Abstract
Altered maternal nutrition and metabolism, restricted utero-placental blood flow and other perturbations in the maternal compartment may disturb critical periods of fetal development resulting in increased susceptibility to develop disease in childhood and adult life. In response to these perturbations, placental structure and function changes, which influence the supply of nutrients, oxygen and methyl donors and alter the secretion of hormones and other signaling molecules into the fetal circulation. Thus, the placenta plays a critical role in modulating maternal-fetal resource allocation, thereby affecting fetal growth and the long-term health of the offspring.
Keywords: Maternal-fetal exchange, nutrient transport, trophoblast, pregnancy, fetal programming
Introduction
Genes and adult life style factors were long believed to be the predominant risk factors for the development of type-2 diabetes and cardiovascular disease. However, a large body of epidemiological data has recently challenged this paradigm by demonstrating that adverse influences during early development, in particular in utero, increase the risk to develop disease in adult life (1). This concept, known as “fetal programming” or “developmental origins of health and disease”, have profound impact on public health strategies for the prevention of major illnesses. A link between an adverse intrauterine environment and subsequent metabolic and cardiovascular health was pioneered by Barker and colleagues who reported associations between low birth weight and the risk to develop type-2 diabetes and cardiovascular disease (1). These epidemiological findings are strongly supported by experimental studies in a wide range of animal models (1, 2). The most commonly used approach to perturb intrauterine environment has been to alter maternal nutrition during pregnancy, such as feeding animals a low protein diet, which has been shown to result in various degrees of disturbance in glucose metabolism and cardiovascular function in the offspring. In addition to altered maternal nutrition, a number of other perturbations of maternal physiology, including administration of corticosteroids or cytokines and experimental reduction of uterine blood flow, have been shown to lead to fetal programming of diabetes and/or hypertension.
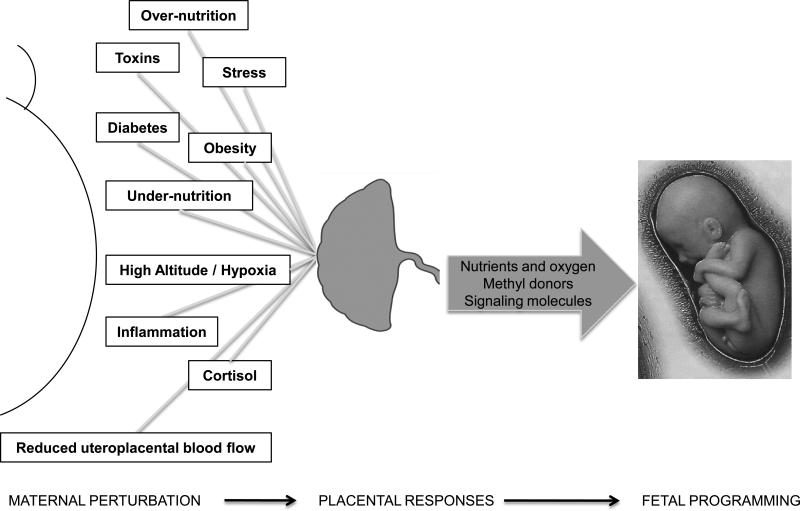
The placenta constitutes the active interface between the maternal and fetal blood circulations and is responsible for a multitude of functions critical for fetal development, including nutrient transport, hormone production and providing an immunological barrier. Perturbations in the maternal compartment, such as hypoxia, stress, obesity, diabetes, toxins, altered nutrition, cytokine and cortisol levels and reduced utero-placental blood flow must be transmitted across the placenta in order to affect the fetus (Fig 1). Emerging evidence suggests that these disturbances in maternal physiology influence placental structure and function leading to changes in the supply of nutrients, oxygen and methyl donors as well as alterations in the secretion of hormones and other signaling molecules into the fetal circulation. Thus, the placenta responds to and modulates perturbations in the maternal environment, thereby playing a key role in transmitting the programming stimuli to the fetus (Fig 1). A better understanding of how the placenta responds to changes in the maternal environment will help unravel the molecular mechanisms underlying fetal programming and will be crucial in the efforts to design strategies for intervention. After briefly reviewing the role of altered placental structure and 11-β-HSD-2 activity in determining the risk of adult disease we will discuss the emerging concept of placental nutrient sensing and how this process is linked to developmental programming. This review is focused on human pregnancy, with discussion of animal models when human data is lacking and when needed to decipher human biology.
