Abstract
Background
Post-stroke subjects with hemiparesis typically utilize a reduced number of modules or co-excited muscles compared to non-impaired controls, with at least one module resembling the merging of two or more non-impaired modules. In non-impaired walking, each module has distinct contributions to important biomechanical functions, and thus different merged module combinations post-stroke may result in different functional consequences.
Methods
Three-dimensional forward dynamics simulations were developed for non-impaired controls and two groups of post-stroke hemiparetic subjects with different merged module combinations to analyze how paretic leg muscle contributions to body support, forward propulsion, mediolateral control and leg swing are altered.
Findings
The potential of the plantarflexors to generate propulsion was impaired in both hemiparetic groups while the remaining functional consequences differed depending on which modules were merged. Paretic leg swing was impaired during pre-swing when Modules 1 (hip abductors and knee extensors during early stance) and 2 (plantarflexors during late stance) were merged and during late swing when Modules 1 and 4 (hamstrings during swing into early stance) were merged. When Modules 1 and 4 were merged, body support during early stance was also impaired.
Interpretation
These results suggest that improving plantarflexor ability to generate propulsion is critical during rehabilitation regardless of module composition. If Modules 1 and 2 are merged, then rehabilitation should also focus on improving paretic leg pre-swing whereas if Modules 1 and 4 are merged, then rehabilitation should also focus on improving early stance body support and late paretic leg swing.
Keywords: Forward dynamics simulation, muscle synergies, gait, biomechanics
1. Introduction
Stroke is a leading cause of long-term disability in the United States that often leaves survivors with various levels of hemiparesis affecting their mobility. These individuals typically walk at slower speeds (e.g., Perry et al., 1995) due to impaired muscle coordination (Den Otter et al., 2007; Turns et al., 2007). However, the type of coordination impairment varies among individuals (e.g., De Quervain et al., 1996; Knutsson and Richards, 1979). Recent studies have suggested that complex muscle activity during walking may be generated using a reduced neural control strategy organized around the co-excitation of multiple muscles, or modules (e.g., Cappellini et al., 2006; Clark et al., 2010; Ivanenko et al., 2004). Furthermore, the coordination impairments observed in post-stroke hemiparetic subjects manifests in different modular patterns (Clark et al., 2010). Recent studies have proposed analyzing modules as a promising technique to identify and monitor muscle coordination impairments and their influence on locomotor performance (Clark et al., 2010; Safavynia et al., 2011).
Four modules have previously been identified in non-impaired walking (Clark et al., 2010) that each have distinct contributions to important biomechanical functions (e.g., support, forward propulsion, mediolateral control and leg swing; Allen and Neptune, 2012; Neptune et al., 2009). Module 1 (early stance module with hip abductor and knee extensor activity) and Module 2 (late stance module with plantarflexor activity) provide body support during early and late stance, respectively. These two modules, combined with Module 4 (late swing into early stance module composed of hamstrings activity), function synergistically to control both forward propulsion and mediolateral ground reaction forces. Modules 3 (early swing module with dorsiflexor and biarticular knee extensor activity) and 4 are both important for controlling ipsilateral leg swing, while Modules 1 and 4 are both important for controlling contralateral leg swing. Since these four modules synergistically control important biomechanical functions during non-impaired walking, altered module compositions may adversely affect walking performance.
Studies have shown that instead of independently activating the same four modules found during non-impaired walking, post-stroke hemiparetic subjects often utilize a reduced number of modules, with at least one of the modules resembling the merging of multiple non-impaired modules (Clark et al., 2010). As the number of independently activated modules decreases, walking performance (e.g., self-selected speed, bilateral symmetry, etc.) also decreases (Bowden et al., 2010; Clark et al., 2010). A consequence of merged modules may be a reduced ability to successfully generate each biomechanical function, resulting in poor walking performance. Which specific functions are affected likely depends on which modules are merged. For example, within those subjects who could independently activate three modules, two common types of module compositions were found (Clark et al., 2010). One type merged Modules 1 and 2 such that the proximal extensors and plantarflexors were co-activated throughout stance. A second type merged Modules 1 and 4 such that the proximal extensors and hamstrings were co-activated from swing into late stance.
It is likely that subjects with these two common types of module compositions would have different neuromotor impairments and benefit from different rehabilitation strategies. Therefore, the purpose of this study was to develop muscle-actuated forward dynamics simulations of walking for non-impaired control subjects and two groups of hemiparetic subjects in which two different sets of non-impaired modules are merged (Group A: merged Modules 1 and 2; Group B: merged Modules 1 and 4) in order to identify the potential influence of these two common types of merged modules on post-stroke hemiparetic walking performance (as measured by contributions to specific biomechanical functions during walking). Identifying the relationship between these two module compositions and biomechanical functions during walking can provide evidence-based rationale for rehabilitation programs designed to target the deficits associated with these specific module compositions.
