an important mechanism contributing to persistent drug-seeking behavior. A variety of studies have demonstrated that, as with food cues, cues associated with drugs will maintain drug-seeking behavior for long intervals between drug delivery events, and support complex drug-seeking behavioral sequences (Arroyo et al., 1998; Di Ciano and Everitt, 2003, 2004; Di Ciano and Everitt, 2005; Everitt and Robbins, 2005; Goldberg and Tang, 1977; Katz, 1979; Kelleher, 1966; Kelleher and Goldberg, 1977; Schindler et al., 2002). Di Ciano and Everitt (2004) found, for example, that a discrete visual CS associated with cocaine can actually reinforce the learning of a novel instrumental action, and maintain responding in the absence of the drug across two months of intermittent testing. Consistent with this, other studies demonstrated that self administration of drugs is more robust when a cue is associated with drug delivery, compared to when drug delivery is unsignaled (Caggiula et al., 2009; Caggiula et al., 2001; Panlilio et al., 1996; Schenk and Partridge, 2001). Many further studies using the traditional extinction-reinstatement procedure showed that animals will reinstate extinguished drug-seeking behaviors in order to receive presentations of a drug-associated CS alone (e.g., de Wit and Stewart, 1981; Shaham et al., 2003).
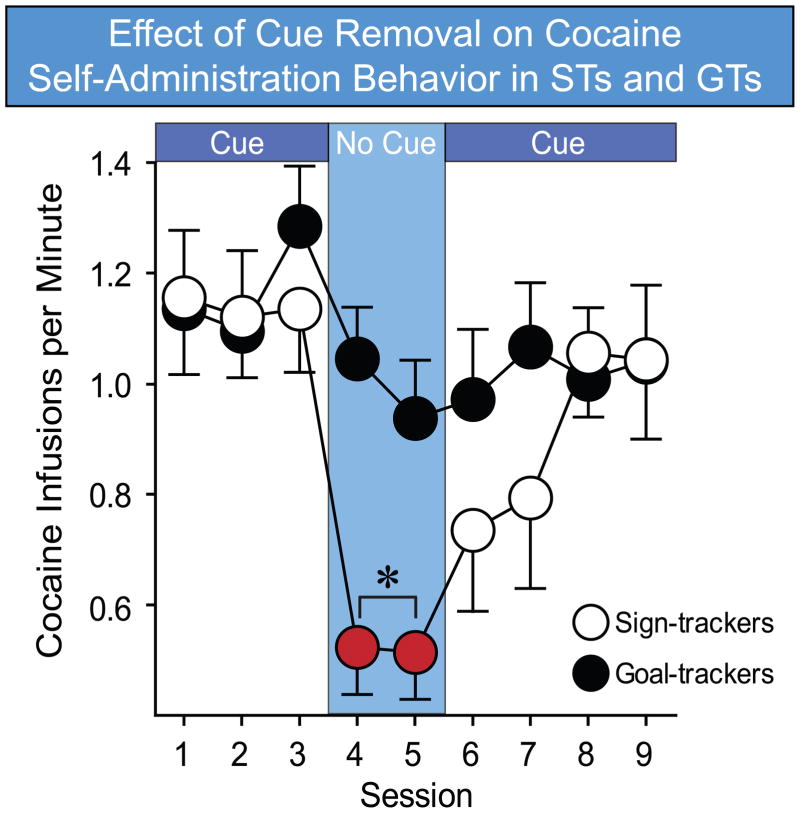
Recently, several studies have demonstrated that there is considerable individual variation in the degree to which drug cues serve as conditioned reinforcers. Barker et al. (2012) found that for mice that exhibited high levels of PIT in a test using food reward, an alcohol cue served as a more effective conditioned reinforcer, as measured by its ability to reinstate alcohol seeking. Additionally, Saunders and Robinson (2010) trained STs and GTs to self administer cocaine, in sessions where a discrete visual cue was explicitly paired with drug infusions. Following the acquisition of stable self-administration behavior, the cocaine cue was removed, though cocaine remained available. This manipulation caused a dramatic reduction in the rate of drug self-administration in STs, but not GTs, suggesting that the cocaine cue had acquired considerable motivational power of its own, but only in STs (Figure 2). Further evidence for such differences came in a follow-up experiment (Saunders and Robinson, 2010), where it was found that a cocaine cue reinforced much greater reinstatement in STs than GTs. Additionally, Yager and Robinson (2012) found that a cocaine cue acquired greater ability to reinstate drug seeking behavior in STs than GTs, even if it had only been paired with cocaine in separate Pavlovian conditioning sessions. Furthermore, using a conditioned cue preference procedure, Meyer et al. (2012b) showed that STs preferred a tactile cue that had been paired with cocaine injections to one paired with saline, while GTs did not show this preference. Importantly, in all of these experiments, both total drug intake and cue exposure were held equivalent across groups. Additionally, using these controlled procedures (e.g., Saunders and Robinson, 2010), STs and GTs were found to acquire self administration behavior equally well, providing further evidence that any behavioral differences were not a reflection of differences in learning. We should note, however, one recent study reported that when total drug intake was not limited by the experimenter, rats that preferentially exhibit sign-tracking responses acquired self administration at a faster rate than rats that preferentially goal-track (Beckmann et al., 2011). However, this effect was found using only low doses and a self-administration training procedure that is a modified version of Pavlovian approach training, so there is as yet no clear evidence that STs and GTs acquire drug self administration differently.
Figure 2.
Effect of cue removal on cocaine self-administration behavior in STs (n=14) and GTs (n=16). STs and GTs have an equivalent rate of self administration when a light cue signaled drug delivery (sessions 1–3, 6–9), but on the two sessions when the light cue was omitted (sessions 4–5), the rate of self-administration in STs, but not GTs, was significantly reduced. Symbols represent the mean ± SEM. *P < 0.05. Data adapted from Saunders and Robinson (2010), with permission.
4.1.3. Conditioned Motivation: Drug Cues
The presence of drug-associated cues in the environment can elicit conditioned motivational states that instigate drug-seeking behavior (Milton and Everitt, 2010). Such cue-evoked conditioned motivational states are thought to result in drug craving, which is an important way that drug cues promote relapse behavior. Additionally, exposure to small amounts of drug itself can instigate craving and relapse (de Wit and Stewart, 1981; Hodgson et al., 1979; Jaffe et al., 1989). As mentioned above, the ability of cues to produce conditioned motivation is often measured in tests of Pavlovian-instrumental transfer, where the presence of a Pavlovian CS invigorates current instrumental behavior. However, until recently there was no clear experimental evidence that a drug-associated cue can produce a PIT effect. In an important study addressing this issue, LeBlanc et al. (2012) demonstrated drug cue PIT, showing that presentations of a cocaine-associated CS acutely increased the rate of ongoing self-administration behavior in rats (see also Cortright et al., 2012). Interestingly, they found that the presence of a Pavlovian cocaine CS invigorated behavior during both the “seeking” and “taking” phases of the self administration behavioral chain, which are analogous to the approach/preparation and consumption phases of drug taking. Though this topic requires more investigation, the results of LeBlanc et al. (2012) suggest that Pavlovian drug cues directly invigorate behavior at multiple points along the progression of drug use.
As with the other incentive motivational properties of drug cues, there are also individual differences in the degree to which drug cues acquire the ability to evoke a conditioned motivational state. For example, Saunders and Robinson (2011a) trained STs and GTs to self administer cocaine, where a discrete visual cue was paired with drug infusions. Next, instead of extinction training, an aversive consequence to drug seeking was introduced to eliminate self-administration behavior. While cocaine was still available, the front two-thirds of the experimental chamber was electrified, with the current increasing over days (see also Cooper et al., 2007). Thus, to make a response that produced a cocaine infusion, the rat was required to cross this electric “barrier”, experiencing foot shock. After the current was high enough so that responding fell to a low level, the ability of the cocaine cue to instigate drug seeking was assessed with a procedure functionally equivalent to PIT. The cocaine cue was presented noncontingently, under extinction conditions, but with the electric barrier still in place. Noncontingent cocaine cue presentations spurred robust reinstatement of drug seeking in STs, but not GTs. In another study, Saunders and Robinson (2011b) trained STs and GTs to self administer cocaine in the absence of any explicitly paired cues. Following extinction, we found that a “priming” injection of cocaine instigated greater reinstatement behavior in STs than GTs. Thus, cocaine and discrete cocaine cues produce a state of conditioned motivation to a greater extent in some rats, and this motivational state is powerful enough to reinstate drug-seeking behavior, even overcoming aversive consequences.
4.2. Individual differences in reward cue responsivity underlie addiction vulnerability
Not all individuals experience temptation to consume drugs in a maladaptive way. Only a small subset of the general population ever becomes addicted to drugs, even though the vast majority of people use a potentially addictive substance at some point in their lives (Anthony et al., 1994). Given the enormous cultural and public health costs associated with addiction and other impulse control disorders, it is important to understand the mechanisms that engender these behaviors in order to understand the individual variation. The studies described above demonstrate that in individuals with a tendency to attribute exaggerated incentive salience to food cues, drug cues also acquire powerful motivational control over behavior – as measured by their ability to instigate approach, maintain drug self administration, and reinstate drug seeking. We propose that this variation is a contributing factor (of many) to individual differences in vulnerability to addiction. Specifically, we hypothesize: individuals for whom drug cues acquire exaggerated incentive salience will find them difficult to resist, and will therefore be more vulnerable to developing the persistent and compulsive patterns of drug seeking characteristic of addiction. Thus, one source of variation in susceptibility to maladaptive drug use may be variation in the ability of drug cues to gain motivational control over behavior. We will now turn our focus to an evaluation of the evidence for this prediction in human studies.
4.3. Human studies
The degree to which humans find drug cues attractive, as measured by their ability to bias attention, relative to neutral cues, predicts subjective craving for drugs, prospective drug use, and likelihood of relapse (Cox et al., 2002; Field and Cox, 2008; Franken et al., 2000; Marissen et al., 2006; Simon et al., 2010; Vezina and Leyton, 2013; Waters et al., 2003). For example, Field and Eastwood (2005) found that when subjects were experimentally manipulated into exhibiting greater attentional bias to alcohol cues, they experienced greater subjective craving, and drank more alcohol during a subsequent taste test. By training subjects to exhibit less attentional bias to alcohol cues, Fadardi and Cox (2009) reduced their subsequent alcohol consumption. Similarly, Attwood et al. (2008) found that smokers could be trained to show more or less attentional bias, and the degree of bias was positively associated with subjective craving. These studies suggest there is a direct correlation between the extent that a drug cue is attractive and attention grabbing and its ability to spur motivation to take drugs. The causal connection between drug-cue attentional bias and drug craving/intake is somewhat unclear, however, because some studies (e.g., Duka and Townshend, 2004; Schoenmakers et al., 2008) have reported that drug exposure increases subsequent attentional bias to drug cues, suggesting there may be a reciprocal relationship. One possibility is that while the development of an attentional bias for drug cues may be essential for those cues to later instigate craving and drug consumption, once attentional bias is established, further drug use can produce conditioned motivation that potentiates the bias.