Fig 1. The critical role of the placenta in fetal programming.
Perturbations in the maternal compartment may disturb critical periods of fetal development, resulting in increased susceptibility to develop disease in adult life (fetal programming). Placental structure and function changes in response to these perturbations, which influences the supply of nutrients, oxygen and methyl donors and alters the secretion of hormones and other signaling molecules into the fetal circulation. Thus, placental responses to restricted utero-placental blood flow or altered maternal nutrition and metabolism may directly contribute to and/or mediate programming of the fetus for future disease.
The link between placental structure and adult disease
Intrauterine growth restriction (IUGR) is often associated with an increased resistance to blood flow in the umbilical artery. Thornburg and colleagues have proposed that this subjects the fetal heart to an increased pressure work load, which leads to an adaptation that may be advantageous in the short-term perspective but could contribute to cardiovascular disease postnatally (3). Thus, the increased placental vascular resistance in IUGR may represent alterations in placental structure that are directly involved in fetal programming of cardiovascular disease (3). Furthermore, recent epidemiological data from follow-up studies of individuals that were exposed to varying degrees of nutrient restriction in utero during the Dutch Famine 1944-1945 (4) and of individuals born in Helsinki, Finland between 1934 and 1944 have demonstrated that placental shape and dimensions are linked to later disease. For example, studies of the Helsinki Birth Cohort have revealed that the relationship between the length and breath of the placenta predict coronary heart disease (5). However, the underlying mechanisms are not known.
Placental 11-β-HSD-2 and developmental programming
Administration of glucocorticoids to pregnant animals and humans causes IUGR and offspring exposed to excess glucocorticoids in utero have an increased risk of developing hypertension and diabetes in adult age. This may not be a direct effect on the fetus, because studies in the sheep show that giving corticosteroids directly to the fetus does not result in IUGR, suggesting that the placenta mediates, at least in part, the effects of corticosteroids on fetal growth. Consistent with this hypothesis, administration of dexamethasone to pregnant rats decreased placental amino acid transport, which may contribute to the restricted fetal growth by limiting amino acid supply.
Circulating levels of cortisol are markedly higher in the mother than in the fetus. This concentration difference is believed to be maintained by placental 11-β hydroxysteroid dehydrogenase type-2, which forms a functional barrier restricting the free transfer of cortisol between the maternal and fetal compartments by converting cortisol to its much less active 11-keto form, cortisone. It has been proposed that attenuation of placental 11β-HSD-2 activity may expose the placenta and fetus to inappropriately high levels of corticosteroids and result in IUGR and fetal programming of adult disease (6). This hypothesis is supported by associations observed between human placental 11β-HSD-2 expression and/or activity and birth weight in several studies. However, placental specific inhibition of 11β-HSD-2 is needed in order to unequivocally determine a cause and effect relationship between low placental 11β-HSD-2 activity and reduced fetal growth. Nevertheless, decreased 11β-HSD-2 activity, resulting in dysfunction in the placental glucocorticoid barrier and exposure of the placenta and fetus to excess corticosteroids, constitutes a potential direct link between altered placental function and fetal programming.
Syncytiotrophoblast function and fetal development
The syncytiotrophoblast (ST) is the transporting epithelium of the human placenta mediating maternal-fetal exchange of respiratory gases, nutrients and waste products. In addition, ST is the predominant source of placental hormone production. There are only two cell layers separating the fetal and maternal circulations in the term human placenta; the fetal capillary endothelium and the ST (Fig 2) and these two cell layers constitute the placental barrier in late pregnancy. Fetal placental capillaries are of the continuous type, allowing the unrestricted passage of molecules of the size of glucose and amino acids between cells through intercellular spaces but restricting the transfer of large molecules such as immunoglobulins. Thus, it is the syncytiotrophoblast cell layer and in particular its two polarized plasma membranes, the microvilllous (MVM) and basal plasma membranes (BM), that constitute the actual barrier for the transfer of molecules such as glucose and amino acids. This provides the rationale for isolating these plasma membranes to study their transport characteristics in vitro.
Fig 2. A model for placental transport of neutral amino acids.
