Abstract
Background
Induction onto buprenorphine during pregnancy may be more challenging than induction onto methadone. This study explores factors predicting withdrawal intensities and compares trajectories of withdrawal during the induction phase between opioid-dependent women randomly assigned to methadone or buprenorphine.
Methods
A secondary analysis was conducted on data from 175 opioid-dependent pregnant women inducted onto buprenorphine or methadone subsequent to stabilization on morphine sulfate. ANOVA analyses were conducted to determine differences between mean peak CINA scores by medication and completion status. General linear mixed models were fitted to compare trajectories of CINA scores between methadone and buprenorphine conditions, and between study dropouts and completers within the buprenorphine condition.
Results
Both buprenorphine and methadone patients experienced withdrawal categorized as minimal by the CINA scoring system. Significant differences in mean peak CINA scores for the first 72 hours of induction were found between the methadone (4.5; SD=0.4) and buprenorphine conditions (6.9; SD=0.4), with buprenorphine patients exhibiting higher mean peak CINA scores [F (3, 165) =9.70, p<0.001]. The trajectory of CINA scores showed buprenorphine patients exhibiting a sharper increase in mean CINA scores than methadone patients [F (1, 233) =8.70, p=0.004]. There were no differences in mean peak CINA scores [F (3, 77) =0.08, p=0.52] or in trajectory of CINA scores [F (1, 166) =0.42, p=0.52] between buprenorphine study dropouts and completers.
Conclusion
While mean peak CINA score was significantly higher in the buprenorphine condition than the methadone condition, neither medication condition experienced substantial withdrawal symptoms. Further research on factors related to successful induction to buprenorphine treatment in pregnant women is needed.
Keywords: pregnancy, opioid dependence, buprenorphine induction, CINA, opioid withdrawal, methodone induction
1. INTODUCTION
The benefits of opioid agonist maintenance medication relative to no medication or detoxification to treat opioid dependence during pregnancy are well-established (Winklbaur et al., 2008; Mattick et al., 2008; Jones et al., 2008; Kaltenbach et al., 1998). Opioid agonist maintenance treatment during pregnancy decreases illicit drug use, lessens risk of infectious diseases, and improves compliance with prenatal care (Kaltenbach et al., 1998; Fajemirokun-Odudeyi et al., 2006; Kandall et al., 1999; McCarthy et al., 2005). Methadone, a long-acting opioid agonist, has been used since the 1970s for treatment of opioid dependence during pregnancy and is currently the recommended standard of care (National Institutes of Health, 1998).
Buprenorphine was approved by the FDA for use to treat opioid-dependent adults in 2002, and has been used to expand treatment options for opioid-dependent individuals. A Cochrane review suggests that among non-pregnant patients, buprenorphine is similarly effective to methadone for management of opioid withdrawal (Gowing et al., 2009). Buprenorphine has been used in Europe since 1995 for the treatment of both opioid dependent pregnant and non-pregnant patients (Auriacombe et al., 2004, Fischer et al., 2000), and began to be investigated in the United States as a treatment option for pregnant women in 1996 (Johnson et al., 2001).
Buprenorphine's pharmacologic and pharmacokinetic characteristics differ from that of methadone. Buprenorphine is a partial agonist with a high binding affinity at the mu opioid receptor and a longer duration of action than methadone. Sublingual absorption of buprenorphine produces a shorter onset of central nervous system action compared to the gastrointestinal absorption rout of methadone. Buprenorphine has a shorter half-life than methadone and the action of buprenorphine is also limited by a “ceiling effect”. This property may produce lower physical dependence than full opioid agonists such as methadone (Jasinski et al., 1978); a better safety profile due to a limit on respiratory depression and sedation (Mattick et al., 2003; Megarbane et al., 2006); and a shorter less difficult taper from maintenance (Mattick et al., 2008). More specific to in utero exposure, buprenorphine has been shown to have lower concentration in maternal and umbilical cord plasma following chronic maintenance dosing in pregnancy, less transplacental transfer to the fetus and less medication in fetal circulation relative to methadone (Gordon et al., 2010; Nanovskaya et al., 2002). The different transplacental pharmacokinetics of methadone and buprenorphine may be a possible mechanism underlying the less severe neonatal abstinence syndrome observed following prenatal exposure to buprenorphine than prenatal exposure to methadone (Jones et al., 2010; Gaalema et al., 2012).
