Abstract
Soman forms a stable, covalent bond with tyrosine 411 of human albumin, with tyrosines 257 and 593 in human transferrin, and with tyrosine in many other proteins. The pinacolyl group of soman is retained, suggesting that pinacolyl methylphosphonate bound to tyrosine could generate specific antibodies. Tyrosine in the pentapeptide RYGRK was covalently modified with soman simply by adding soman to the peptide. The phosphonylated-peptide was linked to keyhole limpet hemocyanin, and the conjugate was injected into rabbits. The polyclonal antiserum recognized soman-labeled human albumin, soman-mouse albumin, and soman human transferrin, but not non-phosphonylated control proteins. The soman-labeled tyrosines in these proteins are surrounded by different amino acid sequences, suggesting that the polyclonal recognizes soman-tyrosine independent of the amino acid sequence. Antiserum obtained after 4 antigen injections over a period of 18 weeks was tested in a competition ELISA where it had an IC50 of 10−11 M. The limit of detection on Western blots was 0.01 μg (15 picomoles) of soman-labeled albumin. In conclusion, a high-affinity, polyclonal antibody that specifically recognizes soman adducts on tyrosine in a variety of proteins has been produced. Such an antibody could be useful for identifying secondary targets of soman toxicity.
Keywords: soman, tyrosine, mass spectrometry, immuno MALDI
Introduction
Organophosphorus toxicants (OP) cause seizures, respiratory arrest, and death when exposure levels are high enough to inhibit greater than 70% of acetylcholinesterase activity. Inhibition of acetylcholinesterase activity causes accumulation of the neurotransmitter acetylcholine followed by overstimulation of acetylcholine receptors and disruption of nerve impulse transmission at cholinergic nerve synapses. 1-4 OP inhibit acetylcholinesterase and butyrylcholinesterase by forming a covalent bond with the active site serine. 5, 6
Proteins in addition to acetylcholinesterase and butyrylcholinesterase are modified by OP exposure.7-9 In vivo studies have identified albumin, acylpeptide hydrolase, and tubulin as proteins covalently modified by exposure to OP.8-12 Based on studies in mice with a fluorescent OP, it is likely that many proteins are modified.13 Proteins with no active site serine are covalently modified on tyrosine14-18, lysine and histidine.19, 20 Our goal was to provide a tool for identifying proteins that react with OP. Such a tool could be used to understand the mechanism of chronic illness from OP exposure.21-23 For this purpose we made a polyclonal antibody that recognizes soman-tyrosine independent of the adjacent amino acid sequence. The OP selected for this study was soman because its large bulk was expected to make a good antigen. Soman adducts on tyrosine are stable and do not age, that is, they do not lose the pinacolyl group.12, 15, 24 Adducts on tyrosine were selected because studies to date suggest that OP adducts on tyrosine occur more frequently than adducts on lysine and histidine.
Antibodies to phosphorylated tyrosine (Tyr-OHPO3−) have been developed by Cell Signaling Technology Inc., (Danvers, MA). Their P-Tyr-100 monoclonal (product #9411) has high affinity and interacts with a broad range of tyrosine phosphorylated proteins independent of the surrounding amino acid sequence. 25 This precedent encouraged us to pursue our goal to raise polyclonal antibodies to soman-tyrosine.
The method by which we made the antigen is one of the special features of our report. We achieved covalent binding of soman to tyrosine in a pentapeptide (RYGRK) simply by incubating the peptide with soman. There was no requirement for the skills of a synthetic chemist. A second unique feature of our work is that the antibody we generated has broad specificity for proteins modified on tyrosine by soman, irrespective of the amino acid sequence surrounding soman-tyrosine.
Methods
Materials
The RYGRK peptide was synthesized by GenScript (Piscataway, NJ). Racemic soman, from DGA Maîtrise NRBC (Vert-Le- Petit) was used in a chemical surety facility. Soman model compounds 26 containing thiocholine and thiomethyl in place of fluoride were synthesized at the Human BioMolecular Research Institute (San Diego, CA). The soman analog containing coumarin in place of fluoride was synthesized by Gareth R. Williams.27 The Imject Immunogen EDC kit with mcKLH #77622 and the AminoLink Coupling Gel #20381 were from Thermo Scientific, Pierce Protein Research Products, Rockford, IL. Peroxidase conjugated AffiniPure Donkey Anti-Rabbit IgG (H+L) code 711-035-152 was from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). LumiGLO Chemiluminescent Substrate System 54-61-00 was from Kierkegaard & Perry Laboratories, Gaithersburg, MD. High binding 96-well flat bottom polystyrene Immulon 2HB microtiter plates, part #3455 were from Thermo Electron Corp. (Milford, MA). Tetramethylbenzidine solution N301 was from Thermo-Fisher. Gelatin from cold water fish skin G7041 (Sigma) and bovine albumin Fraction V code 810532 (ICN via Fisher Scientific) were used in ELISA for blocking. Essentially fatty acid free and globulin free human albumin 98% pure (cat# 05418) and alpha-cyano-4-hydroxycinnamic acid (cat# 70990) were from Fluka (a member of the Sigma-Aldrich group St. Louis, MO). Human transferrin T0665 97% pure was from Sigma. Mouse albumin IR25001-1 and pooled normal human plasma (IPLA-N) were from Innovative Research Inc., Southfield, MI.
Choice of peptide sequence
It was desired to have an antibody that would recognize soman-labeled tyrosine independent of the amino acids near tyrosine. Attempts to label free tyrosine with soman were unsuccessful. A 5-amino acid peptide was selected because very short peptides are unlikely to elicit an immune response against the peptide. The sequence RYGRK contains 3 positively charged amino acids. We speculated that positively charged residues located near tyrosine would facilitate ionization of the phenolic hydroxyl group of tyrosine and therefore make it easier to label tyrosine with soman. A search of the Homo sapiens nonredundant database identified 4 proteins that contain the RYGRK sequence: 1) retinitis pigmentosa GTPAse regulator (AAG00550.1), 2) Chain A, crystal structure of human apo-Eif4aiii (pdb [2HXY]A), 3) Chain A, crystal structure of the exon junction complex at 3.2 Å resolution (pdb[2JOQ]A), and 4) eukaryotic initiation factor 4A-III (NP_055555.1); another name for this protein is KIAA0111. A search of www.plasmaproteomedatabase.org revealed that proteins 1,2, and 4 are present in human plasma, but only protein 4 contains the RYGRK sequence.