The uptake of neutral amino acids from the maternal circulation across the microvillous plasma membrane (MVM) into the syncytiotrophoblast represents the active step of amino acid transport and is largely mediated by the System A and System L amino acid transporters. System A is predominantly expressed in the MVM and transport non-essential neutral amino acids (NEAA) against a concentration gradient energized by the inwardly directed Na+-gradient. System L is an exchanger, which uses the steep outwardly directed concentration gradient of some NEAAs to drive uptake of essential amino acids (EAA) against their concentration gradients. In all these cases, the energy for the uphill transport is ultimately generated by the Na+K+-ATPase. Amino acids are transferred across the basal plasma membrane (BM) by facilitated diffusion driven by the outwardly directed concentration gradient mediated by System L and efflux transporters. ST, syncytiotrophoblast; N, nucleus; ET, endothelial cells of the fetal capillary.
ST function is critical for fetal development and growth because this cell layer determines fetal nutrient supply and secretes an array of hormones and signaling molecules into the maternal circulation, which are essential for normal physiological adaptation of the mother to pregnancy. Moreover, this cell layer is interjected between the maternal and fetal circulations and is therefore ideally positioned to integrate fetal and maternal signals with information from trophoblast nutrient sensing pathways to modulate placental growth and function in response to changes in maternal nutrient supply. This process, which we have called placental nutrient sensing, is described in more detail below.
Regulation of placental nutrient transporters is of particular importance for the placental response to altered maternal nutrient supply. A large number of transporters for glucose, amino acids, fatty acids, ions, and micronutrients are expressed and active in the MVM and BM (reviewed in (7)). In this brief review we will, for the most part, limit our discussion to two key amino acid transporter systems, System A and System L. The justification for this focus is that amino acid availability is an important determinant of fetal growth, these transporters have been reported to be regulated in association to altered fetal growth in the human and in animal models and the molecular mechanisms regulating these transporters have been studied in some detail.
System A is a sodium-dependent transporter mediating the uptake of non-essential neutral amino acids into the cell. All three known isoforms of System A, SNAT1 (SLC38A1), SNAT2 (SLC38A2), and SNAT4 (SLC38A4) are expressed in the placenta. System A activity establishes the high intracellular concentration of non-essential neutral amino acids, which is also used to exchange for extracellular essential amino acids via System L. Thus, System A activity is critical for placental transport of both non-essential and essential amino acids. System L is a sodium-independent amino acid exchanger mediating cellular uptake of essential amino acids including leucine. The System L amino acid transporter is a heterodimer, consisting of a light chain, typically LAT1 (SLC7A5) or LAT2 (SLC7A8), and a heavy chain, 4F2hc/CD98 (SLC3A2). System A is predominantly expressed in the MVM, whereas System L is localized in both ST plasma membranes (Fig 2). Maternal hormones such as insulin, leptin, IGF-I as well as trophoblast mammalian target of rapamycin (mTOR) signaling are important positive regulators of placental amino acid transport, whereas cortisol inhibits placental amino acid transport in vivo (reviewed in (7)).
Placental responses to decreased maternal nutrient supply
Placental transport functions have been studied in human IUGR associated with “placental insufficiency”, a condition believed to be linked to an inability of utero-placental blood flow to increase normally with advancing gestation. Whereas placental glucose transport capacity has been reported to unaltered in IUGR, System A activity has consistently been shown to be lower in MVM isolated from IUGR placentas as compared to controls (reviewed in (8)). Importantly, the most severe cases of IUGR, as defined by abnormal pulsatility index in the umbilical artery and abnormal fetal heart rate tracings, were associated with the most pronounced decreases in MVM System A activity. The activities of transporters of essential amino acids, including System β (transporting taurine) and System L are reduced in MVM and/or BM isolated from IUGR placentas (reviewed in (8)). These in vitro findings are consistent with studies in pregnant women using stable isotopes demonstrating that placental transfer of the essential amino acids leucine and phenylalanine is reduced in IUGR at term. In addition, a reduced placental capacity to transport amino acids is in agreement with studies showing reduced concentrations of amino acids, in particular essential amino acids, in the umbilical vein of IUGR fetuses. Moreover, the activity of MVM lipoprotein lipase (LPL), which mediates the first critical step in transplacental transfer of free fatty acids, is reduced in IUGR. These findings are in line with clinical studies showing lower fetal/maternal plasma ratios for long-chain polyunsaturated fatty acids (LCPUFAs) in IUGR.