Although prenatal exposure to buprenorphine has shown beneficial effects for neonates, the use of buprenorphine may also present some clinical challenges related to retaining patients during induction. Greater attrition has been reported during induction onto buprenorphine relative to methadone in non-pregnant patients and several possible explanations have been suggested. First, a gradual buprenorphine induction may not sufficiently suppress withdrawal symptoms (Fischer, 1999; Petitjean, 2001; Whitley et al., 2010). Second, patients may not have been in adequate opioid withdrawal at the time of induction, resulting in precipitated withdrawal, that is, an acute increase of withdrawal signs and symptoms due to a partial agonist (Whitley et al., 2010). Third, patients may have left treatment due to their inability to manage opioid withdrawal signs and symptoms which lasted beyond the first day's dosing (Whitley et al., 2010). Fourth, the mild withdrawal associated with buprenorphine may make it easier for some patients to discontinue buprenorphine (Mattick et al., 2003). Fifth, as a partial μ opioid agonist without a full μ opioid effect, buprenorphine may be less satisfying to some patients (Mattick et al., 2008). Additionally, an examination of factors associated with complicated buprenorphine inductions has also found that a recent history of benzodiazepine and/or methadone use as well as being buprenorphine naive were important contributors to less successful buprenorphine inductions (Whitley et al., 2010).
More information regarding treatment retention of buprenorphine-inducted patients is needed, including the timing and reasons for discontinuation. Patient history and characteristics that may predict success or premature discontinuation early in buprenorphine treatment would also be valuable information for clinicians in determining the most appropriate medication for a particular patient. In addition, none of the studies above focused on opioid dependent pregnant women.
One of the few studies to date to compare methadone versus buprenorphine maintenance in opioid-dependent pregnant women was the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study. The MOTHER study is an eight-site double-blind, double–dummy flexible dosing parallel-group randomized clinical trial (RCT) investigating the safety and efficacy of maternal and prenatal exposure to methadone and buprenorphine (Jones et al., 2010). The protocol for this study, including induction procedures, is based on that previously published by Jones et al. (2005b) and described in Jones et al. (2012).
Although not statistically significant, more MOTHER participants randomized to buprenorphine did not complete the study than those randomized to methadone. The majority of participants randomized to buprenorphine who dropped out did so during the induction phase, with most citing dissatisfaction with the medication as the reason for discontinuation. The primary purpose of this secondary analysis of MOTHER data is to determine whether withdrawal symptoms during the induction phase differ between the methadone versus buprenorphine-maintained groups, and between dropouts and completers among participants randomized to buprenorphine. A secondary purpose of this study is to identify patient characteristics of MOTHER participants that predict the discontinuation of buprenorphine treatment.
2. METHODS
2.1 The MOTHER Study
This study is a secondary analysis of data from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) protocol not included in the primary outcome study. Analyses of the primary and key secondary outcomes from the MOTHER study have been reported elsewhere (Jones et al., 2010).
2.2 MOTHER Participants
Data were obtained from the 175 opioid-dependent pregnant women who were randomized to methadone or buprenorphine and received at least one dose of double-blind opioid agonist maintenance medication in the MOTHER study (methadone n = 89, buprenorphine n = 86). Participants were between the ages of 18 and 41years of age, inclusive, carried a singleton pregnancy and were randomized between 6 and 30 weeks estimated gestational age (EGA) as confirmed by ultrasound. Exclusion criteria included current benzodiazepine or alcohol abuse or dependence as defined by the Structured Clinical Interview of the DSM-IV (SCID) module E, HIV seropositivity, impending incarceration, non-English speaking (non-German-speaking at the Vienna site), or a medical or psychiatric condition contraindicating study participation as determined by the medically responsible investigator. More complete details regarding participant recruitment and selection and inclusion and exclusion criteria can be found in Jones et al. (2010).
2.3 Procedures
All participants signed a local IRB approved informed consent form for study participation. An extensive screening assessment was conducted to determine eligibility for the study, including demographic information, medical history, psychiatric assessment, nicotine dependence, obstetrical assessment, and a complete blood chemistry. A flexible dosing protocol was implemented. Rapid-release morphine sulfate was administered during the 3–7 day screening period to achieve medical stabilization and facilitate comfortable transition to double-blind maintenance medication. The amount of the first dose of study medication was based on the total amount of morphine received in the 24 hours prior to randomization, (e.g., a participant who received 360 mg of morphine would receive either 60 mg of methadone or 10mg of buprenorphine). The dose conversion ratio used for the transition was the same as used in Jones et al. 2005. The initial dose of study medication was split with the second half of the dose administered 30 minutes-2 hours after the first half. Two optional comfort doses were available each day during induction for participants in both medication conditions. An assessment of withdrawal using the modified Clinical Institute Narcotic Assessment scale (see 2.4 below) was administered every six hours during induction and each time a comfort dose was given. See Jones et al. (2010) for a detailed description of study procedures.