2. Methods
2.1 Experimental data
Kinematic, GRF and EMG data were collected from 14 healthy controls walking at 1.2 m/s (age: mean = 63.1 years. SD = 9.1 years; gender: 2 male, 12 female) and 11 chronic post-stroke hemiparetic subjects walking at their self-selected speed (speed: mean = 0.54 m/s, SD = 0.22 m/s; age: mean = 62.2 years. SD = 11.7 years; gender: 7 male, 4 female; 6 right side hemiparesis; years post-stroke: mean=3.5, SD = 2.7). The hemiparetic data were a subset from Clark et al. (2010) (i.e., those that fell into Group A or Group B). All subjects signed an institutionally approved informed consent and protocol. 3D body-segment kinematics were collected at 100 Hz and GRF and EMG data were collected at 2000 Hz using Vicon Workstation v4.5 software while subjects walked for 30s on an ADAL split-belt treadmill (Techmachine). Kinematics and GRF data were low-pass filtered with a fourth-order Butterworth filter with a cutoff frequency of 6 Hz and 20 Hz, respectively. EMG signals were high-pass filtered with a fourth-order Butterworth filter (40 Hz), demeaned, rectified and low-pass filtered with a fourth-order Butterworth filter (4 Hz). All EMG, GRF and kinematic data were time normalized to 100% of the paretic/left gait cycle. Using non-negative matrix factorization (NNMF, Clark et al., 2010), modules were identified from EMG data collected using bipolar Ag-AgCL surface electrodes from the tibialis anterior, soleus, medial gastrocnemius, vastus medialis, rectus femoris, medial hamstrings, lateral hamstrings and gluteus medius of each leg using a telemetered EMG acquisition system (Konigsberg Instruments).
Walking trials of a representative subject from each hemiparetic group were selected for the simulation analysis based on similar walking speeds and minimum difference between kinematics and GRFs compared to their group average data (see Supplementary Appendix A). The individual gait cycle for each subject with the minimum difference in kinematics and GRFs compared to that subject’s average data was then used for the hemiparetic simulation tracking. Group averaged data was used for control simulation tracking.
2.2 Musculoskeletal model
A previously described 3D musculoskeletal model (Allen and Neptune, 2012) with 23 degrees-of-freedom was developed using SIMM/Dynamics Pipeline (Musculographics, Inc.) and included rigid segments representing the trunk, pelvis and two legs (thigh, shank, talus, calcaneus and toes). The pelvis had six degrees-of-freedom (3 translations, 3 rotations) with the trunk and hip joints modeled using spherical joints. The knee, ankle, subtalar and metatarsophalangeal joints were modeled as single degree-of-freedom revolute joints. Foot-ground contact forces were modeled with 31 independent visco-elastic elements attached to each foot (Neptune et al., 2000). Passive torques representing forces applied by ligaments, passive tissue and joint structures were applied at each joint (Anderson, 1999). The dynamical equations-of-motion were generated using SD/FAST (PTC).
The model was driven by the same 38 Hill-type musculotendon actuators per leg as in Allen and Neptune (2012), but the control scheme used to drive each actuator differed. The control scheme used in the current study is as follows. Modular excitation patterns identified through NNMF were used as excitation inputs to the corresponding muscles (Table 1). Muscles without recorded EMGs but with similar anatomical arrangement, biomechanical function and/or EMG activity were included in these modules (see Supplementary Appendix B). Muscles within each module received the same excitation pattern and timing, but the magnitude was allowed to vary between muscles. Three and four modules were used to control a subset of muscles in the hemiparetic and control simulations, respectively. All other muscles (Table 1) were driven using individual bimodal patterns (Hall et al., 2011). Muscle contraction dynamics were governed by Hill-type muscle properties (Zajac, 1989) and the activation dynamics were modeled by a first-order differential equation (Raasch et al., 1997). Polynomial equations were used to estimate musculotendon lengths and moment arms (Menegaldo et al., 2004).
Table 1.
The 38 individual muscles per leg were combined into 34 groups based on anatomical function. The excitation pattern for each muscle group was either based on experimentally derived modules or defined by a bimodal pattern (Hall et al., 2011). The control group had four modules (C1-C4) which were used to control the muscles on both legs. Each of the hemiparetic groups had three modules (A1-A3, B1-B3) that controlled the muscles on the paretic leg while the nonparetic leg muscles were controlled using bimodal patterns. All muscles within a module received the same timing but the magnitude was allowed to vary.