Preparation of soman-labeled RYGRK peptide
Forty mg of RYGRK (1.47 mM) in 100 mM ammonium bicarbonate pH 8.3, 0.01% sodium azide were treated with a 10-fold molar excess of soman (dissolved in isopropanol) at room temperature for 48 hours. Excess soman was inactivated by raising the pH to 10-11. Soman has a half-life of 2 min at pH 10.8.28 Comparison of peak areas for labeled and unlabeled peptide by MALDI-TOF mass spectrometry (described in the Mass Spectrometry section) showed that 75% of the peptide was labeled on tyrosine. No other residues were labeled. Labeled peptide was separated from unlabeled peptide by HPLC on a Phenomenex C18 Prodigy 5 micron ODS(2), 100 × 4.60 mm column with a gradient of 100% solvent A (0.1% trifluoroacetic acid in water) to 60% solvent B (0.09% trifluoroacetic acid in acetonitrile) in 60 min at a flow rate of 1 mL/min. Fractions containing unlabeled RYGRK (679 m/z) or soman-RYGRK (841 m/z) were identified by MALDI-TOF mass spectrometry. Unlabeled RYGRK eluted at 6-8 min; soman-RYGRK eluted at 21-26 min. Purified peptides were dried in a vacuum centrifuge, redissolved in water, and stored at −80 °C.
Preparation of soman-labeled human albumin, mouse albumin, human transferrin, and human plasma
Solutions of highly purified human albumin, mouse albumin, and human transferrin at a concentration of 1 mg/mL (15 μM) in 20 mM TrisCl pH 8.0 were treated with a ten-fold molar excess of soman for 48 h at room temperature. MALDI-TOF analysis of peptides produced by digestion with pepsin and trypsin showed that about 80% of Tyr 411 of human albumin, 80% of Tyr 411 of mouse albumin, 9% of Tyr 238 and 9% of Tyr 574 of human transferrin were covalently modified by soman.
Human plasma was treated with 200 μM soman for 2 days, digested with pepsin, and the peptides analyzed by MALDI-TOF mass spectrometry. About 1% of Tyr 411 in peptide LVRYTKKVPQVSTPTL was covalently modified by soman. Butyrylcholinesterase activity in human plasma treated with 0.01, 0.02, 0.045, 0.090, and 0.180 μM soman was inhibited 9, 17, 33, 75, and 95%, respectively.
Measuring the concentration of soman-RYGRK
The concentrations of soman-labeled RYGRK and of unlabeled RYGRK were determined by amino acid composition analysis. About 10 micrograms of salt-free peptide were hydrolyzed with 6 M HCl. Amino acids were separated by ion exchange chromatography on a Hitachi L-8800 amino acid analyzer, followed by post-column derivatization with ninhydrin. Aliquots of the same solutions submitted for amino acid composition analysis were measured for absorbance in a Gilford spectrophotometer. A 1 mM solution of unlabeled RYGRK in water pH 2.5 had an absorbance of 0.923 at 265 nm, 1.465 at 275 nm, and 1.277 at 280 nm. In contrast, a 1 mM solution of soman-RYGRK in water pH 2.5 had an absorbance of 0.432 at 265 nm, 0.110 at 275 nm, and 0.009 at 280 nm. Spectral changes in UV absorbance after covalent modification of tyrosine was consistent with the report of others.29 For routine estimates of the concentration of peptide solutions, we measured the absorbance at 265 nm for soman-RYGRK and the absorbance at 275 nm for unlabeled RYGRK.
Mass spectrometry
The MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems, Foster City, CA) was used for estimating the percent labeled peptide and for monitoring HPLC fractions. Mass spectra were acquired in positive reflector mode with delayed extraction. Five hundred laser pulses were accumulated for each measurement using a laser voltage of 3000 volts. Data collection was controlled by 4000 Series Explorer software (v 3.5, Applied Biosystems). Mass calibration was made with Cal Mix 5 (containing bradykinin, 2–9 clip; angiotensin I; Glu fibrinopeptide B; adrenocorticotropic hormone [ACTH], 1–17 clip; ACTH, 18–39 clip; and ACTH, 7–38 clip, Applied Biosystems). The amino acid modified by soman was identified from its MSMS fragmentation spectrum obtained by post-source decay, in the absence of collision gas. A MALDI target plate was spotted with 0.5 μL sample, dried, and overlaid with 0.5 μL of 10 mg/mL alpha-cyano-4-hydroxycinnamic acid in 50% acetonitrile, 0.1% trifluoroacetic acid. Spectra were analyzed with Data Explorer software (v 4.9, Applied Biosystems), which gave the cluster areas for the peptide mass signals. Percent labeled peptide was calculated from the relative cluster areas of labeled and unlabeled peptides in the same MS spectrum. Quantitation based on relative intensities in MALDI-MS spectra gives only a rough estimate of relative concentration even when both peptides are in the same spot and ionize with similar efficiency.
Conjugation to KLH and polyclonal production in rabbits
Mariculture keyhole limpet hemocyanin (KLH) was derivatized with malondialdehyde-acetaldehyde on lysines to enhance immune response.30, 31 Soman-labeled RYGRK (2 mg) was cross-linked to 2 mg of the derivatized KLH via the action of 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride. Much of the KLH precipitated during the linking step. The precipitated protein was washed with 0.083 M sodium phosphate, 0.9 M NaCl pH 7.2. Excess cross-linker was removed from the soluble conjugate with a desalting column. A suspension containing 0.25 mg/mL conjugate in 4 mL of 0.083 M sodium phosphate, 0.9 M NaCl pH 7.2 was sent to Covance Inc. (Denver, PA) for injection into 2 New Zealand White rabbits for production of polyclonal antiserum. Rabbits were injected initially with 0.125 mg (0.5 mL) conjugate. Rabbits were bled after 8, 12, 15, and 18 weeks and boosted with 0.125 mg conjugate 10 days after each bleed.
ELISA for testing rabbit antiserum
Wells in Immulon plates were coated overnight with 1 μg antigen per well in 0.2 mL of 0.1 M sodium bicarbonate/carbonate buffer pH 9.6. Antigens were soman-RYGRK and unlabeled RYGRK peptides. Wells were washed with phosphate buffered saline (pH 7.4) containing 0.05% Tween-20, blocked with 0.3 mL of 1% fish gelatin in phosphate buffered saline (no Tween) for 30 min at room temperature, and washed with phosphate buffered saline, 0.05% Tween-20. Test samples in phosphate buffered saline containing 0.05% Tween-20 were added to the wells in a volume of 0.2 mL per well. The plate was covered with an adhesive sheet and gently mixed for 2 h at room temperature. Wells were washed and incubated with 0.2 mL of horseradish peroxidase conjugated secondary antibody (diluted 1:2000) for 1 h at room temperature. Wells were washed five times before bound antibody was visualized by addition of 0.1 mL 3,3′,5,5′-tetramethylbenzidine solution per well. The reaction was stopped by addition of 0.1 mL of 0.18 M sulfuric acid. The intensity of the yellow color was measured at 450 nm in a microplate reader (Bio-Tek Instruments, Winooski, VT).
Competition ELISA for determination of polyclonal binding affinity
The first step was to determine the appropriate concentration of the polyclonal antibody. It was desired to have an absorbance of 0.4 to 0.5 at 450 nm after 30 min reaction with 3,3′,5,5′-tetramethylbenzidine. An absorbance in this range was expected to respond linearly to a change in bound polyclonal antibody, based on a published protocol.32 Immulon plates coated with 1 μg soman-albumin were treated with various dilutions of polyclonal antiserum. A 1:24,000 dilution of the fourth bleed anti-serum gave an absorbance of 0.40 after 30 min at room temperature.