2.4 Measures
2.4.1 Clinical Institute Narcotics Assessment (CINA)
The original CINA (Peachey and Lei, 1988) consists of 13 items (1 subjective symptom item, 7 objective sign items, and 5 items that included subjective and objective components). Internal consistency α has been previously reported as 0.81 (Peachey and Lei, 1988). The modified version (Center for Substance Abuse Treatment, 2004) contains 11 items with possible scores from a minimum of 0 to a maximum of 31. Total withdrawal score is calculated as the percent of the possible maximum score (total score/31 × 100%).
2.5 Data Analyses
An analysis of variance (ANOVA) was conducted to determine differences between mean peak CINA scores for participants randomized to buprenorphine versus methadone during the first 72 hours of induction onto study medication. An ANOVA was also conducted to identify differences in the buprenorphine group between mean peak CINA scores of those who completed the induction protocol versus those who withdrew. General linear mixed models were fitted to determine differences in the trajectory of CINA scores between methadone and buprenorphine conditions and between study dropouts and completers among the buprenorphine condition using the CINA conducted at the last dose of morphine sulfate and the mean daily CINA scores for the first two days (48 hours) of induction on study medication. Multiple logistic regressions were conducted to determine predictors of study discontinuation among the group randomized to buprenorphine. Estimated gestational age (EGA) week at study entry, demographic factors (education, race, marital status), number of prior drug treatment episodes, receipt of methadone in the 48 hours previous to study entry, first dose of maintenance medication (mg), and drug problem and psychiatric severity, as measured by the Addiction Severity Index (ASI) were included as predictor variables in the model. A multivariate linear regression model was also fitted to determine predictors of peak CINA scores during the first 72 hours among the buprenorphine-maintained patients using the same predictor variables. A three-level factor representing study site [US Urban (Baltimore, MD; Philadelphia, PA; Detroit, MI; Providence, RI) v. US Rural (Burlington, VT; Nashville, TN) v. European (Vienna)], was included in all analyses as a blocking factor to control for variation between study sites. The α level for all analyses was .05.
3. RESULTS
3.1 Participant Characteristics
Descriptive statistics are displayed in Table 1. [Participant characteristics by medication condition can be found in Table 1 in Jones et al. (2010). Average maternal age at study entry was 27.3 (SD=5.9). The majority of the sample was Caucasian (83.4%). Less than one-fifth of the sample reported their status as married (13.1%), and 81.1% had a high school education or less. Average number of times previously treated for substance abuse was 2.9 (SD=3.2). A majority of the sample had previous lifetime experience with medication-assisted treatment, with 65.3% having previously received methadone-maintenance, 42.2% having received treatment with buprenorphine, and approximately one-fourth (26.0%) having prior treatment experience with both medications. Average first dose of study medication for women randomized to methadone was 53.8 mg (range 10–120 mg), and 10.1mg (range 2–30 mg) for women receiving buprenorphine. Of the 28 women randomized to buprenorphine who discontinued participation prior to delivery, 19 withdrew during the first three days of the induction period. Eighteen of these patients reported dissatisfaction with the medication as their reason for withdrawal. For one participant who withdrew during induction, data regarding the reason for discontinuation was missing. [The 20 participants reported as being dissatisfied with the medication in the primary outcomes paper (Jones et al., 2010) reflects data from all participants who withdrew from the study at any point in time.] None of the patients randomized to methadone-maintenance withdrew during the induction period.
Table 1.