| Excitation Pattern Type |
||||
|---|---|---|---|---|
| Muscle Name | Muscle Group | Control | Group A | Group B |
| Iliacus Psoas |
IL IL |
Bimodal | Bimodal | Bimodal |
| Adductor Longus | AL | Bimodal | Bimodal | Bimodal |
| Adductor Brevis | AB | Bimodal | Bimodal | Bimodal |
| Pectineus | PECT | Bimodal | Bimodal | Bimodal |
| Quadratus Femoris | QF | Bimodal | Bimodal | Bimodal |
| Superior Adductor Magnus | AM | |||
| Middle Adductor Magnus | AM | Bimodal | Bimodal | Bimodal |
| Inferior Adductor Magnus | AM | |||
| Sartorius | SAR | Bimodal | Bimodal | Bimodal |
| Rectus Femoris | RF | C1,C3 | A1,A2 | B2, B3 |
| Vastus Medialis | MVAS | C1 | Al | B3 |
| Vastus Lateralis Vastus Intermedius |
LVAS LVAS |
C1 | Al | B3 |
| Anterior Gluteus Medius | GMED1 | Cl | Al | B1,B3 |
| Middle Gluteus Medius | GMED2 | C1 | Al | B1,B3 |
| Posterior Gluteus Medius | GMED3 | C1 | Al | B1,B3 |
| Piriformus | PIRI | Bimodal | Bimodal | Bimodal |
| Gemellus | GEM | Bimodal | Bimodal | Bimodal |
| Anterior Gluteus Minimus | GMIN1 | Cl | Al | B1,B3 |
| Middle Gluteus Minimus | GMIN2 | Cl | Al | B1,B3 |
| Posterior Gluteus Minimus | GMIN3 | Cl | Al | B1,B3 |
| Tensor Fascia Lata | TFL | Bimodal | Bimodal | Bimodal |
| Anterior Gluteus Maximus | GMAX1 | Cl | Al | B1,B3 |
| Middle Gluteus Maximus | GMAX2 | Cl | Al | B1,B3 |
| Posterior Gluteus Maximus | GMAX3 | Cl | Al | B1,B3 |
| Semitendinosus | SM | C4 | A3 | B1,B3 |
| Semimembranosus | ST | C4 | A3 | B1,B3 |
| Gracilis | GRAC | C4 | A3 | B1,B3 |
| Biceps Femoris Long Head | BFLH | C4 | A3 | B1,B3 |
| Biceps Femoris Short Head | BFSH | C4 | A3 | B1,B3 |
| Medial Gastrocnemius | MGAS | C2 | Al | Bl |
| Lateral Gastrocnemius | LGAS | C2 | Al | Bl |
| Soleus | SOL | C2 | Al | Bl |
| Tibialis Posterior | TP | C2 | Bimodal | Bimodal |
| Flexor Digitorum Longus | FD | C2 | Bimodal | Bimodal |
| Tibialis Anterior | TA | C3 | A2 | B2 |
| Extensor Digitorum Longus | ED | C3 | Bimodal | Bimodal |
2.3 Dynamic optimization
Three forward dynamics simulations of 115% of a gait cycle (starting at 15% of the gait cycle prior to paretic/left leg heel-strike) were generated for the (1) non-impaired control group with four independent modules, (2) hemiparetic group A with non-impaired Modules 1 and 2 merged, and (3) hemiparetic group B with non-impaired Modules 1 and 4 merged. A simulated annealing algorithm (Goffe et al., 1994) fine-tuned the muscle excitation patterns and initial joint velocities such that the difference between the simulated and experimentally measured kinematics and GRF walking data were minimized. Quantities included in the cost function were differences in the pelvis translations, trunk, pelvis, hip, knee and ankle joint angles and GRFs. Total muscle stress (muscle force/cross-sectional area of muscle) was also included in the cost function to minimize unnecessary co-contraction. Each bimodal excitation pattern had six optimization parameters (onset, offset and magnitude for the two modes) and each module pattern had two optimization parameters for timing (onset, offset) and a magnitude parameter for each muscle within the module. To improve the tracking optimization convergence, tracking torques were applied at each joint to drive them towards desired experimental kinematics using proportional control (Allen and Neptune, 2012) and were included in the cost function to minimize their magnitudes. The tracking differences, muscle stress and tracking torques were initially weighted equally in the cost function.
2.4 Simulation analyses
Analyses were performed on each simulation for a full gait cycle starting at paretic/left leg heel-strike. The influence of each module was assgged by quantifying the potential of muscles within that module to contribute to forward propulsion (anterior-posterior, AP, GRF), body support (vertical GRF), mediolateral control (ML GRF) and leg swing (average power delivered to the leg during pre-swing and swing) using a perturbation analysis, similar to previously described GRF decomposition and body segmental power analyses (Neptune et al., 2004). First, the total GRF and segmental powers were calculated at time step i. Then, at time step i-1, the muscle force of interest was perturbed IN and the GRFs and segmental powers were recomputed. That muscle’s per unit force contributions to the GRFs and segmental powers were approximated by the difference between the unperturbed and perturbed values. The process was then repeated for each muscle. These per unit force contributions were then scaled by each muscle’s respective experimentally measured module timing pattern to give the potential of a muscle to contribute to each biomechanical function over the gait cycle.