Additionally, a large number of studies have demonstrated that drug cue-induced craving is positively correlated with intensity of abuse history and/or future intake, and likelihood of relapse (for review, see Carter and Tiffany, 1999; Tiffany and Wray, 2012), though the relationship between craving and subsequent drug use is somewhat controversial, as some studies have also demonstrated weak or insignificant correlations between cue-induced craving and drug-related behaviors. Most of these studies, however, measure craving in the laboratory setting, where the context has never been associated with drug use, and thus may not be conducive to the generation of robust craving. Interestingly, recent studies have examined the relationship between craving and drug use in the addict’s “natural environment”. For example, Epstein et al. (2009) monitored use of cocaine and heroin in outpatient subjects, using an ecological momentary assessment method (Stone and Shiffman, 1994), where subjects themselves reported real-time behavioral and subjective data on handheld electronic devices. They found that cocaine use was predicted by a variety of antecedent “triggers”, such as seeing the drug, or being reminded of drug use. Addicts who used more cocaine reported the most intense craving associated with these triggers (though this relationship was less clear for heroin use). Similar positive predictive associations between reported cue-induced craving and subsequent real-life drug use have been found in other studies (Epstein et al., 2010; Preston et al., 2009; Shiffman, 2009; Shiffman et al., 2002).
In addition to biasing attention and instigating craving, drug cues also become desirable. For example, Panlilio et al. (2005) demonstrated that a brief cocaine-associated stimulus maintained robust drug-seeking behavior – on the order of thousands of responses – in subjects with a history of cocaine abuse, even in the absence of actual drug delivery. The ability of the cue to maintain high levels of behavior persisted even though individuals reported being consciously aware that cocaine was not available. Related to this finding, Moeller et al. (2009) found that, relative to healthy controls, cocaine addicts preferentially chose to view images related to cocaine use over pleasant (e.g., smiling faces, nude bodies) non-drug images. Among cocaine addicts in this study, the amount of reported drug use in the past month and subjective arousal upon viewing cocaine images were positively correlated with the intensity of cocaine image preference. In another recent study on abstinent smokers, Freeman et al. (2012) found that presentation of smoking cues “overshadowed” (Mackintosh, 1976) neutral cues, in that smoking cues had greater perceived reward value, even though both sets of cues were equally predictive of the rewarding outcome.
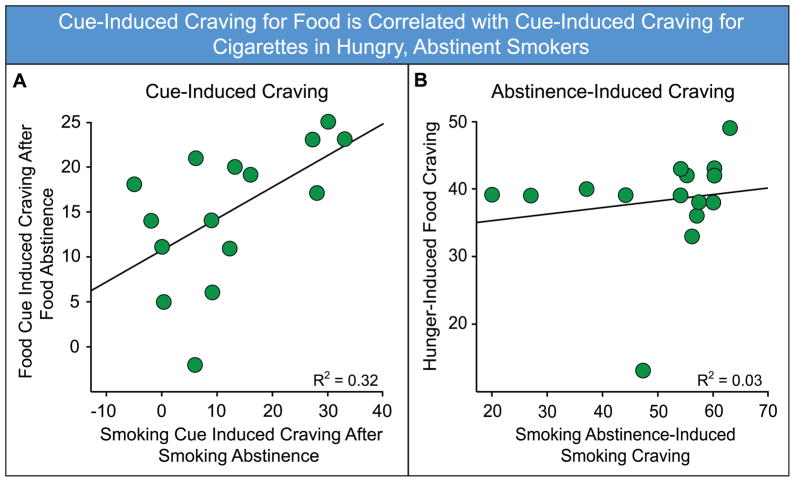
Many of these studies demonstrate there is considerable individual variation in the ability of drugs and drug cues to bias attention, produce craving, and instigate relapse in humans (Abrams et al., 1988; Carpenter et al., 2009; Carter and Tiffany, 1999; de Wit et al., 1986; de Wit et al., 1987; Kirk and de Wit, 2000; Lloyd and Salzberg, 1975; Niaura et al., 1998; Payne et al., 2006). Indeed, there is growing evidence that some humans are more reactive to cues. For example, Mahler and de Wit (2010) examined food and cigarette craving in a groups of smokers (see also Styn et al., 2012). They found that those individuals that showed the highest craving in response to discrete food cues, when hungry, also showed the highest craving to discrete smoking cues, after a period of abstinence (Figure 3). This individual variation parallels that in the rat studies described above (e.g., Saunders and Robinson, 2010; Saunders and Robinson, 2011b), suggesting that some humans may be more “cue reactive”, prone to assigning high incentive salience to certain types of cues in general, regardless of the reward they are associated with.
Figure 3.
Cue-induced craving for food is correlated with cue induced craving for cigarettes in abstinent smokers. A) The amount of craving elicited by food images, when subjects were hungry, correlated with the amount of craving elicited by smoking images, after a period of smoking abstinence. B) Cravings associated by hunger or smoking abstinence alone, in the absence of cues, were not correlated. Data adapted from Mahler and de Wit (2010), with permission.
Together, this research demonstrates the important role drug cues play in human substance use, and highlight how the degree to which cues gain motivational control over behavior increases with increased drug use – experienced drug users show greater attentional bias and cue-induced craving and relapse than new or non users – consistent with an incentive motivational account of addiction (Robinson and Berridge, 1993; 2001; Stewart et al., 1984). In the context of the preclinical studies described above, it appears that similar individual variation in the tendency to attribute incentive salience to reward cues can be found in both humans and non-human animals. Analogous parallels between humans and non-humans are evident in studies of the neural systems that mediate the motivational properties of reward cues, which we will now discuss.
5. Neural mechanisms of Pavlovian reward cue processing
Considerable research suggests that the neural systems recruited by motivationally significant events are very similar across many different classes of rewards, such as food, sex, and drugs (Cardinal et al., 2002; Childress et al., 2008; Haber and Knutson, 2010; Ikemoto, 2010; Ikemoto and Panksepp, 1999; Kalivas and Volkow, 2005; Kelley, 2004a; Kelley, 2004b; Kelley and Berridge, 2002; Kelley et al., 2005; Kenny, 2011; Kuhn and Gallinat, 2011; Nair et al., 2009; Volkow and Wise, 2005). These reward circuits comprise a wide, distributed network, including mesocorticolimbic dopamine pathways, which we will discuss in detail below. Though each may have specific functional roles in different reward-related processes, several brain regions, including the ventral tegmental area (VTA), dorsal and ventral striatum, ventral pallidum, thalamus, habenula, amygdala, and prefrontal/anterior cingulate/orbitofrontal cortex (PFC/ACC/OFC) are all known to be “engaged” by reward-associated cues (Cardinal et al., 2002; Kalivas and Volkow, 2005; Kelley et al., 2005; Koob and Volkow, 2010; Schiltz et al., 2007). Together, these regions constitute a motivational circuit, comprised of cortico-striato-pallido-thalamic loops with extensive reciprocal interregional connectivity (Belin and Everitt, 2008; Belin et al., 2009; Haber et al., 2000; Haber and Knutson, 2010; Kalivas and Volkow, 2005; Zahm, 2000, 2006). Specifically, VTA dopamine neurons project to subcortical targets in the ventral pallidum, amygdala, and nucleus accumbens core and shell, and also to frontal-cortical areas such as the PFC (Beckstead et al., 1979; Britt et al., 2012; Fields et al., 2007; Ikemoto, 2007; Swanson, 1982). The VTA and the adjacent VTA “tail”/rostromedial tegmental nucleus receive GABAergic inputs from the nucleus accumbens, ventral pallidum, and habenula, and glutamatergic inputs from hippocampus and PFC, all of which regulate dopamine signaling (Barrot et al., 2012; Carr and Sesack, 2000; Geisler and Zahm, 2005; Kalivas, 1993; Watabe-Uchida et al., 2012). The nucleus accumbens in particular sits at an important junction within this system, receiving the densest dopamine projections from VTA, as well as having reciprocal connections with the ventral pallidum, amygdala, hippocampus, and PFC/ACC/OFC (Berendse et al., 1992; Brog et al., 1993; Fields et al., 2007; Heimer et al., 1991; Hurley et al., 1991; Ikemoto, 2007; Kelley and Domesick, 1982; Kelley et al., 1982; Nauta et al., 1978; Zahm, 2000). Within the thalamus, the mediodorsal nucleus acts as a relay between these cortical and subcortical structures, as it receives input from the ventral pallidum, and sends projections to frontal cortical areas (Groenewegen, 1988; Ongur and Price, 2000; Ray and Price, 1992).
The ability of cues to act as incentive stimuli is dependent on the functional integrity of this motivational circuit, though the specific cells and systems required for each psychological property of an incentive stimulus are somewhat dissociable (Cardinal et al., 2002; Milton and Everitt, 2010). Conditioned approach: Pavlovian conditioned approach is dependent on neural signaling within the nucleus accumbens, central amygdala, ACC, and OFC (Blaiss and Janak, 2009; Chudasama and Robbins, 2003; Parkinson et al., 1999; Parkinson et al., 2000a; Parkinson et al., 2000b), although the distinction between a ST and GT CR is not always considered in these studies. Conditioned reinforcement: The conditioned reinforcing properties of reward cues are dependent on the ventral striatum, OFC, and basolateral amygdala (Burke et al., 2007, 2008; McDannald et al., 2011; Parkinson et al., 2001). Conditioned motivation: The neural systems supporting the ability of reward cues to produce a conditioned motivational state have been most clearly examined using PIT procedures. These studies suggest that the general and outcome-specific versions of PIT have somewhat dissociable neural substrates. For example, while both forms require an intact VTA, general PIT is dependent on the nucleus accumbens core, central amygdala, and dorsolateral striatum, while outcome-specific PIT requires the nucleus accumbens shell, basolateral amygdala, OFC, mediodorsal thalamus, and dorsomedial striatum (Corbit and Janak, 2007; Corbit and Balleine, 2005, 2011; Corbit et al., 2007; Corbit et al., 2001; Hall et al., 2001; Holland and Gallagher, 2003; Murschall and Hauber, 2006; Ostlund and Balleine, 2007, 2008).