Immulon plates were coated overnight with 1 μg soman-albumin in 0.2 mL of 0.1 M bicarbonate/carbonate buffer pH 9.6, blocked with bovine albumin and washed as described for ELISA. The fourth bleed anti-serum was diluted 1:24,000 by adding 2 μl antiserum to 48 mL phosphate buffered saline containing 0.05% Tween-20. Serial dilutions of soman-RYGRK peptide ranging from 1 × 10−5 to 1 × 10−14 M peptide, were prepared in phosphate buffered saline containing 0.05% Tween-20. The 1:24,000 diluted anti-serum (2.7 mL) was incubated with 0.3 mL of various dilutions of soman-RYRGK for 1 h at room temperature. The antibody/peptide mixture (0.2 mL) was added to the wells and the antibody was allowed to equilibrate with soman-albumin for 2 h at room temperature. Each antibody/peptide mixture, containing various concentrations of soman-RYGRK, was tested in 8 duplicate wells. Unbound antibody was washed off. Bound antibody was detected by incubation with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature with mixing. The activity of horseradish peroxidase was measured with 3,3′,5,5′ tetramethylbenzidine substrate. After 30 minutes the reaction was stopped with 0.18 M sulfuric acid and the absorbance at 450 nm was measured in a microplate reader.
Western blots
Gradient 4-30% polyacrylamide gels were prepared in an SE600 slab gel electrophoresis apparatus (Hoefer Scientific, San Francisco, CA). SDS gels were run for 20 h at 150 volts, constant voltage at 4°C. Proteins were electrophoretically transferred to Immun-Blot PVDF membrane (#162-0177 Bio-Rad Laboratories, Hercules, CA) using a Trans-Blot cell (Bio-Rad). The membrane was blocked with 5% nonfat dry milk dissolved in 20 mM TrisCl pH 7.4, 0.15 M NaCl, 0.2% Tween-20 and hybridized with rabbit antiserum overnight in 10 mL blocking buffer. The first bleed was used at 1:100 dilution; the fourth bleed at 1:2000. The membrane was washed and hybridized with anti-rabbit IgG conjugated to horseradish peroxidase for 1 h at 1:2000 or 1:5000 dilution. Antibody-reactive proteins were visualized with LumiGLO chemiluminescent reagent on x-ray film.
Purification of polyclonal antibody from rabbit antiserum for use on Western blot
The polyclonal rabbit antiserum was purified in two steps. In the first step, antibodies to KLH were removed by binding to agarose beads linked to KLH. Coupling of primary amines in the antigen to aldehyde in the AminoLink agarose gel occurred spontaneously upon mixing. The resulting Schiff base was reduced with cyanoborohydride in phosphate buffered saline to form a stable secondary amine linkage. One mL affinity gel was packed into a column, equilibrated with 10 mM TrisCl pH 7.5, and loaded with 0.5 mL of fourth bleed antiserum that had been diluted with 1 mL of 10 mM TrisCl pH 7.5. In the second step, the unbound material was enriched for the antibody to soman-RYGRK by affinity chromatography on agarose gel linked to soman-RYGRK-keyhole limpet hemocyanin. The agarose beads were washed with buffer and with 0.5 M NaCl in buffer. Antibody was eluted with 8 mL of ImmunoPure IgG elution buffer pH 2.8 (Pierce, 21004) into tubes containing 1 M TrisCl pH 8.5. The purified antibody was dialyzed against 10 mM TrisCl pH 7.5 to decrease the salt concentration. Antibody volume was decreased to 0.5 mL in a vacuum centrifuge. The purified antibody was used for Western blot analysis of soman-treated human plasma in Figure 7 and for immuno MALDI in Figure 8.
Figure 7.
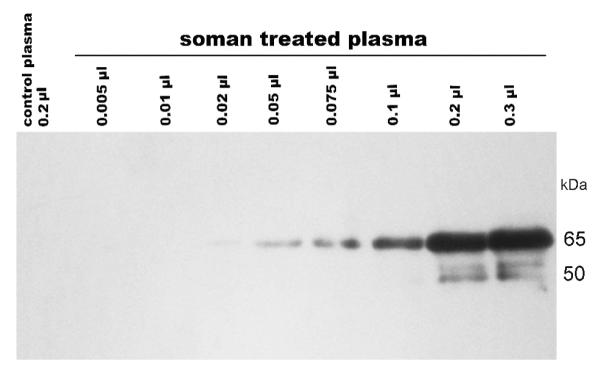
Western blot of soman-treated human plasma. Human plasma that had been treated with 200 μM soman was loaded onto SDS PAGE in volumes of 0.005 to 0.3 μl per lane. The control lane contained 0.2 μl of untreated human plasma. The proteins were transferred from the gel onto a PVDF membrane and the blot was hybridized with purified polyclonal antibody. Values on the right-side of the image indicate the molecular weight at those positions.
Figure 8.
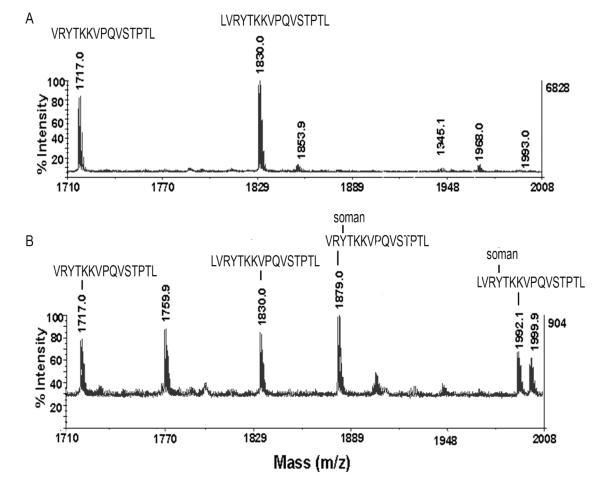
Enrichment of soman-labeled peptides by binding to antibody affinity beads.
Panel A: MALDI-TOF mass spectrum of pepsin-digested, soman-treated human plasma before enrichment. Human plasma, 10 μl treated with 0.2 mM soman, was digested with pepsin. The digest was diluted 400-fold with 0.1% TFA before 1 μl was spotted on a MALDI plate. Unlabeled albumin peptides VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL are prominent at 1717 m/z and 1830 m/z, whereas soman-labeled peptides
VRY411TKKVPQVSTPTL and LVRY411TKKVPQVSTPTL at 1879 m/z and 1992 m/z are barely detectable. Panel B: MALDI-TOF mass spectrum of peptides bound to antibody beads. Ten μl of undiluted peptic digest was bound to 5 μl antibody beads. The beads were washed and 1 μl of beads was spotted on a MALDI plate. The soman-labeled peptides at 1879 and 1992 m/z were enriched 100-fold in Panel B compared to Panel A.