Placental nutrient transport functions in response to decreased maternal nutrient supply have been studied using different approaches in a range of animal models. Reduced utero-placental blood flow induced by uterine artery ligation in the rat and the guinea pig resulted in IUGR and decreased transplacental transport of glucose and amino acids in vivo (reviewed in (9)). There are numerous approaches to induce IUGR in the sheep. A model involving exposure of the ewe to high ambient temperature, which decreases utero-placental blood flow and placental growth resulting in asymmetric IUGR, resembles placental insufficiency in humans. The placental capacity to transport glucose and essential amino acids is reduced in this IUGR model (reviewed in (10). A number of studies in the rat, employing in vivo measurements of transplacental transfer of isotope-labeled substrate analogues, have shown that placental capacity to transport neutral amino acids and glucose is decreased in late pregnancy in response to calorie or protein restriction. Protein restriction in pregnant rats have been shown to decrease the in vitro activity of specific placental amino acid transporters close to term and the decreased placental System A transporter activity preceded the occurrence of IUGR (11). These findings suggest that, in this model, decreased placental amino acid transport is a cause of IUGR, rather than a consequence. Taken together, studies in a range of animal models show that placental nutrient transport is down regulated in IUGR associated with utero-placental insufficiency or maternal nutrient restriction.
Placental responses to increased maternal nutrient supply
Maternal obesity and diabetes are two common clinical conditions that may be associated with an increased maternal nutrient supply to the placenta. In this review we will limit our discussion to studies of fetal overgrowth in maternal obesity and/or diabetes. System A, but not System L, amino acid transport activity was shown to be increased in MVM isolated from placentas of obese Swedish women giving birth to large babies (12). In addition, MVM System A transporter activity in Swedish women with type-1 diabetes, independent of fetal overgrowth, and placental transport of leucine was increased in gestational diabetes (GDM) (13). In another population, however, System A amino acid transport activity was reduced and System L transport activity unaltered in MVM isolated from pregnancies with type-1 diabetes and fetal overgrowth (14). These discrepant findings may be related to differences in methodology or study populations.
Glucose transport activity and expression in the BM are increased in type-1 diabetes (15). Maternal lipoproteins are the predominant source for fetal fatty acids (FFA). Triglyceride hydrolases, such as lipoprotein lipase (LPL) and endothelial lipase (EL) expressed in the MVM release FFA from maternal lipoproteins, and FFA are subsequently transported across the placental barrier mediated by plasma membrane fatty acid transporters (FATP) and cytosolic fatty acid binding proteins (FABP). The activity of placental LPL has been reported to be increased in type-1 diabetes associated with fetal overgrowth (16). In addition, FABP1 protein expression was reported to be up-regulated in the placenta of both GDM and type-1 diabetic women giving birth to large babies (16). Collectively, these findings suggest an increased placental capacity to supply amino acids, glucose and lipids to the fetus in maternal obesity and diabetes associated with fetal overgrowth. However, data is less consistent than for IUGR, in particular with respect to amino acid transport.
There are currently only a few animal models of maternal obesity or diabetes that consistently are associated with fetal overgrowth and in which placental nutrient transport has been explored. In one mouse model of high fat diet resulting in increased maternal adiposity and fetal overgrowth, placental glucose and amino acid transporter expression and in vivo transplacental transport of glucose and amino were reported to be increased (17).
Placental nutrient sensing and maternal nutritional cues
Nutrient restriction in humans and animal models decreases circulating levels of IGF-I, leptin and insulin and increases maternal serum adiponectin concentrations. Maternal obesity, representing a condition of maternal over nutrition, is typically associated with increased insulin, IGF-I and leptin concentrations in the maternal circulation and decreased levels of circulating levels of adiponectin. Thus, the levels of these hormones in the maternal circulation will convey critical information to the placenta with respect to the ability of the maternal supply line to allocate nutrients to fetal growth. Importantly, receptors for most of these hormones are highly expressed on the maternal-facing plasma membrane of the ST and therefore directly accessible to maternal blood, suggesting that maternal hormones regulate trophoblast function. Because IGF-I, insulin and leptin stimulate and adiponectin inhibits the activity of trophoblast amino acid transporters (reviewed in (7)) these hormones provide a direct link between maternal nutritional status and placental nutrient transport. In addition, maternal cortisol increases in stress and maternal undernutrition, which may contribute to decreased placental nutrient transport and restricted fetal growth in these conditions because corticosteroids have been shown to down regulate placental amino acid transport in animals models.