While extensive studies implicate the neural systems mentioned above in mediating the motivational properties of cues, there appear to be large individual differences in the extent to which cues associated with reward engage these neural systems. For example, Flagel et al. (2011a) measured c-fos mRNA expression – an indirect measure of neuronal activity – in the brains of STs and GTs following exposure to a cue that had been paired with food (under extinction conditions). Exposure to the food cue produced significant increases in c-fos mRNA expression in the nucleus accumbens core and shell, dorsal striatum, lateral habenula, lateral septum, OFC, and the paraventricular, mediodorsal, and central medial nuclei of the thalamus in STs, relative to rats who received an equivalent number of unpaired presentations of the CS and US. Interestingly, in GTs c-fos mRNA expression in these regions was not different from unpaired control rats, even though for GTs the food cue was a perfectly effective CS, as indicated by its ability to reliably evoke a GT CR. This suggests that the acquisition of predictive value, via Pavlovian conditioning, is not sufficient for a CS to significantly “engage” these brain reward systems. For that to occur it appears that the cue must also be attributed with incentive salience.
5.1. Cue processing within dopamine systems
Within the larger, distributed reward circuits described above, signaling in dopamine neurons projecting from the VTA to ventral striatal regions such as the nucleus accumbens is thought to be central to motivated behavior. Considerable debate exists, however, about dopamine’s exact role, or roles, in reward processing (Beeler et al., 2012; Berke and Hyman, 2000; Berridge, 2007; Berridge and Robinson, 1998; Bromberg-Martin et al., 2010; Di Chiara, 1998; Ikemoto, 2010; Robinson et al., 2005; Salamone et al., 2007; Saunders and Richard, 2011; Schultz, 2007; Wise, 2004). One view is that phasic signaling of dopamine neurons provides a “prediction-error” signal necessary for learning stimulus-reward associations (Bayer and Glimcher, 2005; Montague et al., 1996; Schultz et al., 1997). This hypothesis stems from electrophysiological recordings of dopamine neurons in the VTA and substantia nigra, as well as electrochemical measurements of actual dopamine release within the nucleus accumbens, showing that a phasic dopamine response that initially occurs to an unexpected reward (US), transfers in time to the CS that predicts reward delivery (Cohen et al., 2012; Day et al., 2007; Pan et al., 2005; Schultz, 1998; Schultz et al., 1997; Waelti et al., 2001). Additionally, these studies suggest that dopamine signaling also modifies learned predictive associations. For example, if a reward is bigger than expected based on the CS’s learned predictive value, dopamine neurons fire more, if it is smaller than expected, they fire less (i.e., a negative prediction error), leading to new learning (Pan et al., 2005; Schultz et al., 1997; Waelti et al., 2001).
Alternatively, others have argued that mesolimbic dopamine is not necessary for learning stimulus-reward associations per se, but for conferring learned reward cues with incentive salience, transforming them into “wanted”, motivationally potent incentive stimuli (Berridge, 2007; Berridge, 2012; Berridge and Robinson, 1998). An important prediction from the incentive salience hypothesis of dopamine is that changes in dopamine signaling can modify the motivational value of learned CSs ‘on-the-fly’, without the need to re-experience CS-US pairing (Zhang et al., 2012; Zhang et al., 2009). This is in contrast to learning-based accounts (Daw et al., 2005; Schultz et al., 1997; Sutton, 1988), which state that dopamine prediction errors update the learned value of a CS incrementally, on a trial-by-trial basis. It has been difficult to separate the potential contribution dopamine makes to learning from its contribution to incentive salience, because reward cues often acquire these properties together. However, recent studies have exploited individual variation in the tendency to attribute cues with motivational value, as discussed above, to dissociate these properties of reward cues (Berridge and Robinson, 2003; Robinson and Flagel, 2009).
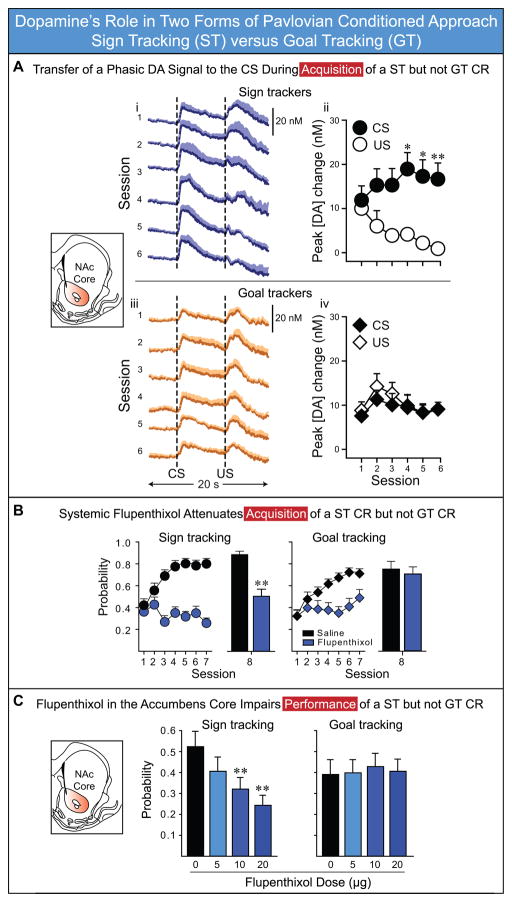
Flagel et al. (2011b) used fast-scan cyclic voltammetry (FSCV) to measure rapid dopamine signaling within the nucleus accumbens core (Phillips et al., 2003b), during Pavlovian training in which a lever-CS was paired with food delivery, independent of any action, as described above. In rats that learned a sign-tracking CR the phasic dopamine signal transferred from the US to the CS, as a function of learning, similar to previous reports (see also Clark et al., 2012; Day et al., 2007). However, in rats that learned a goal-tracking CR, no such US-to-CS transfer occurred (Figure 4), even though for these rats the CS-US association was learned, as indicated by the fact that the CS came to reliably evoke a CR directed at the location of food delivery, as a function of training. Similarly, Parker et al. (2010) found that mice with disrupted phasic dopamine signaling learned a goal-tracking CR normally, even though no clear US-to-CS transfer in dopamine signaling occurred. To test whether dopamine is necessary for learning a ST vs. GT CR, Flagel et al. (2011b) treated rats with systemic injections of the dopamine antagonist flupenthixol prior to each training session, which would block dopamine activity in all brain regions that receive a dopaminergic input. They found that flupenthixol blocked learning of a sign-tracking CR, but it had no effect on learning the CS-US association that underlies a goal-tracking CR (Figure 4; see also Danna and Elmer, 2010).
Figure 4.
Dopamine’s role in two forms of Pavlovian conditioned approach: sign tracking versus goal tracking. A) Transfer of a phasic dopamine signal from the US to the CS during acquisition of a ST CR but not GT CR in outbred rats. Dopamine concentrations in the nucleus accumbens core were measured using FSCV during six days of PCA training. i, iii, Changes in dopamine concentration in response to the CS and US for each day of Pavlovian conditioning for rats that learned a ST CR (n=6) and rats that learned a GT CR (n=5), respectively. ii, iv, Change in the peak amplitude of the dopamine signal in response to the CS and US across training sessions. In STs the phasic dopamine signal in response to the CS increased across days of training, while the response to the US decreased. In GTs there was no change in the dopamine response to the US or CS across days of training. B) Systemic administration of flupenthixol attenuates acquisition of a ST CR but not a GT CR in selectively bred rats. Flupenthixol or saline was administered via i.p. injections to bred high-responder rats (bHRs, left panel; these animals learn a ST CR) and bred low-responder rats (bLRs, right panel; these animals learn a GT CR) prior to the sessions 1–7 of Pavlovian training. Relative to saline, flupenthixol impaired the performance of both ST and GT CRs during sessions 1–7. All rats received a final saline injection before session 8. On the Day 8 drug free test day bHRs (STs) treated with flupenthixol during training (n=22) had a significantly lower probability of making a ST CR than animals in the bHR saline group (n=10), and did not differ from the saline control group on Day 1 of training. This indicates that dopamine is necessary for the acquisition of a ST CR. On the other hand, on the Day 8 drug free test day bLRs (GTs) that received flupenthixol during training (n=16) did not differ from bLR saline rats (n=10) in the probability of making a GT CR, and had a significantly higher probability of making a GT CR than did the saline control animals on the first day of training. This indicates that dopamine was not necessary for the acquisition of a GT CR. C) Flupenthixol in the core of the nucleus accumbens attenuates the performance of a ST CR, but not a GT CR. Flupenthixol or saline was microinjected into the core of the accumbens of rats (N=42) after they had acquired stable Pavlovian conditioned approach behavior. Relative to saline, flupenthixol dose-dependently decreased the probability of animals making a ST CR (left panel), but not the probability of making a GT CR (right panel). Symbols represent the mean ± SEM. *P < 0.05; ** P < 0.01. Data in A and B adapted from Flagel et al. (2011b) and data in C from Saunders and Robinson (2012), with permission. Please see those papers for more detailed analyses.
In their supplemental materials Flagel at al. (2011b) also reported that the performance of already acquired sign- and goal-tracking behavior were both impaired by systemic dopamine antagonism. However, this result is difficult to interpret, because the effects occurred at doses that also produced non-specific reductions in motor activity. Thus, based on this study, the role of dopamine in the performance of sign- and goal-tracking behavior remained unclear. To directly address this issue, and to reduce non-specific effects of dopamine antagonism on behavior, Saunders and Robinson (2012), administered flupenthixol directly into the nucleus accumbens core of rats after they had acquired stable sign- and goal-tracking behavior. The administration of flupenthixol dose-dependently attenuated a sign-tracking CR, but had little to no effect on a goal-tracking CR (Figure 4; see also Di Ciano et al., 2001; Parkinson et al., 2002). Additionally, after administration of flupenthixol into the accumbens, sign-tracking behavior was fully impaired on the very first trial, before new learning via updated prediction-errors could occur. Consistent with the incentive salience hypothesis, this suggests that fluctuations in mesolimbic dopamine signaling can dynamically modify the motivational value of reward cues, without the need to re-experience the CS-US association (see Berridge, 2012; Richard et al., 2013; Zhang et al., 2012; Zhang et al., 2009). Similar learning-independent performance effects of dopamine manipulations have been found in recent studies of Parkinson’s disease patients (e.g., Shiner et al., 2012). Finally, even in STs, dopamine antagonism did not attenuate performance of a different CR, a conditioned orienting response in the direction of the CS, suggesting that even in STs some stimulus-reward associations remained functional after dopamine blockade in the core of the accumbens.
Earlier studies provide further evidence that dopamine is not necessary for stimulus-reward learning. For example, Berridge and Robinson (1998) completely depleted dopamine in the dorsal and ventral striatum of rats using the neurotoxin 6-OHDA, and found they were still able to learn a new value of a food reward just as well as intact control rats. An important series of studies by Richard Palmiter and colleagues similarly demonstrated that genetically engineered dopamine-deficient (DD) mice, whose brains cannot produce dopamine, learned normally on a variety of tasks, such as conditioned place preference (Cannon and Palmiter, 2003; Hnasko et al., 2007; Hnasko et al., 2005; Robinson et al., 2005). From these studies, Robinson et al. (2005) concluded: “dopamine is not necessary for animals to learn to associate salient cues with rewards”…but it “is necessary for reward-related cues to attain motivational significance”.