Immuno MALDI
An immuno MALDI mass spectrometry assay was developed by adapting a protocol described for measurement of angiotensin I peptide in human plasma.33 Purified anti-soman-antibody was dialyzed in phosphate buffered saline to remove primary amines. A 0.5 mg aliquot of purified antibody was linked to 1.5 mL of AminoLink beads using the AminoLink kit from Pierce. The affinity beads were equilibrated with 10 mM NH4HCO3.
The pH of 10 μl of human plasma that had been treated with 0.2 mM soman, was adjusted to pH 2.5 by adding 10 μL of 1% TFA. The diluted plasma was digested for 2 h at 37°C with 1 μL of 1 mg/mL porcine pepsin dissolved in 10 mM HCl. Pepsin was inactivated by raising the pH to 7 by addition of 2.5 μL of 1 M NH4HCO3. Solid material was removed by centrifugation. The clear supernatant was incubated with 10 μL of antibody affinity beads at 4°C overnight with gentle mixing. The beads and liquid were transferred to an empty microcolumn (for example ProbeQuant G-50 microcolumn from Amersham Biosciences). The beads in the microcolumn were washed with 5 × 1 mL of 0.1 M NH4HCO3, 5 × 1 mL of 0.5 M NaCl in 0.1 M NH4HCO3, and 5 × 1 mL of 0.01M NH4HCO3 with 5 second spins in a microfuge. A 1 μL aliquot of the washed bead slurry was spotted on a MALDI plate. After the beads had dried, the spot was overlaid with 1 μL of alpha-cyano-4-hydroxycinnamic acid matrix. Mass spectra were acquired on a MALDI-TOF-TOF 4800 mass spectrometer.
Results
Characterization of soman-labeled RYGRK peptide
Incubating soman with peptide RYGRK for 48 h resulted in covalent attachment of soman to the tyrosine residue of the peptide. About 75% of the peptide acquired an added mass of +162 from O-pinacolyl methylphosphonate. Figure 1 shows the singly charged unlabeled peptide at 679.3 m/z and the soman-labeled peptide at 841.4 m/z. No adduct representing loss of the pinacolyl group from soman was present. Loss of the pinacolyl group would have yielded a peptide with an added mass of +78 at 757.3 m/z. The small unresolved peak with this mass is a metastable ion produced during flight in the mass spectrometer, after the reflector. Fragments produced in the mass spectrometer are characterized by a broad, unresolved peak. The finding of soman-RYGRK adduct in Figure 1 showed that simply adding soman to the peptide in pH 8.3 buffer resulted in covalent binding. There was no need of a chemical cross-linker.
Figure 1.
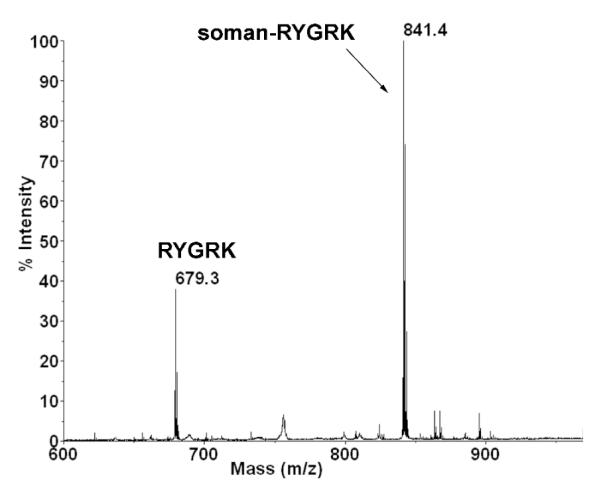
MS spectrum showing relative abundance of unlabeled RYGRK and soman-RYGRK. A reaction mixture of 1.47 mM RYGRK and 14.7 mM soman in 100 mM ammonium bicarbonate was diluted 1:100 before spotting 0.5 μl on a MALDI plate with alpha-cyano-4-hydroxycinnamic acid matrix. The peak at m/z 679.3 is unlabeled peptide RYGRK. The peak at m/z 841.4 is soman-labeled RYGRK. Calculation of cluster areas showed that 75% of the peptide solution was soman-labeled.
The phosphonylation reaction was less efficient with soman model compounds containing thiomethyl, thiocholine, or coumarin leaving groups in place of fluoride (Figure 2). No adduct was formed with the thiomethyl analog. Less than 10% of the peptide was labeled with the coumarin analog, and 20 to 40% with the thiocholine analog. These soman model compounds have been shown to react with acetylcholinesterase and butyrylcholinesterase to make a covalent bond with the active site serine.26, 27, 34
Figure 2.
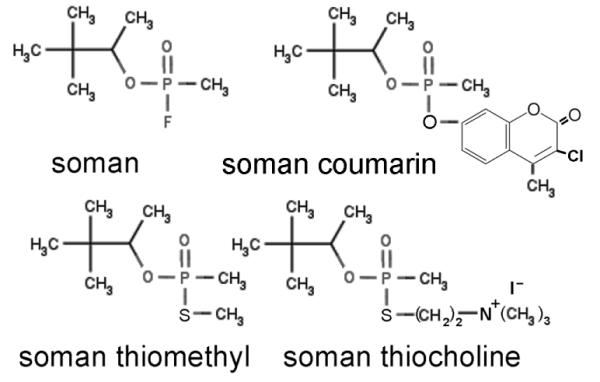
Structures of soman and soman model compounds. Authentic soman has fluoride as a leaving group. The model compounds have coumarin, thiomethyl and thiocholine leaving groups. Tyrosine in peptide RYGRK was modified more readily by soman than by soman model compounds.
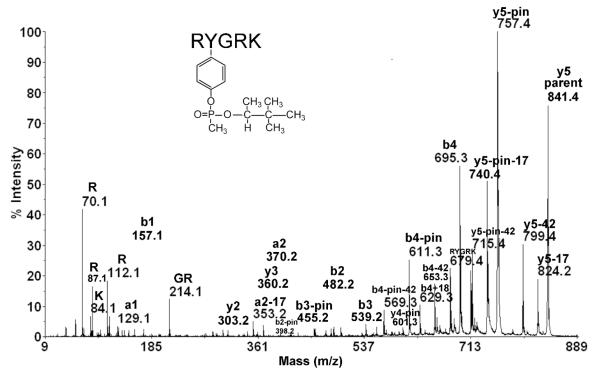
Proof that soman had bound covalently to tyrosine in peptide RYGRK was obtained by analyzing the fragment ions of parent 841.4 in Figure 3. The most intense peak, at 757.4 m/z, is the parent ion after it has lost the pinacolyl group (pin) from soman. Other ions that lost the pinacolyl group (pin) are b2-pin (398.2), b3 pin (455.2), b4 pin (611.3), and y4-pin (601.3). These masses are consistent with soman modification of tyrosine because the amino acid component of their masses (RY, RYG, RYGR and YGRK) all contain tyrosine. Two ions lost both the pinacolyl group (minus 84) from soman and the HN=CN=NH group (minus 42) from arginine; they are b4-pin-42 (569.3) and y5-pin-42 (715.4). Loss of HN=CN=NH (42 m/z) from the guanidine group of arginine has been documented for peptides containing multiple basic amino acids.35 The b1 (157.1), b2 (482.2), b3 (539.2) and b4 ions (695.3) also support the conclusion that the soman-labeled residue is tyrosine. The interval between b1 and b2 is 325 m/z, a value equal to the dehydro-mass of tyrosine (163 m/z) plus the mass of the methyl pinacolyl phospho-adduct (162 m/z). The masses for b3 and b4 are consistent with peptide fragments RYG and RYGR plus the methyl pinacolyl phospho-adduct while the mass of b1 is simply the dehydro mass of the N-terminal amino acid (R). The ion at 679.4 is the intact RYGRK peptide with no modifications, the laser having removed the entire O-pinacolyl methylphosphonate group. The ion at 214.1 is GR from an internal fragment. The ions at 70.1, 87.1, and 112.1 are fragments of arginine. The ion at 84.1 is a fragment of lysine. In summary, the ion masses in Figure 3 support the conclusion that tyrosine in peptide RYGRK has been covalently modified by soman.