3. Results
Each simulation emulated well the tracking data with average kinematic and GRF deviations of 4.6° (experimental SD = 6.2°) and 4.3% body weight (BW, experimental SD = 2.9% BW) for the control simulation, 4.1° (experimental SD = 7.3°) and 3.4% BW (experimental SD = 4.8% BW) for the Group A simulation, and 5.8° (experimental SD = 7.2°) and 3.6% BW (experimental SD = 4.8% BW) for the Group B simulation.
3.1 Forward propulsion
In the non-impaired control subjects, the plantarflexors (SOL+GAS) and knee extensors (RF+VAS) had substantial potential to generate braking (negative AP GRF) while the hamstrings (HAM) and gluteus medius (GMED) generated forward propulsion (positive AP GRF) during the first half of stance (Fig. 1a). When Modules 1 and 2 were merged in Group A and when Modules 1 and 4 were merged in Group B, the potential of SOL+GAS to generate braking during the first half of stance was notably increased compared to the control subjects while both HAM and GMED had increased potential to generate forward propulsion. In Group B, the braking generated by RF+VAS in the first half of stance was greatly reduced compared to the controls and Group A.
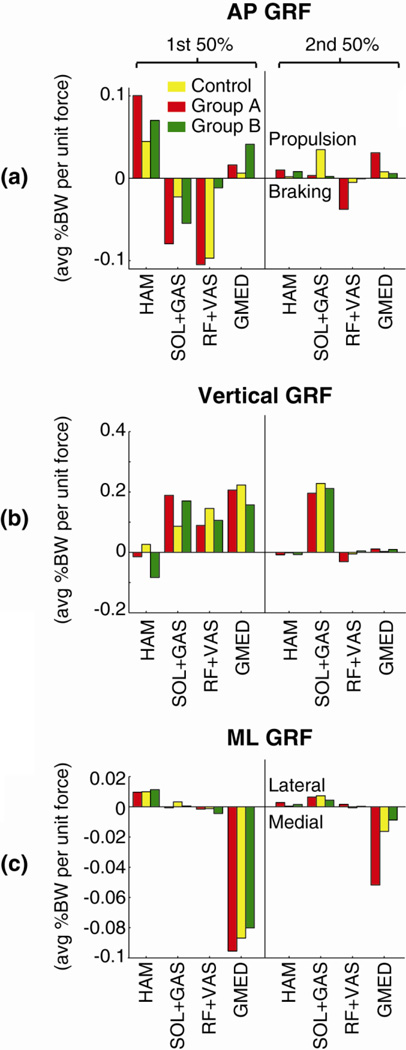
Figure 1.
Average contributions to the GRF (per unit muscle force) during the 1st and 2nd half of stance in the (a) AP direction, (b) vertical direction and (c) ML direction for the non-impaired control (yellow), Group A (red) and Group B (green) simulations. Hemiparetic values in Groups A and B correspond to muscle groups on the paretic leg.
During the second half of stance, SOL+GAS were the primary source of forward propulsion in non-impaired controls (Fig. 1a). In both Group A and Group B, the potential of SOL+GAS to generate forward propulsion was reduced. In Group A, the braking from RF+VAS was extended into the second half of stance. In addition, GMED had an increased potential to generate forward propulsion compared to controls.
3.2 Body support
In the non-impaired controls, GMED, RF+VAS and SOL+GAS each had substantial potential to generate body support (positive vertical GRF) during the first half of stance (Fig. 1b). When Modules 1 and 2 were merged in Group A, the body support generated by RF+VAS was reduced compared to controls while the support generated by SOL+GAS was increased. Similarly, when Modules 1 and 4 were merged in Group B, the body support generated by both RF+VAS and GMED was reduced compared to controls while the support generated by SOL+GAS was increased. In addition, HAM had a small potential to contribute to body support during the first half of stance in both non-impaired controls and Group A but generated negative body support in Group B. Finally, in all simulations, SOL+GAS were the primary generators of body support during the second half of stance.
3.3 Mediolateral control
In the non-impaired controls, GMED generated a medial GRF throughout stance, HAM generated a lateral GRF during the first half of stance and SOL+GAS generated a lateral GRF during the second half of stance (Fig. 1c). While GMED still generated a medial GRF in both Group A and Group B, its potential extended further into stance in Group A such that GMED generated a much larger medial GRF during the second half of stance in Group A compared to the controls.