Several other studies suggest that dopamine controls the degree to which cues act as incentive stimuli. For example, potentiation of dopamine release, via administration of psychostimulant drugs, increases sign-tracking behavior (Hitchcott et al., 1997; Holden and Peoples, 2010; Palmatier et al., 2012; Phillips et al., 2003a; but see Simon et al., 2009), but not goal-tracking behavior (Doremus-Fitzwater and Spear, 2011), and also potentiates the conditioned reinforcing effects of food and drug-associated cues (Collins et al., 2012; Hill, 1970; Kelley and Delfs, 1991; Robbins, 1975, 1976; Taylor and Robbins, 1984). Additionally, injection of amphetamine increases the ability of a Pavlovian CS to spur ongoing food-seeking behavior, as measured by a general PIT procedure (Wyvell and Berridge, 2001), and increases neuronal firing in the ventral pallidum in response to an incentive CS, but not a purely predictive CS (Smith et al., 2011; Tindell et al., 2005; Tindell et al., 2009). This is consistent with reports that administration of dopamine receptor antagonists suppress general PIT effects (Dickinson et al., 2000; Ostlund and Maidment, 2012; Smith and Dickinson, 1998; Wassum et al., 2011), suggesting that dopamine signaling is necessary for Pavlovian CSs to invigorate instrumental responding. Dopamine appears to be somewhat less important for the outcome-selective version of PIT. For example, Yin et al. (2006) found that hyperdopaminergic mice failed to show elevated outcome-specific PIT, relative to wild type control mice (see also, Shiflett, 2012). Furthermore, Ostlund and Maidment (2012) reported that dopamine antagonists did not influence the ability of CSs to bias action selection for a specific outcome. Dopamine’s role in mediating the conditioned motivational effects of CSs may be relatively localized to the ventral striatum, however, as elimination of dopamine cells projecting to the dorsal striatum had no effect on either general or outcome-specific PIT (Pielock et al., 2011).
It is important to acknowledge that dopamine clearly has other functions in the brain besides regulating Pavlovian incentive motivation. For example, dopamine is implicated in arousal, action selection, cognitive flexibility, and behavioral effort, particularly during instrumental conditioning (Beeler et al., 2012; Cools, 2008; Day et al., 2010; Redgrave et al., 1999; Robbins and Everitt, 1992; Salamone et al., 2007; Wassum et al., 2012). We should note, though, that even for instrumental behaviors, dopamine can modulate responding by scaling performance vigor, or by regulating PIT effects, independent of learning (Cagniard et al., 2006; Yin et al., 2006). Also, we have focused our discussion on appetitive cue processing, but dopamine is also involved in processing aversive or dysphoric motivational states (Aragona and Wang, 2009; Badrinarayan et al., 2012; Chaudhury et al., 2012; Faure et al., 2008; Kapur et al., 2005; Lemos et al., 2012; Oleson et al., 2012; Pezze and Feldon, 2004; Pezze et al., 2001; Richard and Berridge, 2011; Roitman et al., 2008; Tye et al., 2012).
Additionally, while we have emphasized dopamine signaling from the VTA to nucleus accumbens, midbrain dopamine neurons in the VTA, as well as substantia nigra, project to a variety of regions outside of the ventral striatum, including the dorsal striatum, amygdala, prefrontal cortex, and hippocampus, and different dopamine neurons have different patterns of activity and functions (Britt et al., 2012; Bromberg-Martin et al., 2010; Fields et al., 2007; Lammel et al., 2011; Li et al., 2012; Margolis et al., 2006; Watabe-Uchida et al., 2012; Witten et al., 2011). Finally, dopamine is but one of many neurotransmitters systems involved in general reward-related processes, and even in mediating the incentive motivational properties of reward cues (Bakshi and Kelley, 1993; Berridge, 2012; Cardinal et al., 2002; Difeliceantonio and Berridge, 2012; Kelley et al., 2002; Mahler and Berridge, 2009; Novak et al., 2010; O'Connor et al., 2010; Puglisi-Allegra and Ventura, 2012; Smith et al., 2010; Ventura et al., 2007; Wassum et al., 2009). Thus in future research it will be necessary to fully investigate the contribution of other systems, such as glutamate, GABA, and endogenous opioids, in individual differences in reward cue processing, as well as their interactions with dopamine.
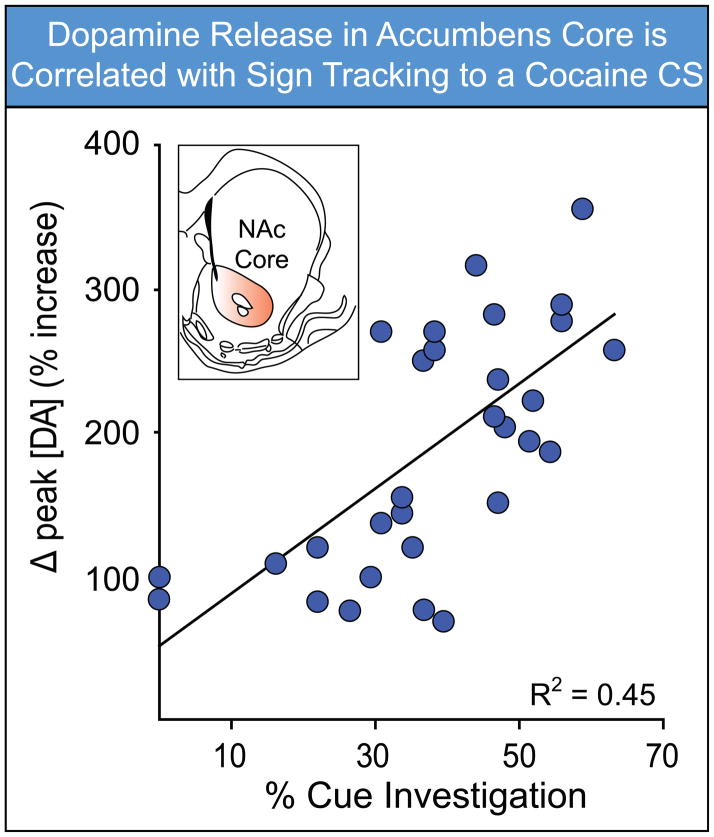
Nevertheless, while a complex and distributed set of brain systems are involved in reward-cue processing, the mesolimbic dopamine system has thus far been an important focal point. Interestingly, there is growing evidence that endogenous individual variation in dopaminergic systems may underlie variation in the tendency to attribute incentive salience to reward cues. Specifically, rats that attribute greater incentive salience to discrete cues, as indicated by sign-tracking behavior, exhibit greater sensitization of stereotyped head movements – thought to reflect sensitization of dopamine pathways (Paulson and Robinson, 1995; Robinson and Becker, 1986) – following a series of cocaine injections (Flagel et al., 2008). In the striatum, STs have higher levels of mRNA for the D1 dopamine receptor, and lower levels of dopamine transporter (DAT) mRNA, than GTs, which has the functional consequence of greater dopamine receptor activation (Flagel et al., 2007). Other studies, using selectively bred rats, have shown that within the nucleus accumbens core STs generate more spontaneous dopamine release events (“transients”), and have a greater number of high affinity dopamine D2 receptors, relative to GTs (Flagel et al., 2010). Finally, variation in dopamine signaling within the nucleus accumbens core is associated with variation in the propensity to approach a reward cue. The best illustration of this thus far is a study by Aragona et al. (2009), who paired a light cue with intravenous infusions of cocaine. They reported that the magnitude of cue-elicited dopamine release in the accumbens core was positively correlated with the propensity to approach the cocaine cue; i.e., to show a sign-tracking CR (Figure 5). Taken together, these studies suggest that variation in dopamine activity is associated with variation in the propensity to attribute incentive salience to reward cues, although this topic requires much more research.
Figure 5.
Dopamine signaling within the nucleus accumbens core is associated with sign tracking to a cocaine cue. Dopamine concentrations within the nucleus accumbens core were measured using FSCV during Pavlovian conditioning of a light CS with i.v. cocaine infusions. On individual trials, the peak change in dopamine concentration was positively correlated with the percent of time rats spent investigating the light CS while it was illuminated (i.e., showed sign tracking behavior). Data modified with permission from Aragona et al. (2009).
Importantly, the role dopamine plays in modulating the incentive salience of reward cues has implications for understanding human disorders such as addiction. Robinson and Berridge (1993; 2000; 2001; 2003; 2008) have argued that changes in the mesolimbic dopamine system associated with drug use play a critical role in the development of persistent drug seeking as seen in addiction. With repeated drug use, brain dopamine systems become hypersensitive, resulting in the exaggerated attribution of incentive salience to drugs and drug cues, making them irrationally desirable and “wanted”. The neurobiological changes associated with repeated drug exposure are long lasting (Paulson et al., 1991; Robinson and Kolb, 2004; Wolf et al., 2004), and thus the threat of relapse persists for a considerable time after the discontinuation of drug use. We will now review evidence of the role of dopamine in processing drug cues in humans.
6. Dopamine regulates drug-cue responsivity in humans
Substantial evidence from human addiction studies, including many by Nora Volkow and colleagues, suggests that brain dopamine systems also play a key role in processing drug-related stimuli in addicts (Ersche et al., 2010a; Franken et al., 2005; Franken et al., 2004; Goldstein et al., 2009; Laruelle et al., 1995; Leyton et al., 2002; Vezina and Leyton, 2013; Volkow et al., 2006; Volkow et al., 1994; Volkow et al., 2008; Wong et al., 2006). Dopamine signaling is often measured by displacement of dopamine at the D2 receptor by the radiolabeled D2 antagonist, raclopride, using PET imaging. For example, Volkow et al. (2006) found that when cocaine addicts view images of cocaine use, dopamine signaling surged within the striatum (see also Wong et al., 2006). Interestingly, the magnitude of cue-evoked dopamine release correlated with subjective craving. Similar striatal dopamine increases have also been shown in response to amphetamine-associated stimuli (Boileau et al., 2007), as well as heroin cues (Zijlstra et al., 2008).
Dopamine signaling in humans also has a broad role in attentional processing of reward cues, including drug cues. Increases in dopamine transmission produce enhancements in performance on behavioral tasks that require selective attention to stimuli, while reductions in dopamine, via pharmacological manipulations, or as seen among Parkinsonian patients, results in selective attentional deficits (Clark et al., 1987; Franken et al., 2005; Hickey et al., 2010a; Nieoullon, 2002; Servan-Schreiber et al., 1998; Stam et al., 1993). A few recent studies have assessed the role of dopamine in attentional bias specifically for drug-related cues. For example, Franken et al. (2004) found that administration of haloperidol, a dopamine receptor antagonist, reduced attentional bias to heroin cues among heroin addicts. Reductions in drug-cue attentional bias were also found in smokers following acute tyrosine/phenylalanine depletion (Hitsman et al., 2008; Munafo et al., 2007). Complementary to this, administration of dopamine agonists increases drug-cue attentional bias (e.g., Ersche et al., 2010a).