Figure 3.
MSMS spectrum identifying tyrosine in peptide RYGRK as the residue modified by soman. The sample was prepared as described in the legend to Figure 1. The singly charged parent ion has a mass of 841.4 m/z. Loss of the pinacolyl group is designated “–pin” for minus pinacolyl. Loss of HN=C=NH from arginine is designated −42. Loss of NH3 is designated −17.
ELISA
Rabbits injected with soman-RYGRK conjugated to keyhole limpet hemocyanin were first bled 8 weeks later. The first bleed was tested by ELISA for binding to soman-RYGRK and unlabeled RYGRK peptide. ELISA showed that at a 1:100 dilution the polyclonal antiserum bound to the soman-labeled peptide, but gave only background signal with unlabeled peptide.
Western blot
The first bleed was also used to test the binding specificity of the polyclonal on a Western blot. Soman-labeled and control unlabeled human albumin, mouse albumin, and human transferrin were loaded onto a SDS polyacrylamide gel in quantities of 5, 7, or 10 μg per lane. Proteins were transferred to a PVDF membrane and hybridized with the first bleed at 1:100 dilution. Figure 4 shows that the polyclonal recognized soman-labeled proteins, but did not recognize non phosphonylated control proteins.
Figure 4.
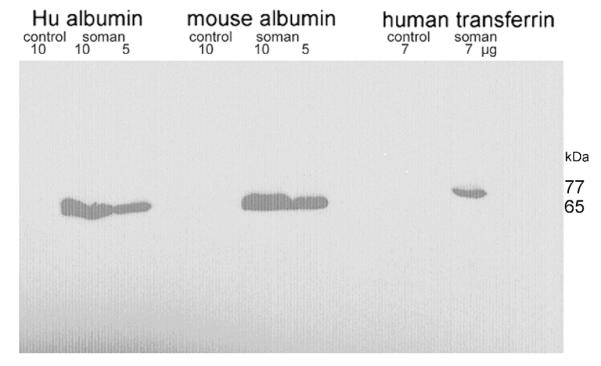
Western blot showing binding of the polyclonal to proteins covalently modified on tyrosine by soman. Lanes containing the soman-labeled proteins, designated “soman”, were compared with the same proteins unlabeled, designated “control”. Control proteins did not bind the antibody. Proteins included human albumin (accession number F6KPG5), mouse albumin (P07724) and human transferrin (TFQTL1). The amount of protein per lane was 5, 7, or 10 μg.
Previous mass spectral studies identified the soman-labeled amino acids in these three proteins as tyrosine.15, 24, 36 These same studies identified the sequences near the labeled tyrosine. Those sequences are given in Table 1. Each sequence is different from the next, and different from the RGYRK sequence used to generate the polyclonal. The fact that the polyclonal recognizes soman-labeled proteins that have different amino acid sequences near the soman-labeled tyrosine and does not recognize the unlabeled control strongly suggests that the antibody is specific for soman-labeled tyrosine and that it does not recognize the adjacent amino acids.
Table 1.
Amino acid sequence near the soman labeled tyrosine
| soman labeled protein | accession number |
sequence near soman labeled tyrosine* |
Reference |
|---|---|---|---|
| Human albumin | gi:122920512 | RY411TKK | 24 |
| Mouse albumin | gi:3647327 | RY435TQK | 36 |
| Human transferrin | gi:4557871 | EY257KDC | 15 |
| Human transferrin | gi:4557871 | EY593ANC | 15 |
| RYGRK | RY2GRK | present report |
The amino acid position of soman labeled tyrosine is indicated by the subscript number. Tyr 411 in human albumin is the residue number for the mature protein, missing the 24 residue signal peptide. Tyr 435 in mouse albumin is analogous to Tyr 411 of human albumin. Residue numbers for human transferrin include 19 amino acids in the signal peptide. The accession numbers from the National Center for Biotechnology Information database are associated with the complete amino acid sequence.
Determination of antibody binding efficiency
The binding efficiency of the polyclonal antiserum (4th bleed) was tested in a competition ELISA experiment that used soman-labeled tyrosine in peptide RYGRK to stop the reaction between the antibody and soman-labeled albumin, as described in the Methods section. Figure 5 shows that the signal intensity from the soman-labeled albumin was inversely related to the amount of soman-RYGRK. The half-effect value indicated that the labeled-peptide binds to the antibody with a very high binding efficiency, an IC50 of ~ 10−11 M. It was concluded that soman-labeled tyrosine in peptide RYGRK competes effectively with soman-labeled tyrosine in human albumin for binding to the polyclonal antibody.
Figure 5.
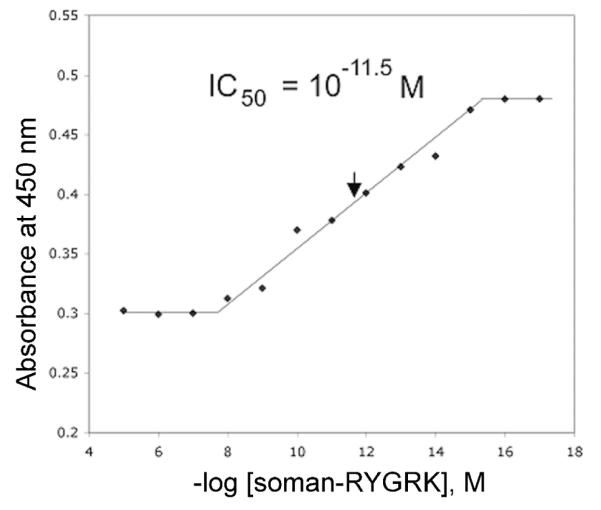
Competition ELISA for determination of the IC50 of the polyclonal to soman-tyrosine. The competition for binding to the antibody was between soman-labeled human albumin and soman-labeled tyrosine in peptide RYGRK. The polyclonal antiserum (fourth bleed) was diluted 1:24,000. The IC50 was 1 × 10−11.5 M. Each point is the average of 8 replicates. The assay was repeated 4 times. Data are presented in semi-log format with soman-RYGRK values on the x-axis being shown as the negative logarithms of their molar concentrations.