Sample Demographic and Background Characteristics (N = 175)
| Variable | Mean (SD) | Percent |
|---|---|---|
| Maternal age | 27.3 (5.9) | |
| EGA week at study entry | 17.2 (6.1) | |
| Education | ||
| Less than high school | 40.0% | |
| Completed high school | 41.1% | |
| Some college | 17.2% | |
| Race | ||
| Caucasian | 83.4% | |
| African-American | 13.7% | |
| Other | 2.9% | |
| Married | 13.1% | |
| Prior methadone treatment | 65.3% | |
| Methadone during prior 48 hours* | 48.0% | |
| Prior buprenorphine treatment | 42.2% | |
| Prior treatment both medications | 26.0% | |
| Number prior treatment | 2.9 (3.2) |
Note: EGA = estimated gestational age; Prior treatment refers to a lifetime treatment experience with an opioid-agonist; Please see Table 1 in Jones et al., 2010 for a comparison of characteristics in methadone and buprenorphine patients.
Received methadone in the 48 hours prior to study entry
3.2 Results of CINA scores
3.2.1 Peak CINA
Significant differences in mean peak CINA scores for the first 72 hours of induction were found between the methadone (4.5; SD=0.4) and buprenorphine-assigned groups (6.9; SD=0.4), with buprenorphine patients exhibiting higher mean peak CINA scores [F (3, 165) =9.70, p<0.001]. No relationship between first dose and peak CINA score was found when the total sample of methadone and buprenorphine-treated patients were examined (b=0.01; SE=0.01; p=0.51).
3.2.2 CINA Trajectories
Figure 1 displays the trajectory of CINA scores in the methadone and buprenorphine groups for the last dose of morphine sulfate and the mean CINA scores for the first 48 hours following the first dose of methadone or buprenorphine. Results from the general linear mixed model analysis show that the trajectory of CINA scores differed significantly between methadone and buprenorphine conditions [F (1, 233) =8.70, p=0.004]. Patients randomized to buprenorphine have a sharper initial increase in CINA scores which levels off at the second time point, while scores of methadone patients continue to increase more gradually until the third time point, prior to leveling off (Figure 1). CINA scores of patients receiving buprenorphine remain marginally higher than those of methadone-maintained patients at all-time points.
Figure 1.
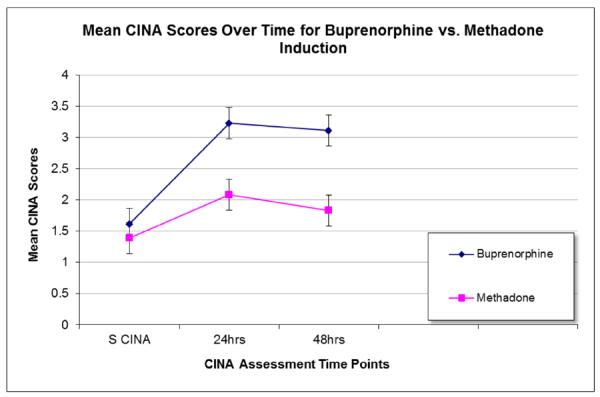
Estimated mean CINA scores over time for in the buprenorphine and methadone treatment medication conditions (n=172). S CINA is the CINA conducted at the last dose of morphine sulfate (n=172). Mean CINA scores for the first two 24-hour periods of induction are reported following the first dose of methadone or buprenorphine (24 hours n=170; 48 hours n=149).
3.2.3 Buprenorphine Condition
Examining the group randomized to buprenorphine, no differences in mean peak CINA score were found between the group of women who discontinued participation (7.7; SD=0.9) and the group of women who completed the protocol (6.5; SD=0.5) [F (3, 77) =0.76, p=0.52]. Although the buprenorphine-maintained patients who withdrew appeared to have a sharper mean initial increase in CINA scores than did the induction completers when transitioning from morphine to buprenorphine maintenance (Figure 2), the trajectories appear similar thereafter, and no significant differences were found between those who completed and those who withdrew [F(1, 166)=0.42, p=0.52]. The only variable that predicted peak CINA score among the buprenorphine-treated group was first dose of study medication, with those requiring a higher first dose of medication exhibiting higher peak CINA score (b=0.04; SE= 0.02; p=0.03).
Figure 2.

Estimated mean CINA scores for buprenorphine patients who completed protocol (n=58) in comparison to patients who discontinued prior to completion (n=28). S CINA is the CINA conducted at the last dose of morphine sulfate (n=84). Mean CINA scores for the first two 24-hour periods of induction are reported following the first dose of buprenorphine (24 hours n=81; 48 hours n=68).