Dopamine signaling may also serve to regulate the responses of other brain regions associated with attentional bias for drug cues, as recently demonstrated by Luijten et al. (2012). They found that, among smokers, haloperidol administration reduced smoking cue-evoked brain activity within ACC and dorsolateral PFC. After haloperidol administration, smoker’s cue-induced brain activity was identical to non-smoker controls. Consistent with this, Hermann et al. (2006) found that administration of the dopamine receptor antagonist anisulpride reduced alcohol cue-induced brain activity in the ACC and OFC in alcoholics, such that they were no longer different from control subjects. Indeed, many brain regions that receive dopaminergic innervation, such as the ACC, PFC, ventral striatum, and amygdala are implicated in attentional bias for drug-related cues (Ersche et al., 2010a; Hester and Garavan, 2009; Janes et al., 2010; Luijten et al., 2012; Luijten et al., 2011), and striatal dopamine signaling, particularly in the ventral striatum, has been suggested to serve as an interface between so called “bottom-up” incentive motivational processes and “top-down” cognitive control of behavior (Aarts et al., 2010; Cools, 2008). Thus, it is possible that dopamine is involved in both the formation of attentional bias for drug cues, by “marking” them with incentive salience, and also in the maintenance of that bias, in part by regulating drug-cue detection that occurs in other brain regions. This has yet to be directly tested, however, and as Luijten et al. (2012) state, it will be important to “examine whether individual differences in dopaminergic activation…are associated with differences in attentional bias-related brain activation”. Given that largely overlapping brain circuits are involved in the detection and processing of cues associated with several classes of drugs (Kalivas and Volkow, 2005; Kuhn and Gallinat, 2011), dopamine likely has a fundamental role in drug-cue processing in humans.
The interaction between dopamine systems and other brain regions is complex, and not unidirectional. Dopamine systems, including the VTA, as well as its target regions, are also under regulation from fronto-cortical regions (Parikh and Sarter, 2008; Phillips et al., 2008; Richard and Berridge, 2012; Takahashi et al., 2011; Volkow et al., 2005; Volkow et al., 2007). An extensive literature has implicated abnormal activity in frontal cortical brain systems in addiction-like behaviors (Bolla et al., 2004; Bolla et al., 2003; Feil et al., 2010; Goto et al., 2010; Hester and Garavan, 2004; Kalivas and Volkow, 2005; Lucantonio et al., 2012). It remains unclear, however, the extent that dysfunction within frontal cortical circuits seen in addicts is a cause or consequence of long-term drug use, and, of course, both could be true. Further research is needed to better understand how variation in frontal cortical activity may interact with dopaminergic variation to underlie maladaptive reward seeking.
6.1. Individual differences in human dopamine systems
Several recent studies have found a relationship between individual differences in human dopamine systems, measured by brain activation patterns, and measures of incentive motivation like reward anticipation and craving (Aarts et al., 2010; Buckholtz et al., 2010a; Buckholtz et al., 2010b; Cools, 2008; Dagher and Robbins, 2009; Franken et al., 2005; Tomer et al., 2008; Treadway et al., 2012; van Schouwenburg et al., 2010). Leyton et al. (2002), for example, found individual differences in amphetamine-induced dopamine release within ventral striatum, and the magnitude of release was positively correlated with measures of subjective drug “wanting”. The results of this study are somewhat unique because this variability was found among healthy subjects, not experienced addicts, suggesting that an exaggerated responsivity of dopamine systems to drugs may be involved in predisposing certain people to have stronger drug craving.
Genetic variation in the dopamine systems of humans may explain some of the individual differences in reward-cue related brain activity. For example, Dreher et al. (2009) found that individuals with a polymorphism at the catechol-O-methyltransferase (COMT; a dopamine metabolizing enzyme) gene that produces a reduction in COMT enzyme activity, exhibited higher levels of activity in PFC and ventral striatum during cued reward anticipation and reward delivery. Similar elevations in brain activity were found among people with a polymorphism of the DAT gene that produces reduced DAT expression (Dreher et al., 2009). Other studies have shown similar relationships between DAT polymorphisms and drug cue-evoked brain activity in PFC and ventral striatum, as well as dorsal striatum, insula, ACC, and OFC (Aarts et al., 2010; Franklin et al., 2009; Wetherill et al., 2012). Though the exact mechanism that mediates these effects is unclear, changes in reward-related brain activity in individuals carrying certain dopamine polymorphisms is presumed to be due to functional differences in dopamine release, reuptake, and/or metabolism.
Several studies have now addressed the relationship between dopamine-related polymorphisms and drug-cue induced neural activation and/or drug-related behaviors (Bogdan et al., 2012; Dagher and Robbins, 2009; Foll et al., 2009; Kreek et al., 2005; McClernon et al., 2007; Noble, 2000). Many, though not all (e.g., Guindalini et al., 2008) of these studies find that polymorphisms thought to produce elevated dopamine signaling are associated with greater drug cue-induced brain activity, and behavioral measures such as drug anticipation and/or craving, and drug use. Some of the strongest relationships between variation in dopamine-related genes and reward-related brain activity have been found when researchers analyzed multilocus genetic profiles, which consider the cumulative impact of multiple polymorphisms (Bogdan et al., 2012; Nikolova et al., 2011; Stice et al., 2012). People with multiple allelic variants that either increase or decrease dopamine activity tend to have the greatest or least cue-related neural activity, respectively. Thus, single polymorphisms themselves may not always contribute to individual differences in cue-evoked brain activity, and specific combinations may be necessary, indicating that a full genetic account of cue responsivity differences will be quite complex. Importantly, however, multilocus genetic profiling studies specifically examining responsivity to drug cues remain to be done.
We have focused here on compulsive drug use, but it is important to point out that individual differences in reward-cue processing have implications for understanding vulnerability to other maladaptive behaviors, such as over-eating (hyperphagia). Obesity brought on by binge eating is a growing problem, and now over a third of adults in the United States are considered obese (Flegal et al., 2010; Ogden et al., 2007). Exposure to food-associated cues can override satiety signals to promote overeating (Cornell et al., 1989), an effect that is more pronounced in obese individuals (Jansen, 1998; Jansen et al., 2003). Especially important for the subject of this review, there are individual differences in the ability of food cues to attract attention and motivate craving and eating. Individual differences in the ability of food cues to attract attention, motivate craving and eating, and elicit brain activity have now been reported in several studies (Carnell and Wardle, 2009; Fedoroff et al., 1997; Franken and Muris, 2005; Schachter and Gross, 1968; Tapper et al., 2010; Tetley et al., 2009). For example, Beaver et al. (2006) found that people reporting higher reward pursuit/seeking tendencies, as measured by the BAS/BIS scale, showed greater activity in ventral pallidum, ventral striatum, amygdala, midbrain, and OFC in response to viewing images of appetizing foods. Interestingly, Lawrence et al. (2012) reported recently that individual variation in food-cue induced activity in the nucleus accumbens predicted subsequent food intake, independent of subjective desire to eat. Several studies have now also shown that, compared to healthy controls, obese individuals show elevated striatal activation in response to food cues (e.g., Rothemund et al., 2007). Additionally, there is a relatively high comorbidity between obesity and abuse of some drugs, especially alcohol (Grucza et al., 2010), suggesting that overlapping factors contribute to variation in vulnerability to both overeating and drug addiction. Indeed, while the existence of “food addiction” is debated (Avena et al., 2012; Davis and Carter, 2009), some are now calling for the implementation of public health strategies known to be successful at reducing rates of drug addiction to the treatment of compulsive overeating (Gearhardt et al., 2011).
Finally, we wish to point out that while the focus of this review is on individual differences in the propensity to attribute reward cues with incentive motivational properties, there are other relevant sources of individual differences relevant to compulsive reward-seeking disorders. For example, individuals vary greatly on measures of impulsive behavior (Belin et al., 2008; Dalley et al., 2011; de Wit, 2009; Evenden, 1999; Winstanley et al., 2010) and novelty seeking (Bardo et al., 1996; Cami and Farre, 2003; Dagher and Robbins, 2009; Tomer, 2008), which have been studied extensively in preclinical and clinical populations as factors that may underlie variability in vulnerability to addiction-like disorders. Interestingly, variation in incentive salience attribution is associated with variation in these traits, in that STs tend to show more impulsive actions and novelty seeking, relative to GTs (Beckmann et al., 2011; Flagel et al., 2010; Lovic et al., 2011; Tomie et al., 1998). While details of the relationships between these traits are currently unknown, they all share the common characteristic of being associated with hyperreactivity to environmental cues. Furthermore, dopamine system differences have been associated with individual variation in each of these traits (Buckholtz et al., 2010a; Buckholtz et al., 2010b; Dalley and Roiser, 2012; Ersche et al., 2010b; Flagel et al., 2009; Leyton et al., 2002; Tomer et al., 2008), suggesting that related neural systems may underlie different behavioral manifestations of cue responsivity. Thus, investigation of the factors – genetic, epigenetic, environmental, and neural-systems-level – that facilitate individual variation in the tendency to attribute incentive salience to cues may shed light on other traits associated with psychopathology.
7. Implications for treatment: A focus on individual differences
The factors underlying individual variation in vulnerability to maladaptive reward seeking are complex. We have summarized some of the literature on variation that results in certain individuals attributing potentially maladaptive levels of motivational value to reward-associated cues, which may be a factor underlying individual differences in vulnerability to addiction and related disorders. Preclinical studies have been useful for furthering understanding the psychological and neural mechanisms associated with such aberrant reward-cue processing, and it will be important to exploit this information to improve treatment strategies in humans. We hope that in the development of future treatments, clinicians will consider 1) individual differences in the psychological factors that control pathological motivation for drugs, and 2) that in susceptible individuals drug cues may be especially insidious in instigating and maintaining drug-seeking behavior.
Some preliminary evidence suggests manipulating attentional bias to drug cues via attentional control therapies may be an effective method for reducing some of the behavioral control drug cues have over addicts (Attwood et al., 2008; Fadardi and Cox, 2009; Schoenmakers et al., 2010). These studies demonstrate that by training addicts to explicitly avoid paying attention to drug cues, or by extensively extinguishing drug cues by presenting them repeatedly and in different contexts without drug exposure, a cue’s relapse-provoking abilities may be lessened. Additionally, drug cue “reappraisal” procedures, where subjects are instructed to reinterpret the meaning of a cue, to make it less motivationally significant, may be effective at reducing cue-induced craving (Zhao et al., 2012). Intriguingly, these studies suggest that addicts may be able to exert some cognitive control over cue-induced urges, but important concerns exist over how generalizable and enduring such behavioral therapies are. Additionally, pharmacological interventions, particularly those targeting brain dopamine systems, have thus far shown some promise for diminishing drug-cue responsivity, though results are mixed (Cools, 2008; Ersche et al., 2010b), in part due to large individual differences in patient responses. Thus, a careful consideration of variation from subject to subject, behaviorally and neurobiologically, will be important for future development of targeted, patient-tailored interventions.