Limit of detection of pure soman-albumin on Western blot
The limit of detection for soman-labeled human albumin with the fourth bleed antiserum was estimated by Western blot analysis. The Western blot in Figure 6 shows that as little as 0.01μg of soman-labeled human albumin (80% labeled) was detected with the polyclonal antibody. This is equivalent to about 1.3×10−13 moles of labeled-albumin (that is: 0.01 μg soman-labeled albumin × 1×10−6 g/μg ÷ 6×104 g albumin/mole × 0.8 fraction labeled = 1.3×10−13 moles detection limit). This, in turn, is equivalent to 200 μL plasma containing soman-labeled albumin at a concentration of 6×10−10 M or 0.0001% of the concentration of albumin in plasma (600 μM) (that is: 1.3×10−13 moles albumin detection limit ÷ 6×10−4 moles albumin/liter divided by 2×10−4 liter sample volume = 1.0×10−6 fraction albumin labeled or 0.0001%; then 600×10−6 M albumin × 1×10−6 fraction albumin labeled = 6×10−10 M labeled albumin). The limit of detection on a Western blot of 0.01 μg (1.3×10−13 moles) of soman-albumin is consistent with the IC50 value of 1 × 10−11 M determined by ELISA competition.
Figure 6.
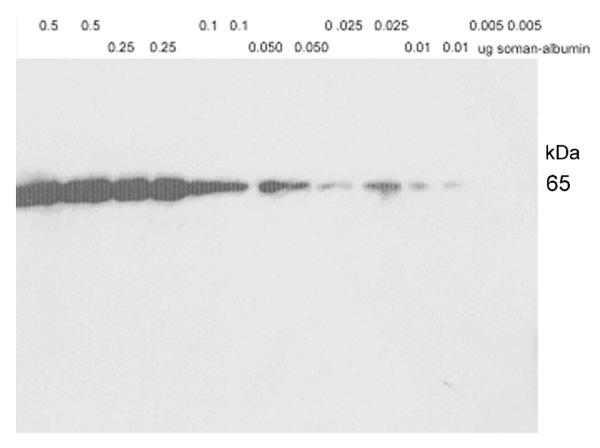
Limit of detection of soman-labeled human albumin on a Western blot. Lanes were loaded in duplicate with 0.5 to 0.005 μg of soman-labeled albumin. The blot was hybridized with fourth bleed antiserum diluted 1:2000. Samples were detected via chemiluminescence using horseradish peroxidase linked to anti-rabbit IgG. The limit of detection was 0.01 μg of soman-labeled human albumin.
Western blot for detection of soman treated plasma
Human plasma has a protein concentration of about 50-70 mg/mL, of which albumin constitutes about 40 mg/mL. The high protein concentration limits the amount of plasma that can be loaded into one lane of an SDS gel to about 0.2 μL (significantly higher loadings distorted the albumin band). In the previous section, the limit of detection was shown to be equivalent to the amount of albumin in 200 μl of serum if labeling were 0.0001%. If only 0.2 μL of serum can be loaded onto a gel (i.e., a 1000-fold lower volume), Western blotting can be expected to detect soman exposure only if the soman concentration is great enough to modify at least 0.1% of plasma albumin.
Treating human plasma with 200 μM soman for 2 hours labeled 1% of the albumin on Tyr 411. When various volumes of this plasma preparation were loaded onto an SDS gel (0.005 to 0.3 μL), transferred to PVDF membrane and hybridized with purified polyclonal antibody, a strong signal was obtained in the albumin band for the 0.2 μL sample (Figure 7). It was essential to use purified polyclonal antibody for detection of soman-labeled protein in plasma. The limit of detection was 0.02 μL of plasma, a volume estimated to contain 0.01 μg of soman-labeled albumin (6×10−4 moles albumin/liter × 0.01 (1%) fraction labeled × 2×10−8 liter sample size × 6×104 g albumin/mole = 7×10−9 g or 0.007 μg). This result is consistent with the limit of detection determined for pure human albumin labeled with soman. Weaker bands at about 50 kDa for IgG also appeared in Figure 7, suggesting that IgG was also modified by soman.
Immuno MALDI
Purified polyclonal antibody covalently linked to agarose beads was used to enrich for soman-labeled peptides in human plasma. Figure 8 compares the mass spectrum of pepsin-digested plasma before enrichment on affinity beads (panel A) with the mass spectrum of the same pepsin-digested plasma after enrichment (panel B). Soman-labeled albumin peptides VRY411TKKVPQVSTPTL and LVRY411TKKVPQVSTPTL at 1879 m/z and 1992 m/z were enriched 100- to 1000-fold by binding to the antibody affinity beads. The presence of the non-adducted peptides shows that the antibody-agarose beads are not entirely specific for soman adducts on tyrosine. The nonspecific binding could be either to the antibody or to the agarose beads. The plasma in Figure 8 had been treated with 0.2 mM soman resulting in 1% labeling of Tyr 411 of albumin. This concentration of soman is 1000-fold higher than the lethal concentration. The immuno MALDI method was also tested with plasma that had been treated with 1000-fold lower concentrations of soman. The soman-labeled peptide was not detectable in a 400 μL digest.
Discussion
We have created a polyclonal antibody with excellent affinity and specificity for soman-labeled tyrosine. Our experimental design introduced a method for making OP-adducts on tyrosine that required no skills in synthesis of organic molecules. Simply mixing the peptide with soman resulted in covalent addition of soman to tyrosine. Other OP and other peptides also make stable adducts (data not shown). Thus, we have introduced a general method for making antigens to OP-tyrosine.
Use of soman-labeled tyrosine as the antigen for an antibody-based assay to detect proteins labeled by soman is a major paradigm change. It is a new alternative to the soman-labeled serine in cholinesterase approach for preparing antibodies to soman-labeled amino acids, that has heretofore proven refractory. Our results establish that soman forms a covalent bond with tyrosine in proteins and that an antibody can be made to specifically detect soman-tyrosine adducts.
Racemic soman is an equimolar mixture of four stereoisomers. Albumin treated with racemic soman has pinacolylmethylphosphonate on Tyr 411.24 Experiments with 31P NMR showed that albumin has no enantiomeric preference for the four stereoisomers of soman.24 The soman-labeled RYGRK antigen was also made with racemic soman, which means the antigen contains all 4 soman stereoisomers on tyrosine. If the polyclonal antibody were specific for one soman stereoisomer, the binding affinity measured with a mixture of soman-RYGRK would be 4-fold weaker than the actual binding affinity.