Placental nutrient sensing and mTOR signaling
Trophoblast cells have an array of nutrient-sensing signaling pathways, including AMP-activated protein kinase (AMPK), amino acid response (AAR) signal transduction pathway, mammalian target of rapamycin complex 1 (mTORC1), Glycogen synthase-3 (GSK-3) and the hexosamine signaling pathway, which regulate cell metabolism in response to altered nutrient levels. Of these nutrient sensors, the evidence supporting a critical role for mTORC1 in placental nutrient sensing is particularly compelling. mTORC1 is a serine/threonine kinase, which controls cell growth, proliferation and metabolism in response to nutrient availability and growth factor signaling. mTOR is highly expressed in the ST of the human placenta and mTORC1 in cultured primary human trophoblast cells is regulated by glucose and AA concentrations as well as by growth factor signaling.
The first line of evidence consistent with an important role for mTORC1 in placental nutrient sensing is that mTORC1 has a multitude of upstream regulators, including free fatty acids, amino acids, glucose, ATP and oxygen. It is likely that the placental concentrations of some of these nutrients are changed in conditions such as placental insufficiency, maternal under nutrition or obesity. For example, phosphorylation of placental AMPK is markedly decreased, indicating high ATP levels, in maternal obesity (12). Moreover, the majority of cases of idiopathic normotensive IUGR have been reported to have placental hypoxic-ischemic histological lesions, consistent with placental hypoxia. mTOR is activated by insulin and IGF-I and placental insulin/IGF-I signaling is inhibited in IUGR. In addition, leptin signaling activates and cortisol inhibits mTOR. Thus, mTOR signaling links maternal metabolic hormones and local nutrient levels to placental growth and function.
The second argument to support an important role for mTORC1 in placental nutrient sensing is that mTORC1 regulates trophoblast amino acid transport. Nutrient transport is a primary function of the ST and nutrient uptake by this cell not only determines placental growth but also has a major influence on fetal nutrient availability and growth. Using cultured primary human trophoblast cells we reported that mTORC1 is a positive regulator of the System A and System L amino acid transporters, which are critical for the transport of EAAs to the fetus (18). Thus, mTORC1 signaling links maternal nutrient availability to fetal growth by modulating the flux of amino acids across the placenta.
A third line of evidence consistent with an important role for mTORC1 signaling in placental nutrient sensing is that the activity of this signaling pathway is altered in pregnancy complications associated with abnormal fetal growth and in animal models where maternal nutrient availability has been altered experimentally. Placental mTORC1 activity is inhibited in human IUGR and activated in placentas of large babies born to obese mothers. Furthermore, placental mTORC1 activity has been reported to be decreased in hyperthermia-induced IUGR in the sheep, in response to a maternal low protein diet in the rat and maternal calorie restriction in the baboon. It is well established that circulating adiponectin is inversely correlated to BMI, thereby representing an important nutritional cue carrying information on the maternal nutritional status. Interestingly, maternal adiponectin inhibits placental mTORC1 signaling, which may represent an endocrine link between maternal adipose tissue, placental function and fetal growth (19).