8. Conclusion
“Those who restrain desire do so because theirs is weak enough to be restrained.” (William Blake, The Marriage of Heaven and Hell, c. 1790–1793)
An important question arises from the exclamation of the character in Oscar Wilde’s play “Lady Windermere’s Fan”, quoted at the beginning of this article. That is, why do some individuals have great difficulty restraining desires aroused by temptations? Why are some people unable to stop eating when they feel full, or can never limit themselves to just one drink? William Blake, above, provides us with somewhat of an answer, in pointing out that the strength of such desires may not be the same for everyone. Scientifically, we are just beginning to understand the basis of individual variation in the ability to resist temptations for rewards, but we suggest that one important factor, of many likely factors, is the extent to which reward-associated cues acquire incentive motivational properties. In those for whom reward cues become powerful incentives, cues will evoke desires that are difficult to suppress, potentially motivating maladaptive patterns of reward seeking, and these individuals will therefore be more vulnerable to compulsive behavioral disorders, such as addiction.
Individuals exhibit large variation in the strength of their reward temptations
Reward cues acquire powerful motivational properties in only a subset of individuals
Mesolimbic dopamine is causally involved in regulating cue motivational value
“Cue reactive” individuals will be susceptible to developing binge eating and addiction
Acknowledgments
The research by the authors reviewed here was supported by National Institute on Drug Abuse grants to B.T.S. (F31 DA030801) and T.E.R. (R37 DA004294 and P01 DA031656). We thank Shelly Flagel and Paul Meyer for helpful comments. We dedicate this manuscript to Ann Kelley, who, in a career cut short by untimely death, contributed many foundational discoveries about the neuroscience of motivated behavior. Her work informs much of our current understanding about brain reward circuitry. All of us who knew her personally (TER) will long remember not only her contributions to science, but as someone who’s presence brightened any meeting or symposium she attended, because of her energetic, warm and delightful personality. She was, by any measure, a wonderful colleague and friend.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Benjamin T. Saunders, Email: btsaunde@umich.edu.
Terry E. Robinson, Email: ter@umich.edu.
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Frontiers in behavioral neuroscience. 2009:3. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Attwood AS, O'Sullivan H, Leonards U, Mackintosh B, Munafo MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103:1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci. 2012;13:514–514. doi: 10.1038/nrn3212-c1. [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics with the nucleus accumbens core and shell. J Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1253–1260. [PubMed] [Google Scholar]
- Balleine B. Asymmetrical interactions between thirst and hunger in Pavlovian-instrumental transfer. Q J Exp Psychol B. 1994;47:211–231. [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR. Low prefrontal PSA-NCAM confers risk for alcoholism-related behavior. Nat Neurosci. 2012 doi: 10.1038/nn.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking Dopamine Systems: A New GABA Master Structure for Mesolimbic and Nigrostriatal Functions. The Journal of Neuroscience. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Research. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Frazier CR, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci. 2012;6:49. doi: 10.3389/fnint.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon doparnine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behavioural Brain Research. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Graaf YGD, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. The Journal of Comparative Neurology. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations. In: Medin DL, editor. Psychology of Learning and Motivation: Advances in Research and Theory. Academic Press; San Diego: 2001. pp. 223–278. [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bindra D. Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychological Review. 1968;75:1–22. [Google Scholar]
- Bindra D. How adaptive behavior is produced: A perceptual-motivation alternative to response reinforcement. Behav Brain Sci. 1978;1:41–91. [Google Scholar]
- Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Earlbaum; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Bogdan R, Carré JM, Hariri AR, Cryan JF, Reif A. Toward a Mechanistic Understanding of How Variability in Neurobiology Shapes Individual Differences in Behavior. Curr Top Behav Neurosci. 2012;12:361–393. doi: 10.1007/7854_2011_182. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC. Reinforcement, expectancy and learning. Psychol Rev. 1972;79:394–409. [Google Scholar]
- Bray S, Rangel A, Shimojo S, Balleine B, O'Doherty JP. The neural mechanisms underlying the influence of pavlovian cues on human decision making. J Neurosci. 2008;28:5861–5866. doi: 10.1523/JNEUROSCI.0897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland K, Breland M. The misbehavior of organisms. American Psychologist. 1961;16:681–684. [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. The Journal of Comparative Neurology. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon's key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010a;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010b;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Miller DN, Schoenbaum G. Conditioned reinforcement can be mediated by either outcome-specific or general affective representations. Front Integr Neurosci. 2007;1:2. doi: 10.3389/neuro.07.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Miller DN, Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–344. doi: 10.1038/nature06993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M, Domjan M. Sign tracking versus goal tracking in the sexual conditioning of male Japanese quail (Coturnix japonica) J Exp Psychol Anim Behav Process. 1996;22:297–306. doi: 10.1037//0097-7403.22.3.297. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Sensory Pleasure. The Quarterly Review of Biology. 1979;54:1–29. doi: 10.1086/410981. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacology Biochemistry and Behavior. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M. Drug Addiction. New England Journal of Medicine. 2003;349:975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Reward without dopamine. Journal of Neuroscience. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carnell S, Wardle J. Appetitive traits in children. New evidence for associations with weight and a common, obesity-associated genetic variant. Appetite. 2009;53:260–263. doi: 10.1016/j.appet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, DeSantis S, Gray KM, LaRowe SD, Upadhyaya HP. Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addict Behav. 2009;34:536–541. doi: 10.1016/j.addbeh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the Rat Prefrontal Cortex to the Ventral Tegmental Area: Target Specificity in the Synaptic Associations with Mesoaccumbens and Mesocortical Neurons. The Journal of Neuroscience. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2012 doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O'Brien CP. Prelude to Passion: Limbic Activation by "Unseen" Drug and Sexual Cues. Plos One. 2008;3:7. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Cole J, Goudie A, Field M. Components of behavioural impulsivity and automatic cue approach predict unique variance in hazardous drinking. Psychopharmacology. 2012;219:501–510. doi: 10.1007/s00213-011-2396-z. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, Geffen GM, Geffen LB. Catecholamines and attention. II: Pharmacological studies in normal humans. Neurosci Biobehav Rev. 1987;11:353–364. doi: 10.1016/s0149-7634(87)80007-6. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Hollon NG, Phillips PE. Pavlovian valuation systems in learning and decision making. Curr Opin Neurobiol. 2012 doi: 10.1016/j.conb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD, Adamo SA. Cuttlefish (Sepia officinalis: Cephalopoda) hunting behavior and associative learning. Anim Cogn. 2005;8:27–30. doi: 10.1007/s10071-004-0228-9. [DOI] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology (Berl) 2012;219:123–135. doi: 10.1007/s00213-011-2382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA. Associations between the discriminative stimulus and the reinforcer in instrumental learning. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:155–164. [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008;14:381–395. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L, Janak P. Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of Pavlovian stimuli on instrumental responding. J Neurosci. 2007;27:13977–13981. doi: 10.1523/JNEUROSCI.4097-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26:3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiology & Behavior. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- Cortright JJ, Sampedro GR, Neugebauer NM, Vezina P. Previous Exposure to Nicotine Enhances the Incentive Motivational Effects of Amphetamine via Nicotine-Associated Contextual Stimuli. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P. Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology (Berl) 2007;192:231–241. doi: 10.1007/s00213-007-0704-4. [DOI] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson's disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacol Biochem Behav. 2010;96:40–47. doi: 10.1016/j.pbb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GC, Cleland GG. Topography of signal-centered behavior in the rat: Effects of deprivation state and reinforcer type. J Exp Anal Behav. 1982;38:291–304. doi: 10.1901/jeab.1982.38-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in initiation, maintenance and extinction of drug-seeking behavior. Pavlovian Journal of Biological Science. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4:5–10. [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual-differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug and Alcohol Dependence. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11:52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- DeJong W. Relapse prevention: an emerging technology for promoting long-term drug abstinence. Int J Addict. 1994;29:681–705. doi: 10.3109/10826089409047904. [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacology. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. European Journal of Neuroscience. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. Journal of Psychopharmacology. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behavioral Neuroscience. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology 47, Supplement. 2004;1:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. European Journal of Pharmacology. 2005;526:186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Dickinson A, Dawson GR. Pavlovian processes in the motivational control of instrumental performance. Quarterly Journal of Experimental Psychology B Comparative and Physiological Psychology. 1987;39:201–213. [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Difeliceantonio AG, Berridge KC. Which cue to 'want'? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res. 2012;230:399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behav Neurosci. 2011;125:661–667. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addictive Behaviors. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010a;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug Addiction Endophenotypes: Impulsive Versus Sensation-Seeking Personality Traits. Biol Psychiatry. 2010b;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK. Discriminative conditioning. I. A discriminative property of conditioned anticipation. Journal of Experimental Psychology. 1943;32:150–155. [Google Scholar]
- Estes WK. Discriminative conditioning. II. Effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. Journal of Experimental Psychology. 1948;38:173–177. doi: 10.1037/h0057525. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research Reviews. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM. Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend. 2009;101:137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Fantino E. Conditioned reinforcement: Choice and information. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Prentice-Hall; Englewood Cliffs, NJ: 1977. [Google Scholar]
- Fantino E, Case DA. Human observing: Maintained by stimuli correlated with reinforcement but not extinction. J Exp Anal Behav. 1983;40:193–210. doi: 10.1901/jeab.1983.40-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff IDC, Polivy J, Herman CP. The Effect of Pre-exposure to Food Cues on the Eating Behavior of Restrained and Unrestrained Eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl) 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Kiernan A, Eastwood B, Child R. Rapid approach responses to alcohol cues in heavy drinkers. J Behav Ther Exp Psychiatry. 2008;39:209–218. doi: 10.1016/j.jbtep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcohol. 2005;40:504–510. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011a;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011b;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: Influence on cocaine sensitization. Behavioural Brain Research. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. JAMA: The Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foll BL, Gallo A, Strat YL, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behavioural Pharmacology. 2009;20:1–17. doi: 10.1097/FBP.1090b1013e3283242f3283205. [DOI] [PubMed] [Google Scholar]