3.4 Leg swing
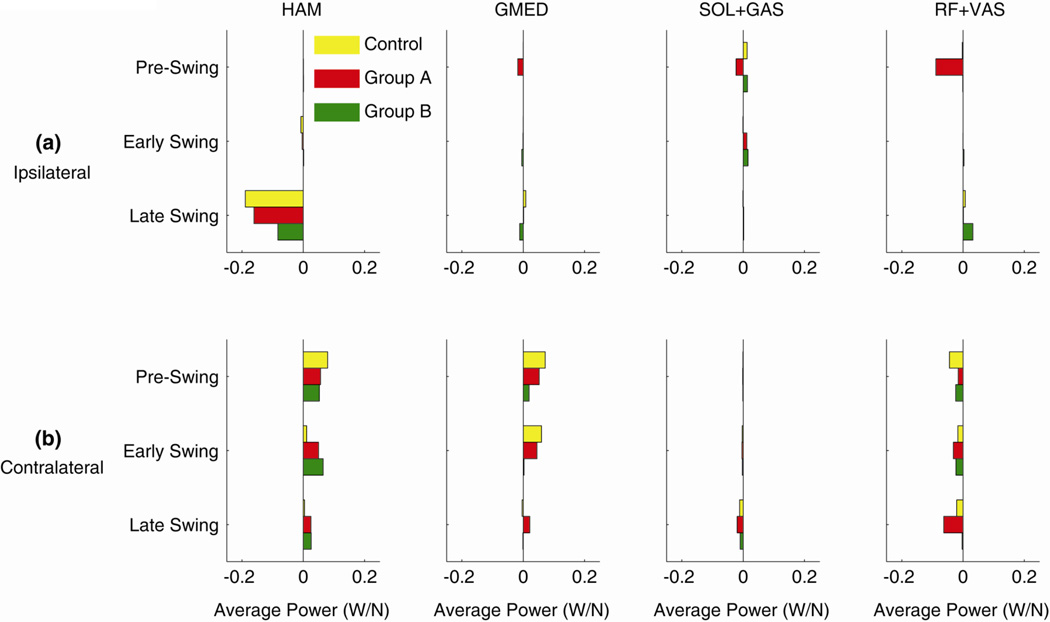
In the non-impaired controls, only HAM and SOL+GAS contributed to ipsilateral leg swing (Fig. 2a); the plantarflexors delivered energy to the leg in pre-swing while HAM absorbed energy from the leg during late swing prior to heel-strike. When Modules 1 and 2 were merged in Group A, HAM function remained the same while those muscles in the merged module (GMED, SOL+GAS and RF+VAS) all absorbed energy from the leg during pre-swing. When Modules 1 and 4 were merged in Group B, SOL+GAS function remained the same while HAM absorbed less energy from the leg, GMED absorbed energy from the leg and RF+VAS generated energy to the leg during late swing.
Figure 2.
Average power (per unit muscle force) delivered to the leg during the (a) ipsilateral/paretic and (b) contralateral/non-paretic pre-swing, early swing and late swing phases. Values for Groups A and B correspond to muscle groups on the paretic leg.
Both HAM and GMED delivered energy to the contralateral leg during pre-swing and into swing in all simulations (Fig. 2b). HAM primarily delivered energy to the contralateral leg prior to swing in the controls, but in both Group A and Group B this was extended throughout swing. GMED delivered energy to the contralateral leg during pre-swing and early swing in controls and also when Modules 1 and 2 were merged in Group A. However, when Modules 1 and 4 were merged in Group B, GMED delivered less energy to the contralateral leg during pre-swing and had a minimal effect during swing. In all groups SOL+GAS had minimal contributions to contralateral leg swing control while RF+VAS absorbed energy from pre-swing into late-swing.
4. Discussion
Muscle activity post-stroke can be explained using fewer modules than found in non-impaired control subjects, with two or more of the non-impaired modules often merged together (Clark et al., 2010). Moreover, the different coordination impairments observed post-stroke have been found to manifest in different combinations of merged modules. Understanding how different module compositions post-stroke influence walking performance has important implications for the design of targeted locomotor interventions aimed at improving rehabilitation outcomes. This study examined the effects of two commonly merged modules on walking performance post-stroke: the merging of (A) the gluteus medius and knee extensors in Module 1 with the plantarflexors in Module 2, and (B) the gluteus medius and knee extensors in Module 1 with the hamstrings in Module 4.