Placental nutrient sensing: An integrated model for the regulation of placental function
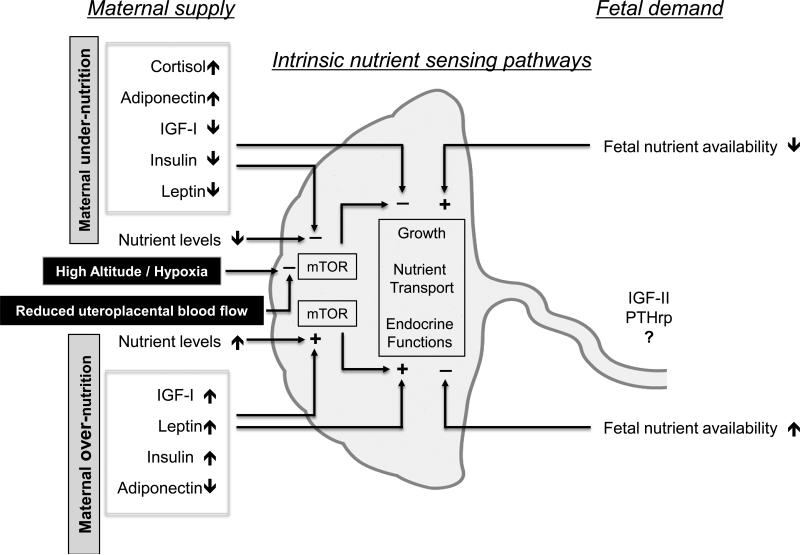
We have proposed a model (“maternal supply model”) in which maternal signals are important regulators of placental transport in response to changes in maternal nutrient supply, which (when defined broadly) also include compromised utero-placental blood flow and hypoxia (Fig 3). In this model (8, 9), the placenta responds to maternal nutritional cues, resulting in down-regulation of placental nutrient transporters in response to maternal under-nutrition or restricted utero-placental blood flow. Therefore, fetal nutrient availability becomes limited and fetal growth decreases. The maternal supply model therefore represents a mechanism by which fetal growth is matched to the ability of the maternal supply line to allocate resources to the fetus. In this model, changes in placental growth and nutrient transport directly contribute to, or cause, alterations in fetal growth. Maternal signals conveying nutritional information to the placenta may include metabolic hormones such as insulin, cortisol, leptin, and adiponectin, which are known to be influenced by maternal nutrition and regulate placental transport (Fig 3). Other signals proposed to be sensed by the placenta are oxygen and nutrient levels.
Fig 3. Placental nutrient sensing.
The placenta integrates maternal and fetal nutritional signals with information from intrinsic nutrient sensors such as mammalian target of rapamycin (mTOR) signaling. This process, which we call placental nutrient sensing, results in a coordinated response, including altered placental growth, nutrient transport and endocrine functions, to balance fetal demand with the ability of the mother to support pregnancy. Thus, the placenta plays a critical role in modulating maternal-fetal resource allocation, thereby affecting fetal growth and the long-term health of the offspring. See text for detailed explanation. IGF-II, insulin-like growth factor II; PTHrp, parathyroid hormone related peptide. Adapted from (9) with permission from Cambridge University Press.
However, there is a fundamentally distinct model for how placental transporters are regulated in response to changes in the ability of the maternal supply line to deliver oxygen and nutrients to the placenta. The fetal demand model, which is predominantly based on elegant mouse studies, postulates that placental function is primarily controlled by fetal demand (reviewed in (20)). In response to maternal under-nutrition or restricted utero-placental blood flow, resulting in decreased placental transfer and limited fetal nutrient availability, the fetal demand model predicts that the fetus signals to the placenta to up-regulate placental growth and nutrient transport. This model represents a classical homeostatic mechanism by which the fetus compensates for changes in nutrient availability by regulating nutrient supply (i.e., placental transport) in the opposite direction, thereby correcting for deficiencies.
As discussed previously, key placental transporters for amino acids, lipids and ions are, in general, down-regulated in human IUGR, which is inconsistent with a homeostatic or fetal demand model for regulation of placental transport. However, most of these studies were performed at term and it cannot be excluded that compensatory changes consistent with fetal demand signals are present earlier in pregnancy, as has been shown in mouse models of IUGR. Most published data indicate that placental capacity to transport free fatty acids and, possibly, glucose is increased in diabetic women, particularly in cases of fetal overgrowth. Thus, the available information from IUGR and fetal overgrowth in humans is in general agreement with the maternal supply model for regulation of placental transport.
We propose an integrated model for the regulation of placental function accounting for both the maternal supply and fetal demand models (Fig 3). The key feature in this integrated model is placental nutrient sensing where the placenta integrates a multitude of maternal and fetal nutritional cues with information from intrinsic nutrient sensing signaling pathways to balance fetal demand with the ability of the mother to support the pregnancy by regulating maternal physiology, placental growth and nutrient transport (Figure 3) (9). As discussed previously, there is compelling evidence to suggest that trophoblast mTOR signaling is one important component of the placental nutrient sensor. We propose that these mechanisms have evolved due to the evolutionary pressures of maternal under-nutrition. Although these regulatory loops may function in the “reverse” direction in response to over-nutrition, it is possible that these responses may not be as readily apparent in maternal obesity or diabetes as in response to maternal under-nutrition. Matching fetal growth to maternal resources in response to maternal under-nutrition will produce an offspring that is smaller in size but who, in most instances, will survive and be able to reproduce.