Comparison to published antibodies against soman
The polyclonal in the present report was designed to detect any protein modified on tyrosine by soman. It exhibits an IC50 of 10−11 M and a detection limit on Western blots of 15 picomoles, with soman-labeled albumin. Such an antibody has not previously been made though antibodies for detection of soman exposure 37 or for detoxication of soman poisoning have been produced.38-40 Monoclonal mAb-HSA-GD specifically detects human albumin covalently modified by soman on tyrosine 411.37 Monoclonal BE2 was produced by mice immunized with a p-aminophenol derivative of soman bound to bovine albumin through a diazo linkage, a structure similar to soman-tyrosine.39 The BE2 monoclonal recognizes intact racemic soman with an IC50 value of 0.1 mM. Monoclonal #2-ID8.2 was produced by mice immunized with soman-joined to human albumin through the pinacolyl group.39 The #2-ID8.2 monoclonal recognizes proteins adducted by soman, sarin, tabun, and VX with IC50 values ranging from 0.2 to 10 μM. The #2-ID8.2 monoclonal, after conjugation to colloidal gold nanoparticles, has been incorporated into a lateral flow point-of-care test for exposure to nerve agents.41
Suggestions for future work
It is anticipated that by using the strategy described in this paper a monoclonal antibody could be generated with a binding affinity similar to the polyclonal in this report, namely 10−11 M, to specifically bind proteins labeled on tyrosine by soman. A monoclonal produced in rabbit is likely to have a higher binding affinity than a monoclonal produced in mouse.42 Such a monoclonal could be used to selectively enrich soman-labeled proteins. For example, the monoclonal immobilized on magnetic beads coated with Protein G could be used to immunopurify soman-tyrosine labeled proteins from a large volume of plasma.43, 44 Organophosphorus pesticides make adducts on tyrosine in proteins.14, 15, 18 Therefore, monoclonals that recognize proteins modified by organophosphorus pesticides could be produced. The enriched preparations could be analyzed by Western blotting and by mass spectrometry to identify the modified proteins. Knowledge of the identity of the OP-modified proteins could lead to an understanding of chronic illness from exposure to nerve agents and organophosphorus pesticides.
Acknowledgment
We thank Laurey Steinke and Michelle Fontaine of the Protein Structure core facility at the University of Nebraska Medical Center for amino acid composition analysis. Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
Funding This work was funded by the National Institute of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke awards [Grants NS058183 and, NS058038 to JC; U01 NS058056 to OL]. NIH Cancer Center [Grant P30CA036727 to the Eppley Cancer Center, directed by Kenneth Cowan]. NIH/National Institutes for Alcoholism and Alcohol Abuse M.E.R.I.T. Award [Grant 2 R01/5R37AA078-17 to LWK and GMT]. U.S. Army Medical Research and Materiel Command [W81XWH-07-2-0034 to OL]. U.S. Army Medical Research Institute of Chemical Defense [V91ZLK-06-R-0029 to JZ]. Direction Générale de l’Armement of the French Ministry of Defense [DGA grant 03co010-05/PEA01 08 7 to PM; DGA/PEA 08co501 and BioMeDef PDH-2-NRBC-3-C-301 to FN]. GRW was supported by the UK Ministry of Defence. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the United States, British, or French governments.
Abbreviations
- ACTH
adrenocorticotropic hormone
- KLH
keyhole limpet hemocyanin
- MALDI-TOF
matrix assisted laser desorption ionization-time of flight mass spectrometry
- MSMS
tandem mass spectrometry
- m/z
ratio of mass to charge
- OP
organophosphorus compound
- pin
pinacolyl group of soman
References
- (1).Grob D, Garlick WL, Harvey AM. The toxic effects in man of the anticholinesterase insecticide parathion (p-nitrophenyl diethyl thionophosphate) Bull. Johns Hopkins Hosp. 1950;87:106–129. [PubMed] [Google Scholar]
- (2).Namba T, Nolte CT, Jackrel J, Grob D. Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Am. J. Med. 1971;50:475–492. doi: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- (3).Marrs TC. Organophosphate poisoning. Pharmacol. Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-m. [DOI] [PubMed] [Google Scholar]
- (4).Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MH, Szinicz L, Dawson AH, Buckley NA. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- (5).Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- (6).Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- (7).Black RM, Harrison JM, Read RW. The interaction of sarin and soman with plasma proteins: the identification of a novel phosphonylation site. Arch. Toxicol. 1999;73:123–126. doi: 10.1007/s002040050596. [DOI] [PubMed] [Google Scholar]
- (8).Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol. Pharmacol. 2000;58:577–583. doi: 10.1124/mol.58.3.577. [DOI] [PubMed] [Google Scholar]
- (9).Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol. Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Li B, Ricordel I, Schopfer LM, Baud F, Megarbane B, Nachon F, Masson P, Lockridge O. Detection of adduct on tyrosine 411 of albumin in humans poisoned by dichlorvos. Toxicol. Sci. 2010;116:23–31. doi: 10.1093/toxsci/kfq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).van der Schans MJ, Hulst AG, van der Riet-van Oeveren D, Noort D, Benschop HP, Dishovsky C. New tools in diagnosis and biomonitoring of intoxications with organophosphorothioates: Case studies with chlorpyrifos and diazinon. Chem. Biol. Interact. 2012 Nov 1; doi: 10.1016/j.cbi.2012.10.014. 2012. doi:pii: S0009-2797(12)00220-7. 10.1016/j.cbi.2012.10.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- (12).Williams NH, Harrison JM, Read RW, Black RM. Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch. Toxicol. 2007;81:627–639. doi: 10.1007/s00204-007-0191-8. [DOI] [PubMed] [Google Scholar]
- (13).Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol. Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- (14).Schopfer LM, Grigoryan H, Li B, Nachon F, Masson P, Lockridge O. Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: characteristic mass fragments and a new binding motif for organophosphates. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:1297–1311. doi: 10.1016/j.jchromb.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Li B, Schopfer LM, Grigoryan H, Thompson CM, Hinrichs SH, Masson P, Lockridge O. Tyrosines of human and mouse transferrin covalently labeled by organophosphorus agents: a new motif for binding to proteins that have no active site serine. Toxicol. Sci. 2009;107:144–155. doi: 10.1093/toxsci/kfn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Li B, Ricordel I, Schopfer LM, Baud F, Megarbane B, Nachon F, Masson P, Lockridge O. Detection of adducts on tyrosine 411 of albumin in humans poisoned by dichlorvos. Toxicol. Sci. 2010;116:23–31. doi: 10.1093/toxsci/kfq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Grigoryan H, Li B, Anderson EK, Xue W, Nachon F, Lockridge O, Schopfer LM. Covalent binding of the organophosphorus agent FP-biotin to tyrosine in eight proteins that have no active site serine. Chem. Biol. Interact. 