4.1 Group A
In non-impaired controls, Modules 1 and 2 are the primary contributors to the AP GRFs, with Module 1 generating the early stance braking (negative AP GRF) and Module 2 the late stance propulsion (positive AP GRF) that are typical during steady-state walking (Allen and Neptune, 2012; Neptune et al., 2009). A common post-stroke change in muscle coordination is that these two modules are no longer independently activated (Clark et al., 2010). Given their important but opposing contributions to the AP GRFs, it is not surprising that a consequence of this loss of independent drive was a reduction in forward propulsion generation from the paretic leg. This reduction is consistent with the reduced paretic leg propulsion found experimentally within this group (Clark et al., 2010) and our simulation results reveal that the underlying mechanism was a combination of both increased braking from the merged module and an impaired capacity of the plantarflexors to generate propulsion in late stance (Fig. 1a). The increased braking was a direct result of the altered excitation timing in the merged module such that Module 1 muscles had prolonged activity into late stance and Module 2 muscles were active in early stance (Fig. 3a). The prolonged activity of the knee extensors and gluteus medius in the merged module resulted in their contributions to braking extending into the second half of stance (Fig. 1a). Similarly, the early plantarflexor activity resulted in an increase in their contributions to braking in early stance (Fig. 1a). Furthermore, the plantarflexors also had a reduced capacity to generate forward propulsion during the second half of stance, which is consistent with recent muscle-actuated simulations of post-stroke hemiparetic walking that found reduced forward propulsion from the plantarflexors (Hall et al., 2011; Peterson et al., 2010).
Figure 3.
Module timing for the hemiparetic Group A (a and b) and Group B (c and d) simulations.(a) The merged module in Group A controls the plantarflexors (SOL+GAS) and the gluteus medius (GMED) and knee extensors (RF+VAS) which are separately controlled in the non-impaired control simulation.(b) Even though the hamstrings (HAM) are controlled with their own module in Group A, the excitation pattern is extended further into stance compared to non-impaired controls.(c) The merged module in Group B controls HAM and GMED and RF+VAS which are separately controlled in the non-impaired control simulation.(d) Even though SOL+GAS are controlled with their own module in Group B, the excitation pattern differs from controls and they are excited earlier in stance.
Some of this reduction in forward propulsion by the paretic leg appears to be compensated for by increased hamstrings activity. While the hamstrings module remained independently activated in this group, its timing differed slightly with extended activity further into stance (Fig. 3b). Similar prolonged hamstrings activity has previously been documented in other groups of post-stroke hemiparetic subjects (e.g., Den Otter et al., 2007; Knutsson and Richards, 1979; Shiavi et al., 1987), with Den Otter et al. (2007) suggesting that the prolonged hamstring activity, in conjunction with prolonged knee extensor activity, may be a compensatory mechanism used to overcome insufficient plantarflexor strength and provide additional body support. Our simulations results do not support this hypothesis as the hamstrings had only very minor contributions to body support (Fig. 1b). In contrast, their contribution to forward propulsion increased (Fig. 1a), suggesting that they may be utilized as a compensatory mechanism to counteract the increased braking from the knee extensors and plantarflexors. However, even with this increased hamstrings contribution to forward propulsion, the net forward propulsion from the paretic leg was reduced.
Another consequence of merging Modules 1 and 2 was altered paretic leg swing (Fig. 2a), which may explain the atypical relationship between propulsion asymmetry and step length asymmetry that occurs within this group. Reduction in forward propulsion from the paretic leg was previously found to be associated with high step length asymmetry values such that paretic steps lengths were longer than nonparetic step lengths (Balasubramanian et al., 2007). However, most Group A subjects exhibited symmetric step lengths despite their reduced paretic leg propulsion (see Appendix A, Table Al). The current results suggest that this may be due to increased absorption of leg energy prior to and during swing, which would act to reduce leg advancement during swing. In non-impaired walking, the gluteus medius and knee extensors are not active during the swing phase and thus have minimal contributions to leg swing control. When their activity was extended into pre-swing in Group A, they absorbed energy from the leg prior to swing. Similarly, the plantarflexors had altered contributions to paretic leg swing. In non-impaired walking, the biarticular plantarflexors (GAS) are important for swing initiation (e.g., Neptune et al., 2001) and transfer energy to the leg during pre-swing (Fig. 4a). In Group A, GAS no longer generated energy to the paretic leg during pre-swing and instead absorbed leg energy similar to the uniarticular plantarflexors (SOL, Fig. 4b). While reduced forward propulsion generation in this group appears to be the main deficit, these results suggest that improving the ability to independently control Module 1 and Module 2 may not only improve forward propulsion but may also improve leg swing.
Figure 4.
Average power delivered to the ipsilateral/paretic leg and trunk during pre-swing by the uniarticular (SOL) and biarticular (GAS) plantarflexors for (a) non-impaired controls and (b) Group A.