- Franken IH, Booij J, van den Brink W. The role of dopamine in human addiction: from reward to motivated attention. Eur J Pharmacol. 2005;526:199–206. doi: 10.1016/j.ejphar.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriks VM, Stam CJ, Van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol. 2004;14:503–508. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Franken IH, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addict Behav. 2000;25:99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O'Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TP, Morgan CJA, Beesley T, Curran HV. Drug cue induced overshadowing: selective disruption of natural reward processing by cigarette cues amongst abstinent but not satiated smokers. Psychological Medicine. 2012;42:161–171. doi: 10.1017/S0033291711001139. [DOI] [PubMed] [Google Scholar]
- Gamzu E, Schwam E. Autoshaping and automaintenance of a key-press response in squirrel monkeys. J Exp Anal Behav. 1974;21:361–371. doi: 10.1901/jeab.1974.21-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. The Journal of Comparative Neurology. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Tang AH. Behavior maintained under second-order schedules of intravenous morphine injection in squirrel and rhesus monkeys. Psychopharmacology. 1977;51:235–242. doi: 10.1007/BF00431630. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Honorio Carrillo J, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009;29:6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Krueger RF, Racette SB, Norberg KE, Hipp PR, Bierut LJ. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry. 2010;67:1301–1308. doi: 10.1001/archgenpsychiatry.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindalini C, Laranjeira R, Collier D, Messas G, Vallada H, Breen G. Dopamine-beta hydroxylase polymorphism and cocaine addiction. Behav Brain Funct. 2008;4:1. doi: 10.1186/1744-9081-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. European Journal of Neuroscience. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hall JF. Studies in secondary reinforcement: I. Secondary reinforcement as a function of the frequency of primary reinforcement. Journal of Comparative and Physiological Psychology. 1951;44:246–251. doi: 10.1037/h0056337. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins H. Monograph of the Psychonomic Society. Austin: 1974. Sign-tracking: The stimulus-reinforcer relation and directed action. [Google Scholar]
- Hebb DO. Drives and the C. N. S. (conceptual nervous system) Psychological Review. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacology Biochemistry and Behavior. 2009;93:270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. J Neurosci. 2010a;30:11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward guides vision when it's your thing: trait reward-seeking in reward-mediated visual priming. PLoS One. 2010b;5:e14087. doi: 10.1371/journal.pone.0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, van Zoest W. Reward creates oculomotor salience. Current Biology. 2012;22:R219–R220. doi: 10.1016/j.cub.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Hill RT. Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulation. In: Costa E, Garattini S, editors. Amphetamine and related compounds. Raven; New York: 1970. pp. 781–795. [Google Scholar]
- Hitchcott PK, Harmer CJ, Phillips GD. Enhanced acquisition of discriminative approach following intra-amygdala d-amphetamine. Psychopharmacology. 1997;132:237–246. doi: 10.1007/s002130050341. [DOI] [PubMed] [Google Scholar]
- Hitsman B, MacKillop J, Lingford-Hughes A, Williams TM, Ahmad F, Adams S, Nutt DJ, Munafo MR. Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology (Berl) 2008;196:611–621. doi: 10.1007/s00213-007-0995-5. [DOI] [PubMed] [Google Scholar]
- Hnasko T, Sotak B, Palmiter R. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Rankin H, Stockwell T. Alcohol dependence and the priming effect. Behaviour Research and Therapy. 1979;17:379–387. doi: 10.1016/0005-7967(79)90009-3. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Wright A, Kouvaraki M, Duka T. The role of drug expectancy in the control of human drug seeking. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:484–496. doi: 10.1037/0097-7403.33.4.484. [DOI] [PubMed] [Google Scholar]
- Holden JM, Peoples LL. Effects of acute amphetamine exposure on two kinds of Pavlovian approach behavior. Behav Brain Res. 2010;208:270–273. doi: 10.1016/j.bbr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34:1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of behavior: an introduction to behavior theory. Appleton-Century; Oxford, England: 1943. [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. The Journal of Comparative Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eating Behaviors. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Jenkins HM, Moore BR. The form of the auto-shaped response with food or water reinforcers. J Exp Anal Behav. 1973;20:163–181. doi: 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P, Volkow N. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Research Reviews. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Katz JL. A comparison of responding maintained under second-order schedules of intramuscular cocaine injection or food presentation in squirrel monkeys. J Exp Anal Behav. 1979;32:419–431. doi: 10.1901/jeab.1979.32-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT. Conditioned reinforcement in second-order schedules. J Exp Anal Behav. 1966;9:475–485. doi: 10.1901/jeab.1966.9-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Goldberg SR. Fixed-interval responding under second-order schedules of food presentation or cocaine injection. J Exp Anal Behav. 1977;28:221–231. doi: 10.1901/jeab.1977.28-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Gollub LR. A review of positive conditioned reinforcement. J Exp Anal Behav. 1962;5:543–597. doi: 10.1901/jeab.1962.5-s543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley A, Delfs J. Dopamine and conditioned reinforcement. Psychopharmacology. 1991;103:187–196. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004a;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews. 2004b;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology & Behavior. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJH. The amygdalostriatal projection in the rat: an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- Killeen PR. Complex dynamic processes in sign tracking with an omission contingency (negative automaintenance) J Exp Psychol Anim Behav Process. 2003;29:49–61. doi: 10.1037/0097-7403.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Individual differences in the priming effect of ethanol in social drinkers. J Stud Alcohol. 2000;61:64–71. doi: 10.15288/jsa.2000.61.64. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain: An interdisciplinary approach. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank MD, O'Neill S, Squarey K, Jacob J. Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 2008;196:397–405. doi: 10.1007/s00213-007-0971-0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kruse JM, Overmier JB, Konz WA, Rokke E. Pavlovian conditioned stimulus effects upon instrumental choice behavior are reinforcer specific. Learning and Motivation. 1983;14:165–181. [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lajoie J, Bindra D. An interpretation of autoshaping and related phenomena in terms of stimulus-incentive contingentcies alone. Canadian Journal of Psychology. 1976;30:157–173. [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF, Innis RB. SPECT imaging of striatal dopamine release after amphetamine challenge. Journal of Nuclear Medicine. 1995;36:1182–1190. [PubMed] [Google Scholar]
- Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT. Pavlovian-to-Instrumental Transfer in Cocaine Seeking Rats. Behavioral Neuroscience. 2012 doi: 10.1037/a0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BAS, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PEM. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012 doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang H-L, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Structure and Function. 2012:1–18. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Lindesmith A. Addiction and opiates. 2. Aldine; Chicago: 1968. [Google Scholar]
- Lloyd RW, Jr, Salzberg HC. Controlled social drinking: an alternative to abstinence as a treatment goal for some alcohol abusers. Psychol Bull. 1975;82:815–842. [PubMed] [Google Scholar]
- Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220:91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process. 1983;9:225–247. [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, Hester R, Smits M, Pepplinkhuizen L, Franken IH. Brain Activation Associated with Attentional Bias in Smokers is Modulated by a Dopamine Antagonist. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M, Franken IH. Neurobiological substrate of smoking-related attentional bias. Neuroimage. 2011;54:2374–2381. doi: 10.1016/j.neuroimage.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Mackintosh N. Overshadowing and stimulus intensity. Learning & Behavior. 1976;4:186–192. doi: 10.3758/bf03214033. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. Academic Press; London: 1974. [Google Scholar]
- Mahler SV, Berridge KC. Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- McClernon F, Hutchison K, Rose J, Kozink R. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology. 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Lucantonio F, Burke KA, Niv Y, Schoenbaum G. Ventral striatum and orbitofrontal cortex are both required for model-based, but not model-free, reinforcement learning. J Neurosci. 2011;31:2700–2705. doi: 10.1523/JNEUROSCI.5499-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012a;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012b;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltz H. Contemporary instinct theory and the fixed action pattern. Psychological Review. 1965;72:27–47. doi: 10.1037/h0020275. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Mannie ZN, Cowen PJ, Harmer CJ, McTavish SB. Effects of acute tyrosine depletion on subjective craving and selective processing of smoking-related cues in abstinent cigarette smokers. J Psychopharmacol. 2007;21:805–814. doi: 10.1177/0269881107077216. [DOI] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learning & Memory. 2006;13:123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- Nadler N, Delgado MR, Delamater AR. Pavlovian to instrumental transfer of control in a human learning task. Emotion. 2011;11:1112–1123. doi: 10.1037/a0022760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH, Smith GP, Faull RLM, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Kristiansen TS, Fosseidengen JE, Ferno A, van den Bos R. Sign- and goal-tracking in Atlantic cod (Gadus morhua) Anim Cogn. 2008;11:651–659. doi: 10.1007/s10071-008-0155-2. [DOI] [PubMed] [Google Scholar]
- Noble EP. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. European Psychiatry. 2000;15:79–89. doi: 10.1016/s0924-9338(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Novak M, Halbout B, O'Connor EC, Rodriguez Parkitna J, Su T, Chai M, Crombag HS, Bilbao A, Spanagel R, Stephens DN, Schutz G, Engblom D. Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci. 2010;30:11973–11982. doi: 10.1523/JNEUROSCI.2550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Crombag HS, Mead AN, Stephens DN. The mGluR5 antagonist MTEP dissociates the acquisition of predictive and incentive motivational properties of reward-paired stimuli in mice. Neuropsychopharmacology. 2010;35:1807–1817. doi: 10.1038/npp.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States--no statistically significant chance since 2003–2004. NCHS data brief. 2007:1–8. [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond Dopamine Release in the Nucleus Accumbens Predicts Conditioned Punishment and Its Successful Avoidance. The Journal of Neuroscience. 2012;32:14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in pavlovian but not instrumental conditioning. Journal of Neuroscience. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. Journal of Neuroscience. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37:508–519. doi: 10.1038/npp.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfai TP. Activating action tendencies: The influence of action priming on alcohol consumption among male hazardous drinkers. J Stud Alcohol. 2006;67:926–933. doi: 10.15288/jsa.2006.67.926. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology. 2012:1–13. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio L, Schindler C, Weiss S. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Nemeth-Coslett R, Katz JL, Henningfield JE, Solinas M, Heishman SJ, Schindler CW, Goldberg SR. Human cocaine-seeking behavior and its control by drug-associated stimuli in the laboratory. Neuropsychopharmacology. 2005;30:433–443. doi: 10.1038/sj.npp.1300599. [DOI] [PubMed] [Google Scholar]
- Paredes-Olay C, Abad M, Gámez M, Rosas J. Transfer of control between causal predictive judgments and instrumental responding. Learning & Behavior. 2002;30:239–248. doi: 10.3758/bf03192833. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc Natl Acad Sci U S A. 2010;107:13491–13496. doi: 10.1073/pnas.1007827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J, Roberts A, Everitt B, Di Ciano P. Acquisition of instrumental conditioned reinforcement is resistant to the devaluation of the unconditioned stimulus. Q J Exp Psychol B. 2005;58:19–30. doi: 10.1080/02724990444000023. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC. The role of the primate amygdala in conditioned reinforcement. J Neurosci. 2001;21:7770–7780. doi: 10.1523/JNEUROSCI.21-19-07770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behavioural Brain Research. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-Amphetamine. Journal of Neuroscience. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. European Journal of Neuroscience. 2000a;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: Further evidence for limbic cortical-ventral striatopallidal systems. Behavioral Neuroscience. 2000b;114:42–63. [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Dover; New York, NY: 1927. [Google Scholar]