2009;180:492–498. doi: 10.1016/j.cbi.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents. Chem. Biol. Interact. 2008;175:180–186. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Grigoryan H, Li B, Xue W, Grigoryan M, Schopfer LM, Lockridge O. Mass spectral characterization of organophosphate-labeled lysine in peptides. Anal. Biochem. 2009;394:92–100. doi: 10.1016/j.ab.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Liyasova MS, Schopfer LM, Lockridge O. Cresyl saligenin phosphate, an organophosphorus toxicant, makes covalent adducts with histidine, lysine, and tyrosine residues of human serum albumin. Chem. Res. Toxicol. 2012;25:1752–1761. doi: 10.1021/tx300215g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Beseler CL, Stallones L, Hoppin JA, Alavanja MC, Blair A, Keefe T, Kamel F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ. Health Perspect. 2008;116:1713–1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum. Exp. Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- (23).Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li B, Nachon F, Froment MT, Verdier L, Debouzy JC, Brasme B, Gillon E, Schopfer LM, Lockridge O, Masson P. Binding and hydrolysis of soman by human serum albumin. Chem. Res. Toxicol. 2008;21:421–431. doi: 10.1021/tx700339m. [DOI] [PubMed] [Google Scholar]
- (25).Heibeck TH, Ding SJ, Opresko LK, Zhao R, Schepmoes AA, Yang F, Tolmachev AV, Monroe ME, Camp DG, 2nd, Smith RD, Wiley HS, Qian WJ. An extensive survey of tyrosine phosphorylation revealing new sites in human mammary epithelial cells. J. Proteome Res. 2009;8:3852–3861. doi: 10.1021/pr900044c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Barakat NH, Zheng X, Gilley CB, MacDonald M, Okolotowicz K, Cashman JR, Vyas S, Beck JM, Hadad CM, Zhang J. Chemical synthesis of two series of nerve agent model compounds and their stereoselective interaction with human acetylcholinesterase and human butyrylcholinesterase. Chem. Res. Toxicol. 2009;22:1669–1679. doi: 10.1021/tx900096j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Briseno-Roa L, Hill J, Notman S, Sellers D, Smith AP, Timperley CM, Wetherell J, Williams NH, Williams GR, Fersht AR, Griffiths AD. Analogues with fluorescent leaving groups for screening and selection of enzymes that efficiently hydrolyze organophosphorus nerve agents. J. Med. Chem. 2006;49:246–255. doi: 10.1021/jm050518j. [DOI] [PubMed] [Google Scholar]
- (28).Ward JR, Yang YC, Wilson RB, Burrows WD, Ackerman LL. Base-catalyzed hydrolysis of 1,2,2-trimethylpropyl methylphosphonofluoridate - an examination of the saturation effect. Bioorg. Chem. 1988;16:12–16. [Google Scholar]
- (29).Riordan JF, Wacker WEC, Vallee BL. N-Acetylimidazole: a reagent for determination of “free” tyrosyl residues of proteins. Biochemistry. 1965;4:1758–1765. [Google Scholar]
- (30).Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin. Exp. Res. 1998;22:1731–1739. [PubMed] [Google Scholar]
- (31).Willis MS, Klassen LW, Tuma DJ, Sorrell MF, Thiele GM. Adduction of soluble proteins with malondialdehyde-acetaldehyde (MAA) induces antibody production and enhances T-cell proliferation. Alcohol Clin. Exp. Res. 2002;26:94–106. [PubMed] [Google Scholar]
- (32).Hunter KW, Jr., Lenz DE, Brimfield AA, Naylor JA. Quantification of the organophosphorus nerve agent soman by competitive inhibition enzyme immunoassay using monoclonal antibody. FEBS Lett. 1982;149:147–151. doi: 10.1016/0014-5793(82)81091-0. [DOI] [PubMed] [Google Scholar]
- (33).Reid JD, Holmes DT, Mason DR, Shah B, Borchers CH. Towards the development of an immuno MALDI (iMALDI) mass spectrometry assay for the diagnosis of hypertension. J. Am. Soc. Mass Spectrom. 2010;21:1680–1686. doi: 10.1016/j.jasms.2010.01.024. [DOI] [PubMed] [Google Scholar]
- (34).Gilley C, MacDonald M, Nachon F, Schopfer LM, Zhang J, Cashman JR, Lockridge O. Nerve agent analogues that produce authentic soman, sarin, tabun, and cyclohexyl methylphosphonate-modified human butyrylcholinesterase. Chem. Res. Toxicol. 2009;22:1680–1688. doi: 10.1021/tx900090m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Shields SJ, Bluhm BK, Russell DH. Fragmentation chemistry of [M + Cu]+ peptide ions containing an N-terminal arginine. J. Am. Soc. Mass Spectrom. 2000;11:626–638. doi: 10.1016/S1044-0305(00)00128-8. [DOI] [PubMed] [Google Scholar]
- (36).Lockridge O, Schopfer LM. Review of tyrosine and lysine as new motifs for organophosphate binding to proteins that have no active site serine. Chem. Biol. Interact. 2010;187:344–348. doi: 10.1016/j.cbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Chen S, Zhang J, Lumley L, Cashman JR. Immunodetection of serum albumin adducts as biomarkers for organophosphorus exposure. J. Pharmacol. Exp. Ther. 2013;344:531–541. doi: 10.1124/jpet.112.201368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Brimfield AA, Hunter KW, Jr., Lenz DE, Benschop HP, Van Dijk C, de Jong LP. Structural and stereochemical specificity of mouse monoclonal antibodies to the organophosphorous cholinesterase inhibitor soman. Mol. Pharmacol. 1985;28:32–39. [PubMed] [Google Scholar]
- (39).Johnson JK, Cerasoli DM, Lenz DE. Role of immunogen design in induction of soman-specific monoclonal antibodies. Immunol. Lett. 2005;96:121–127. doi: 10.1016/j.imlet.2004.08.003. [DOI] [PubMed] [Google Scholar]
- (40).Jia P, Wang Y, Yu M, Wu J, Yang R, Zhao Y, Zhou L. An organophosphorus hapten used in the preparation of monoclonal antibody and as an active immunization vaccine in the detoxication of soman poisoning. Toxicol. Lett. 2009;187:45–51. doi: 10.1016/j.toxlet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- (41).Vandine R, Babu UM, Condon P, Mendez A, Sambursky R. A 10-minute point-of-care assay for detection of blood protein adducts resulting from low level exposure to organophosphate nerve agents. Chem. Biol. Interact. 2012 Nov 27;2012 doi: 10.1016/j.cbi.2012.11.011. pii: S0009-2797(12)00255-4. doi: 10.1016/j.cbi.2012.11.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- (42).Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl. Acad. Sci. USA. 1995;92:9348–9352. doi: 10.1073/pnas.92.20.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Sporty JL, Lemire SW, Jakubowski EM, Renner JA, Evans RA, Williams RF, Schmidt JG, van der Schans MJ, Noort D, Johnson RC. Immunomagnetic separation and quantification of butyrylcholinesterase nerve agent adducts in human serum. Anal. Chem. 2010;82:6593–6600. doi: 10.1021/ac101024z. [DOI] [PubMed] [Google Scholar]
- (44).Marsillach J, Richter RJ, Kim JH, Stevens RC, MacCoss MJ, Tomazela D, Suzuki SM, Schopfer LM, Lockridge O, Furlong CE. Biomarkers of organophosphorus (OP) exposures in humans. Neurotoxicology. 2011;32:656–660. doi: 10.1016/j.neuro.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]