3.3 Predictor Variables
Among the group treated with buprenorphine, two variables predicted study discontinuation. Receipt of methadone during the 48 hours prior to study entry (OR=6.16; 95% CI= 1.62–23.5; p=0.008) and higher drug problem severity, as assessed by the ASI, predicted higher likelihood of study withdrawal (OR=33.7; 95% CI=28.45 – 40.00; p=0.004). No relationship was found for demographic variables, number of prior treatment episodes, first dose of study medication, or psychiatric severity.
4. DISCUSSION
Successful patient induction and retention on long-acting opioid agonist medication is an important clinical issue in the treatment of opioid dependency. The differing pharmacological properties of buprenorphine offer some significant clinical advantages over methadone, such as improved safety profile, longer duration of action, and a milder withdrawal when the medication is discontinued (Mattick et al., 2003; Megarbane et al., 2006). However, buprenorphine may have an induction period that is more complex to manage, particularly in patients recently exposed to long-acting full opioid agonists (Mattick et al., 2008; Whitley et al., 2010). This secondary analysis compared withdrawal signs and symptoms during the induction process for opioid-dependent pregnant patients receiving either methadone or buprenorphine. It also examined factors that predicted greater withdrawal signs and symptoms during induction for patients treated with buprenorphine, as well as successful completion of induction.
While mean peak CINA score was significantly higher in the buprenorphine condition than the methadone condition, the mean peak CINA score for both the methadone and buprenorphine conditions were within the range of scores classified as minimal withdrawal, less than one-fourth of the potential maximum withdrawal symptoms (the maximum score on the CINA is 31). Thus, this statistically significant finding may be of limited clinical relevance. It is clear that neither group experienced substantial withdrawal symptoms. Further, this low average withdrawal score is similar to the average CINA scores of non-pregnant patients inducted onto buprenorphine without complications (DiPaula et al., 2002). Nor did those participants who withdrew have higher CINA scores than those who completed the protocol among the buprenorphine-treated group. However, the trajectory of more rapid increase in withdrawal symptoms among the buprenorphine-treated patients compared to the methadone-treated patients may have disconcerted patients in the buprenorphine group. Although not significant, this higher rate of increase in withdrawal symptoms also appears to have been more pronounced among those participants who discontinued the induction protocol versus those participants who completed within the buprenorphine condition. It may be that for pregnant women enrolled in a RCT, modest differences in even mild withdrawal scores are important in determining dropout. As such, more intensive and more repetitive patient education around expectations for the induction process may help to improve retention rates for buprenorphine treatment.
Individuals who require higher maintenance doses of medication may exhibit greater withdrawal symptoms during induction on buprenorphine. The reason for this is not clear, but is consistent with previous complicated buprenorphine inductions (Whitley et al., 2010) and with concerns that induction onto buprenorphine is sometimes conducted too slowly (Fischer, 1999; Petitjean, 2001), resulting in a higher attrition rate. The differing safety profiles of methadone and buprenorphine make a slower induction necessary for methadone than is required for buprenorphine. However, due to differences in half life, an induction this slow may not be appropriate for buprenorphine. As first dose of buprenorphine (or methadone) was based on the amount of morphine sulfate required for patient comfort in the previous 24 hours, this difference in attrition rates may also simply reflect that individuals requiring higher maintenance doses, that is, a greater pre-existing opioid physiological dependence, may have more withdrawal symptoms during the induction process until they reach a stabilizing dose. Or conversely, our induction protocol may have in some cases precipitated withdrawal and higher CINA scores. It may be that administering the initial dose in small increments given repeatedly throughout the day, e.g. 2–4 mg every 2–3 hours may be a more successful approach. Finally, the higher CINA's among patients receiving buprenorphine may reflect the shorter half-life of buprenorphine. More rapidly declining blood levels in fast metabolizers may necessitate higher doses to maintain an effective blood level of buprenorphine.