Finally, the prolonged contribution of the gluteus medius to the medial GRF during the second half of stance (Fig. 1c) suggests that the organization of ML control of the GRFs is altered in this group. While this could be a potential source of instability in ML control since it is the ML GRFs that prevent the center-of-mass from moving too far beyond the base of support, it is possible that other muscle groups compensate for this increased ML GRF contribution. For example, the hip adductors have previously been shown to produce a lateral GRF (Allen and Neptune, 2012; Pandy et al., 2010) and if they were co-active with the merged module they would counteract the increased medial GRF from the gluteus medius. Alternatively, it is possible that nonparetic leg muscles may compensate since the second half of stance includes a double support period in which both legs are in contact with the ground and can contribute to ML control. While we cannot specifically conclude that ML stability is impaired in this group of subjects, the results of this study suggest that some reorganization of ML center-of-mass control has occurred.
4.2 Group B
When Modules 1 and 4 were merged together in Group B, forward propulsion, body support and ipsilateral leg swing were all affected. In non-impaired walking, the hamstrings are only activated into early stance where they generate body support (Fig. 1b). However, in the merged module of Group B, this high hamstrings activity was extended further into stance with the knee extensors (Fig. 3c), which is a common co-activation pattern among post-stroke hemiparetic subjects (e.g., Knutsson and Richards, 1979; Shiavi et al., 1987). Instead of the hamstrings generating body support later into stance as would be expected, they acted to impede body support (i.e., negative body support, Fig. 1b). While not intuitive, this was due to altered kinematics that put the hamstrings in a position where they acted to flex the knee more than extend the hip, which has the overall effect of lowering the center-of—mass and compromising body support.
Paretic leg swing control also appeared affected when Modules 1 and 4 were merged. In the control subjects the gluteus medius and knee extensors were activated only near the very end of swing, and thus have minimal contributions to ipsilateral leg swing control. Consequently, when merged with Module 4, their activity began earlier in swing (Fig. 3c) and their contributions to paretic leg swing were altered: gluteus medius acted to decelerate the leg (absorb energy) while the knee extensors acted to accelerate the leg (generate energy) prior to heel-strike. In addition, hamstrings potential to decelerate the leg prior to heel-strike was reduced. Thus it appears that subjects who merge Modules 1 and 4 may have difficulty decelerating the swinging paretic leg prior to heel-strike. While the individual contributions to nonparetic leg swing were altered such that the gluteus medius had less potential to transfer energy to the nonparetic leg prior to and during swing than in controls, the hamstrings compensated through their extended contribution into swing (Fig. 2b) such that overall nonparetic leg swing control was not altered.
Finally, despite their independent recruitment, the plantarflexors in Group B were prematurely activated in stance compared to controls (Fig. 3d), which is a common abnormality following stroke (Den Otter et al., 2007; Knutsson and Richards, 1979). This premature activity could be due to either impaired plantarflexor recruitment or a compensatory strategy to overcome the effects of merging Modules 1 and 4. Given that the plantarflexors are critical generators of body support, it is possible that they were activated earlier to counteract the negative body support from the hamstrings in order to provide needed body support. Similar to the plantarfiexors in Group A, the plantarfiexors in Group B also had a reduced potential to generate forward propulsion due to a poor mechanical advantage. Therefore, while the muscles in the merged module did not impair forward propulsion generation, the overall effect left the body in such a position that the plantarfiexors were unable to generate proper forward propulsion during late stance.
4.3 Potential Limitations
One potential limitation of this study was that the tracking torques in both the Group A and Group B simulations were not completely eliminated. However, the unit force perturbation analysis used in this study is insensitive to the value of the tracking torques. This analysis identifies the potential of a muscle to contribute to specific biomechanical functions rather than its absolute contribution. While the total effect from all muscles within a merged module muscles depends on the relative amount to which they are recruited, their individual function would remain the same.
Another limitation of this study was that we did not include hip adductor muscles in any of our modules. We have previously shown this muscle group is important for the achievement of non-sagittal plane subtasks (e.g., mediolateral control;Allen and Neptune, 2012) and others have shown there to be a coupling between hip adduction and knee extension post-stroke (e.g., Cruz and Dhaher, 2008) that may adversely affect walking performance. We did not record muscle activity from the hip adductors and due to the heterogeneity of stroke we expect that this muscle group may be differentially affected post-stroke. Therefore, we could not assume any type of excitation pattern for this muscle group that would allow us to place it in a module. Future study should include the adductor muscles in the module analysis to provide a more complete assessment of the non-sagittal plane subtasks. However, the addition of these muscles should not the affect how the non-sagittal plane subtasks are altered post-stroke when the modules are merged.
Another limitation is that we only examined how changes in module composition post-stroke affect a select set of biomechanical subtasks. These were chosen based on our previous analyses showing clear relationships between each non-impaired module and these specific biomechanical subtasks (Allen and Neptune, 2012; Neptune et al., 2009). However, it is possible that the production of other subtasks (such as limb stiffness, ground clearance during swing, etc.) may play a heightened role post-stroke. In addition, we have previously shown that impaired generation of the subtasks examined in this study is related to reduced walking performance post-stroke (Hall et al., 2011; Peterson 2010). Therefore, even if modules are reorganized post-stroke to produce different task-level goals, it is important to understand how these modules impair the specific subtasks studied here to improve walking performance.