- Pavlov IP. The reply of a physiologist to psychologists. Psychological Review. 1932;39:91–127. [Google Scholar]
- Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict Behav. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Perone M, Baron A. Reinforcement of human observing behavior by a stimulue correlated with extinction or increased effort. J Exp Anal Behav. 1980;34:239–261. doi: 10.1901/jeab.1980.34-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Heidbreder CA, Feldon J, Murphy CA. Selective responding of nucleus accumbens core and shell dopamine to aversively conditioned contextual and discrete stimuli. Neuroscience. 2001;108:91–102. doi: 10.1016/s0306-4522(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Setzu E, Hitchcott PK. Facilitation of appetitive Pavlovian conditioning by d-amphetamine in the shell, but not the core, of the nucleus accumbens. Behavioral Neuroscience. 2003a;117:675–684. doi: 10.1037/0735-7044.117.4.675. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003b;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Pielock SM, Lex B, Hauber W. The role of dopamine in the dorsomedial striatum in general and outcome-selective Pavlovian-instrumental transfer. European Journal of Neuroscience. 2011;33:717–725. doi: 10.1111/j.1460-9568.2010.07561.x. [DOI] [PubMed] [Google Scholar]
- Pithers RT. The roles of event contingencies and reinforcement in human autoshaping and omission responding. Learning and Motivation. 1985;16:210–237. [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology (Berl) 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Ventura R. Prefrontal/accumbal catecholamine system processes high motivational salience. Front Behav Neurosci. 2012;6:31. doi: 10.3389/fnbeh.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain–prefrontal cortex topography. The Journal of Comparative Neurology. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Raymond JE, O'Brien JL. Selective visual attention and motivation: the consequences of value learning in an attentional blink task. Psychol Sci. 2009;20:981–988. doi: 10.1111/j.1467-9280.2009.02391.x. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning - It's not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Skucy JC. Effect of response-independent reinforcers during extinction. Journal of Comparative and Physiological Psychology. 1969;67:381–389. [Google Scholar]
- Rescorla RA, Solomon RL. 2-Process learning theory - Relationships between Pavlovian conditioning and instrumental learning. Psychological Review. 1967;74:151. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Prefrontal Cortex Modulates Desire and Dread Generated by Nucleus Accumbens Glutamate Disruption. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2012.12.008. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs. A test of Hill's hypothesis. Psychopharmacology. 1975;45:103–114. [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functions of dopamine in the dorsal and ventral striatum. Sem Neurosci. 1992;4:119–127. [Google Scholar]
- Robinson S, Sandstrom S, Denenberg V, Palmiter R. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Roitman M, Wheeler R, Wightman R, Carelli R. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Richard JM. Shedding light on the role of ventral tegmental area dopamine in reward. J Neurosci. 2011;31:18195–18197. doi: 10.1523/JNEUROSCI.4924-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: Implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cue evokes relapse in the face of adverse consequences preferentially in rats prone to attribute incentive salience to reward cues. Society for Neuroscience Abstracts 2011a [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011b;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S, Gross LP. Manipulated time and eating behavior. Journal of Personality and Social Psychology. 1968;10:98–106. doi: 10.1037/h0026285. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology (Berl) 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. Nebraska symposium on motivation; 1959; Oxford, England: Univer. Nebraska Press; 1959. pp. 1–42. [Google Scholar]
- Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl) 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend. 2010;109:30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Williams DR. The role of the response-reinforcer contingency in negative automaintenance. J Exp Anal Behav. 1972;17:351–357. doi: 10.1901/jeab.1972.17-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Carter CS, Bruno RM, Cohen JD. Dopamine and the mechanisms of cognition: Part II. D-amphetamine effects in human subjects performing a selective attention task. Biol Psychiatry. 1998;43:723–729. doi: 10.1016/s0006-3223(97)00449-6. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiflett MW. The effects of amphetamine exposure on outcome-selective Pavlovian-instrumental transfer in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner T, Seymour B, Wunderlich K, Hill C, Bhatia KP, Dayan P, Dolan RJ. Dopamine and performance in a reinforcement learning task: evidence from Parkinson's disease. Brain. 2012;135:1871–1883. doi: 10.1093/brain/aws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich HC, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49:1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology (Berl) 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms: an experimental analysis. Appleton-Century; Oxford, England: 1938. [Google Scholar]
- Skinner BF. Superstition in the pigeon. J Exp Psychol. 1948;38:168–172. doi: 10.1037/h0055873. [DOI] [PubMed] [Google Scholar]
- Smith JW, Dickinson A. The dopamine antagonist, pimozide, abolishes Pavlovian-instrumental transfer. Journal of Psychopharmacology. 1998;12:A6. [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic hotspots: Generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 62–73. [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Visser SL, Op de Coul AA, De Sonneville LM, Schellens RL, Brunia CH, de Smet JS, Gielen G. Disturbed frontal regulation of attention in Parkinson's disease. Brain. 1993;116 ( Pt 5):1139–1158. doi: 10.1093/brain/116.5.1139. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci. 2012;32:10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavorial medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973;27:168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- Styn MA, Bovbjerg DH, Lipsky S, Erblich J. Cue-induced cigarette and food craving: A common effect? Addictive Behaviors. 2012 doi: 10.1016/j.addbeh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS. Learning to predict by the methods of temporal differences. Mach Learn. 1988;3:9–44. [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Wilson RC, Toreson K, O'Donnell P, Niv Y, Schoenbaum G. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat Neurosci. 2011;14:1590–1597. doi: 10.1038/nn.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Seymour B, Dayan P, Dolan R. Human pavlovian-instrumental transfer. J Neurosci. 2008;28:360–368. doi: 10.1523/JNEUROSCI.4028-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper K, Pothos EM, Lawrence AD. Feast your eyes: Hunger and trait reward drive predict attentional bias for food cues. Emotion. 2010;10:949–954. doi: 10.1037/a0020305. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52:614–620. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Thewissen R, Havermans RC, Geschwind N, van den Hout M, Jansen A. Pavlovian conditioning of an approach bias in low-dependent smokers. Psychopharmacology (Berl) 2007;194:33–39. doi: 10.1007/s00213-007-0819-7. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. Annals of the New York Academy of Sciences. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. An ecological approach to learning. Learning and Motivation. 1984;15:321–333. [Google Scholar]
- Timberlake W, Lucas GA. The basis of superstitious behavior: chance contingency, stimulus substitution, or appetitive behavior? J Exp Anal Behav. 1985;44:279–299. doi: 10.1901/jeab.1985.44-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Pecina S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: "wanting" what was never "liked". J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toates F. Motivational Systems. Cambridge University Press; Cambridge, UK: 1986. [Google Scholar]
- Tomer R. Attentional bias as trait: correlations with novelty seeking. Neuropsychologia. 2008;46:2064–2070. doi: 10.1016/j.neuropsychologia.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein R, Wang G, Wong C, Volkow N. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neuroscience and Biobehavioral Reviews. 1996;20:505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl) 1998;139:376–382. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacology Biochemistry and Behavior. 2000;65:509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Lincks M, Nadarajah SD, Pohorecky LA, Yu L. Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behav Brain Res. 2012;226:571–578. doi: 10.1016/j.bbr.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychological Review. 1969;76:264–281. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2012 doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behavioural Brain Research. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Vansteenwegen D, Van den Bergh O, Beckers T. Conditioned craving cues elicit an automatic approach tendency. Behaviour Research and Therapy. 2008;46:1160–1169. doi: 10.1016/j.brat.2008.05.010. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg M, Aarts E, Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des. 2010;16:2026–2032. doi: 10.2174/138161210791293097. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.01.018. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Telang F, Fowler J, Logan J, Childress A, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, Burr G, Cooper T, Wolf AP. Imaging endogenous dopamine competition with [ 11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma YM, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: Relevance to addiction. Journal of Neuroscience. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wassum K, Ostlund S, Maidment N, Balleine B. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT. Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol Psychiatry. 2012;71:846–854. doi: 10.1016/j.biopsych.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Lohoff FW, Ehrman R, O'Brien CP, Childress AR, Franklin TR. Neural correlates of attentional bias for smoking cues: modulation by variance in the dopamine transporter gene. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence: Implications of a conditioning theory for research and treatment. Archives of General Psychiatry. 1973;28:611. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wilcove WG, Miller JC. CS-USC presentations and a lever: human autoshaping. J Exp Psychol. 1974;103:868–877. doi: 10.1037/h0037388. [DOI] [PubMed] [Google Scholar]
- Williams DR, Williams H. Auto-maintenance in the pigeon: sustained pecking despite contingent non-reinforcement. J Exp Anal Behav. 1969;12:511–520. doi: 10.1901/jeab.1969.12-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Woods JH, Schuster CR. Opiates as reinforcing stimuli. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. Appleton-Century-Crofts; New York: 1971. [Google Scholar]
- Wyckoff LB. The role of observing responses in discrimination learning. Part I. Psychological Review. 1952;59:431–442. doi: 10.1037/h0053932. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: Increased cue-triggered "wanting" for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Zhuang X, Balleine B. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem. 2006;85:283–288. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Young PT. The role of affective processes in learning and motivation. Psychological Review. 1959;66:104–125. doi: 10.1037/h0045997. [DOI] [PubMed] [Google Scholar]
- Young PT. Hedonic organization and regulation of behavior. Psychological Review. 1966;73:59–86. doi: 10.1037/h0022630. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional anatomical macrosystems. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Zener K. The significance of behavior accompanying conditioned salivary secretion for theories of the conditioned response. American Journal of Psychology. 1937;50:384–403. [Google Scholar]
- Zhang J, Berridge KC, Aldridge JW. Computational models of incentive-sensitization in addiction: Dynamic limbic transformation of learning into motivation. In: Gutkin B, Ahmed SH, editors. Computational Neuroscience of Drug Addiction. Springer; New York, NY: 2012. pp. 189–203. [Google Scholar]
- Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW. A neural computational model of incentive salience. PLoS Comput Biol. 2009;5:e1000437. doi: 10.1371/journal.pcbi.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LY, Tian J, Wang W, Qin W, Shi J, Li Q, Yuan K, Dong MH, Yang WC, Wang YR, Sun LL, Lu L. The Role of Dorsal Anterior Cingulate Cortex in the Regulation of Craving by Reappraisal in Smokers. PLoS One. 2012;7:e43598. doi: 10.1371/journal.pone.0043598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18:262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]