These data underscore that pregnant women are similar to non-pregnant patients (Whitley et al., 2010) with greater drug problem severity and exposure to a long-acting full opioid agonist (in this case methadone) immediately prior to study entry increasing the likelihood of discontinuation during buprenorphine induction. Drug problem severity, as measured by the ASI, is a composite of a number of factors, including previous treatment episodes and amount of drug use. Receipt of methadone during the 48 hours prior to study entry also predicted study discontinuation among patients inducted on buprenorphine. It is important to clarify that receipt of methadone during the 48 hours prior to study entry is not an indicator that participants entered the study maintained on methadone. This variable reflects the fact that some women received methadone when entering treatment and prior to being consented for the study in order to avoid consenting women who were experiencing opioid withdrawal at the time of consent. Their discontinuation may reflect greater dissatisfaction with the “feel” of a partial opioid agonist after the recent experience of maintenance with a full opioid agonist, as others have suggested (Mattick et al., 2008; Whitley et al., 2010). These findings are consistent with previous research with non-pregnant patients that methadone maintenance prior to buprenorphine induction may be a factor in less successful inductions onto buprenorphine (Whitley et al., 2010). Whitely et al. (2010) also report that lack of experience with buprenorphine treatment predicts less successful inductions onto buprenorphine. Although not included as a potential predictor in the model in the present study due to limitations of sample size, it is notable that 11 of the 19 patients in the buprenorphine condition that discontinued study participation during induction had no prior experience with buprenorphine treatment.
4.1 Conclusions
Further research on the induction stage of buprenorphine treatment, particularly for pregnant women, is needed. While it is clear that both methadone and buprenorphine-treated patients in the MOTHER study experienced minimal withdrawal symptoms during induction, differences in peak CINA scores and the trajectory of withdrawal symptoms were found between the two medication groups. These differences may account for the non-significant yet markedly different attrition rate among buprenorphine-treated patients than among methadone-treated patients. Additional study on patient characteristics that may predict successful retention in buprenorphine treatment may be helpful for clinicians in prescribing the medication most likely to be effective for a particular patient. Finally, different medication induction protocols may help improve retention rates.
Table 2.
Results of logistic regression analysis predicting study discontinuation among buprenorphine-maintained patients (n=86)
| Variable | Odds ratio (SE) | z | p | Confidence Interval |
|---|---|---|---|---|
| EGA week | 0.99 (0.05) | −0.16 | 0.87 | 0.60 – 4.12 |
| Education | 0.78 (0.14) | −1.35 | 0.18 | 0.54 – 1.11 |
| Race | 1.00 (0.80) | −0.01 | 1.00 | 0.20 – 4.86 |
| Marital status | 1.48 (1.26) | 0.47 | 0.64 | 0.28 – 7.79 |
| Methadone 48 hours1 | 6.16 (4.20) | 2.67 | 0.008* | 1.62 – 23.46 |
| First dose2 | 1.00 (0.01) | 0.28 | 0.78 | 0.98 – 1.02 |
| Prior treatment | 2.40 (2.26) | 0.94 | 0.35 | 0.39 – 15.00 |
| ASI Drug severity3 | 33.72 (12.18) | 2.89 | 0.004* | 28.45 – 40.00 |
| ASI Psychiatric severity4 | 0.10 (0.16) | −1.39 | 0.17 | 0.004 – 2.62 |
Note:
p< 0.01;
Received methadone in the 48 hours prior to study entry;
1st dose of buprenorphine;
Addiction Severity Index (ASI) Drug severity composite score at study entry;
ASI Psychiatric severity composite score at study entry.
Acknowledgment
We would like to thank Kevin O'Grady for his kind assistance in the revision of this paper.
Role of Funding Source Funding for this study was provided by grants from the National Institute on Drug Abuse: RO1 DA015778 to Brown University; RO1 DA015764 to Johns Hopkins University; RO1 DA018417 to the Medical University of Vienna; RO1 DA015738 to Thomas Jefferson University; RO1 DA018410 and MO1 RR109 to the University of Vermont; RO1 DA017513 and MO1 RR00095 to Vanderbilt University; and RO1 DA15832 to Wayne State University; The MOTHER clinical trial was registered with Clinical Trials.gov (Identifier: NCT00271219; Title: RCT Comparing Methadone and Buprenorphine in Pregnant Women).
NIDA had no further role in the study design; collection, analysis and interpretation of data; writing of the paper; and decision where to submit this paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors AMH and KK planned this secondary analysis study. AMH conducted the statistical analyses and wrote the initial draft of the manuscript. SHH, HEJ, and KK provided extensive revisions to the initial draft. All authors provided comments and suggestions to subsequent drafts and have approved the final manuscript.
Conflict of Interest HE Jones discloses that she has received reimbursement for time and travel from Reckitt Benckiser. G Fisher discloses that she has received honorarium and travel support from Reckitt Benckiser and Sheringh Plough. The remaining authors report no conflict of interest.