5. Conclusions
When modules are merged post-stroke their unique functional roles can no longer be utilized, and thus walking performance is impaired. The plantarflexors had reduced potential to generate forward propulsion regardless if this module was merged with Module 1 (Group A) or was independently activated (Group B), which is consistent with a previous study that found the plantarflexors were impaired in all subjects regardless of step length asymmetry (Allen et al., 2011) (note, the Group A subject had a symmetric step length ratio of 0.501 while the Group B subject had a low step length asymmetry ratio of 0.443, see Supplementary Appendix A). This suggests that improving forward propulsion from the plantarflexors should be a focus of rehabilitation in both groups of subjects. However, while the plantarflexors were independently activated by their own module in Group B, they were co-excited with the knee extensors and gluteus medius in Group A. This group of subjects would also benefit from rehabilitation programs that separately focus on each muscle group.
The remaining functional consequences depended on the specific merged modules, which highlights how subjects with each of these two common altered module compositions would benefit from different types of rehabilitation efforts. Group A exhibited impaired paretic leg swing during pre-swing, therefore rehabilitation that focuses on improving the energy delivered to the paretic leg prior to swing may be beneficial for this group. On the other hand, Group B exhibited impaired paretic leg swing prior to heel strike and rehabilitation that focuses on improving the deceleration of the paretic leg prior to heel-strike may be beneficial for this group. In Group B, body support was also compromised during early stance, and thus these subjects may benefit from rehabilitation programs that include additional focus on improving body support. Future study should be performed on other types of merged module compositions, such as those who could only independently activate two modules, to understand their different effects on walking performance and provide guidelines for rehabilitation strategies to improve their walking performance.
Supplementary Material
Acknowledgements
This work was supported by NIH Grant R01 HD46820 and the National Science Foundation Graduate Research Fellowship Program. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NICHD or NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture. 2011;33(4):538–543. doi: 10.1016/j.gaitpost.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Neptune RR. Three-dimensional modular control of human walking. J Biomech. 2012;45(12):2157–2163. doi: 10.1016/j.jbiomech.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FC. Doctoral Dissertation. The University of Texas at Austin; 1999. A dynamic optimization solution for complete cycle of normal gait. [Google Scholar]
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24(4):328–337. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol. 2006;95(6):3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103(2):844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz TH, Dhaher YY. Evidence of abnormal lower-limb torque coupling after stroke: an isometric study. Stroke. 2008;39(1):139–147. doi: 10.1161/STROKEAHA.107.492413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quervain LA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78(10):1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture. 2007;25(3):342–352. doi: 10.1016/j.gaitpost.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Goffe WL, Ferrier GD, Rogers J. Global optimization of statistical functions with simulated annealing. J Econometrics. 1994;60(1–2):65–99. [Google Scholar]
- Hall AL, Peterson GL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech. 2011;26(5):509–915. doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556(Pt 1):267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain. 1979;102(2):405–430. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- Menegaldo LL, de Toledo Fleury A, Weber HI. Moment arms and musculotendon lengths estimation for a three-dimensional lower-limb model. J Biomech. 2004;37(9):1447–1453. doi: 10.1016/j.jbiomech.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Wright IC, Van Den Bogert AJ. A Method for Numerical Simulation of Single Limb Ground Contact Events: Application to Heel-Toe Running. Comput Methods Biomech Biomed Engin. 2000;3(4):321–334. doi: 10.1080/10255840008915275. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34(11):1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004;19(2):194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: A simulation study. J Biomech. 2009;42(9):1282–1287. doi: 10.1016/j.jbiomech.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy MG, Lin YC, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech. 2010;43(11):2055–2064. doi: 10.1016/j.jbiomech.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010;43(12):2348–2355.1. doi: 10.1016/j.jbiomech.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE, Ma B, Levine WS. Muscle coordination of maximum-speed pedaling. J Biomech. 1997;30(6):595–602. doi: 10.1016/s0021-9290(96)00188-1. [DOI] [PubMed] [Google Scholar]
- Safavynia SA, Torres-Oviedo G, Ting LH. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top Spinal Cord Inj Rehabil. 2011;17(1):16–24. doi: 10.1310/sci1701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiavi R, Bugle HJ, Limbird T. Electromyographic gait assessment, Part 2: Preliminary assessment of hemiparetic synergy patterns. J Rehabil Res Dev. 1987;24(2):24–30. [PubMed] [Google Scholar]
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88(9):1127–1135. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17(4):359–411. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.