Conclusions and future perspectives
Long-term health is critically dependent on the availability of nutrients during fetal life, which is determined by placental transport. Our understanding of the role of the placenta in fetal nutrition has evolved from the view that the placenta constitutes a selective passive filter to the recognition that the placenta adapts to changes in maternal nutrition by responding to maternal nutritional cues, fetal demand signals and intrinsic nutrient sensing signaling pathways. The complexity of these regulatory pathways is only beginning to be appreciated as placental nutrient sensing. A better understanding of the molecular mechanisms involved in placental nutrient sensing may help to identify critical links between maternal nutrition, fetal growth and developmental programming. In addition, this knowledge is essential when designing novel intervention strategies. However, our current understanding of these processes is limited, at best, presenting great challenges and opportunities for the future. For example, there is a lack of information on the (1) molecular identity of fetal demand signals, (2) the mechanisms by which lipids are transported across the placenta and the role of placental lipid transport in programming of obesity and diabetes, (3) how multiple placental nutrient sensing signaling pathways are integrated, and (4) how signals from the placenta to the mother influence maternal-fetal resource allocation.
Acknowledgments
sources of funding: This work was supported by NIH grants HD065007, HD068370, DK089989 and HD071306.
Footnotes
Conflicts of interest The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 2.Armitage JA, Khan IY, Taylor PD, et al. Developmental programming of metabolic syndrome by maternal nutritional imbalance; how strong is the evidence from expermental models in animals. J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornburg KL, Louey S. Fetal roots of cardiac disease. Heart. 2005;91:867–868. doi: 10.1136/hrt.2004.047407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Abeelen AF, de Rooij SR, Osmond C, et al. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011;32:694–698. doi: 10.1016/j.placenta.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson JG, Kajantie E, Thornburg KL, et al. Mother's body size and placental size predict coronary heart disease in men. Eur Heart J. 2011;32:2297–303. doi: 10.1093/eurheartj/ehr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards CRW, Benediktsson R, Lindsay R, et al. Dysfunction of the placental glucocorticoid barrier: a link between the foetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 7.Lager S, Powell TL. Regulation of transport across the placenta. J Pregnancy. 2012 doi: 10.1155/2012/179827. Epub 2012 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human Placental Transport in Altered Fetal Growth: Does the Placenta Function as a Nutrient Sensor? - A Review. Placenta. 2006;27(SupplA):S91–97. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Gaccioli F, Lager S, Powell TL, et al. Placental transport in response to altered maternal nutrition. J DOHaD. 2013;4:101–115. doi: 10.1017/S2040174412000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnault TRH, Friedman JE, Wilkening RB, et al. Fetoplacental transport and utilization of amino acids in IUGR- A review. Placenta. 2005;26(Suppl A):S52–S62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Jansson N, Pettersson J, Haafiz A, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–46. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson N, Rosario FJ, Gaccioli F, et al. Activation of Placental mTOR Signaling and Amino Acid Transporters in Obese Women Giving Birth to Large Babies. J Clin Endocrinol Metab. 2013;98:105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson T, Ekstrand Y, Björn C, et al. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 14.Kuruvilla AG, D'Souza SW, Glazier JD, et al. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest. 1994;94:689–695. doi: 10.1172/JCI117386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin dependent diabetes. Am J Obstet Gynecol. 1999;180:163–168. doi: 10.1016/s0002-9378(99)70169-9. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson AL, Waterman IJ, Wennergren M, et al. Triglyceride hydrolase activities and expression of fatty acid binding proteins in human placenta in pregnancies complicated by IUGR and diabetes. J Clin Endocrinol Metab. 2004;89:4607–4614. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- 17.Jones HN, Woollett LP, Barbour N, et al. High fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/Bl6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosario FJ, Kanai Y, Powell TL, et al. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591:609–625. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosario FJ, Schumacher MA, Jiang J, et al. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590:1495–1509. doi: 10.1113/jphysiol.2011.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibley CP, Brownbill P, Dilworth M, et al. Review: Adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta. 2010;31(Suppl):S70–4. doi: 10.1016/j.placenta.2009.12.020. [DOI] [PubMed] [Google Scholar]