References
- Auriacombe M, Fatséas M, Dubernet J, Daulouède JP, Tignol J. French field experience with buprenorphine. Am. J. Addict. 2004;13(Suppl. 1):S17–S28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment . Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2004. (Treatment Improvement Protocol (TIP) Series 40). DHHS Publication No. (SMA) 04-3939. [PubMed] [Google Scholar]
- DiPaula BA, Schwartz R, Montoya ID, Barrett D, Tang C. Heroin detoxification with buprenorphine on an inpatient psychiatric unit. J. Subst. Abuse Treat. 2002;23:163–169. doi: 10.1016/s0740-5472(02)00244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajemirokun-Odudeyi O, Sinha C, Tutty S, Pairaudeau P, Armstrong D, Phillips T, Lindow SW. Pregnancy outcome in women who use opiates. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;126:170–175. doi: 10.1016/j.ejogrb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stuhlinger G, Pezawas L, Aschauer HN, Kasper S. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- Fischer G, Johnson RE, Jagsh R, Peternell A, Weninger M, Langer M, Aschauer HN. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95:239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- Gaalema DE, Scott TL, Heil SH, Coyle MG, Kaltenbach K, Badger GJ, Arria A, Stine SM, Martin PR, Jones HE. Differences in the profile of neonatal abstinence syndrome signs in methadone vs. buprenorphine exposed neonates. Addiction. 2012;107(Suppl. 1):53–62. doi: 10.1111/j.1360-0443.2012.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AQ, Lopatko O, Somogyi A, Foster D, Wjite J. (R)- and (S)-methadone concentration ratios in maternal and umbilical cord plasma following chronic maintenance dosing in pregnancy. Br. J. Clin. Pharmacol. 2010;70:895–902. doi: 10.1111/j.1365-2125.2010.03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2009:CD002025. doi: 10.1002/14651858.CD002025. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. Obstet. Gynecol. Clin. North Am. 1998;25:139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Kandall SR, Doberczak TM, Jantunen M, Stein J. The methadone-maintained pregnancy. Clinics Perinatol. 1999;26:173–183. [PubMed] [Google Scholar]
- Jasinski DR, Oevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch. Gen. Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, Kissin WB, Alpan G, Lantz ME, Cone EJ, Wilkins DG, Golden AS, Huggins GR, Lester BM. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Jones HE, Fischer G, Heil S.H. Heil, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, O'Grady KE, Arria AM. Maternal Opioid Treatment: Human Experimental Research (MOTHER)-approach, issues, and lessons learned. Addiction. 2012;107(Suppl. 1):28–35. doi: 10.1111/j.1360-0443.2012.04036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, Jasinski DR, Milo L. Randomized controlled study transitioning opioid-dependent pregnant women from short-acting morphine to buprenorphine or methadone. Drug Alcohol Depend. 2005;78:33–38. doi: 10.1016/j.drugalcdep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil S, Stine S, Coyle M, Arria A, O'Grady K, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, O'Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am. J. Addict. 2008;17:372–386. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Ali R, White J, O'Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind with 405 opioid-dependent patients. Addiction. 2003;98:441–452. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;3:CD002209. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Leamon ML, Parr MS, Anania BA. High dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2005;193:606–610. doi: 10.1016/j.ajog.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Hreiche R, Pirnay S, Marie N, Baud FJ. Does high-dose buprenorphine cause respiratory depression? Possible mechanisms and therapeutic consequences. Toxicol. Rev. 2006;25:79–85. doi: 10.2165/00139709-200625020-00002. [DOI] [PubMed] [Google Scholar]
- Nanovskaya TN, Deshmukh S, Brooks M, Ahmed M. Transplacental transfer and metabolism of buprenorphine. J. Pharmacol. Exp. Ther. 2002;3:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Treatment of Opiate Addiction. JAMA. 1998;280:1938–1943. [PubMed] [Google Scholar]
- Peachy JE, Lei H. Assessment of opioid dependence with naloxone. Br. J. Addict. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- Petitjean S, Stohler R, Deglon JJ, Livoti S, Waldvogel D, Uehlinger C, Ladewig D. Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alcohol Depend. 2001;62:97–104. doi: 10.1016/s0376-8716(00)00163-0. [DOI] [PubMed] [Google Scholar]
- Whitley SD, Sohler NL, Kunins HV, Giovanniello A, Li X, Sacajiu G, Cunningham CO. Factors associated with complicated buprenorphine inductions. J. Subst. Abuse Treat. 2010;39:51–57. doi: 10.1016/j.jsat.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103:1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]


