Abstract
Objectives
To evaluate performance of kidney function estimation equations and to determine the frequency of drug dose discordance in an older population.
Design
Cross-sectional analysis of data from community-dwelling volunteers randomly selected from the Baltimore Longitudinal Study of Aging from January 1, 2005–December 31, 2010.
Subjects
Two hundred sixty-nine men and women with a mean ± SD age of 81 ± 6 years, mean serum creatinine concentration (Scr) of 1.1 ± 0.4 mg/dl, and mean measured 24-hour creatinine clearance (mClcr) of 53 ± 13 ml/minute.
Measurements and Main Results
Kidney function was estimated by using the following equations: Cockcroft-Gault (CG), Modification of Diet in Renal Disease Study (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). The performance of each equation was assessed by measuring bias and precision relative to mClcr. Dose calculation errors (discordance) were determined for 10 drugs requiring renal dosage adjustments to avoid toxicity when compared to the FDA-approved dosages. The CG equation was the least biased estimate of mClcr. The MDRD and CKD-EPI equations were significantly positively biased compared to CG (mean ± SD 34 ± 20% and 22 ± 15%, respectively, p<0.001) and mClcr (29 ± 47% and 18 ± 40%, respectively, p<0.001). Rounding low Scr values (< 1.0 mg/dl) up to an arbitrary value of 1.0 mg/dL resulted in CG values (44±10 mL/min) that were significantly lower than mClcr (56±12 mL/min, p<0.001) and CG (56±15 mL/min, p<0.001). The MDRD and CKD-EPI equations had median dose discordance rates of 28.6% and 22.9%, respectively.
Conclusion
The MDRD and CKD-EPI equations significantly overestimated creatinine clearance (mClcr and CG) in elderly individuals. This leads to dose calculation errors for many drugs, particularly in individuals with severe renal impairment. Thus, GFR-estimating equations should not be substituted in place of the CG equation in older adults for the purpose of renal dosage adjustments. In addition, the common practice of rounding or replacing low Scr values with an arbitrary value of 1.0 mg/dL for use in the CG equation should be avoided. Additional studies that evaluate alternative eGFR equations in the older populations that incorporate pharmacokinetic and pharmacodynamic outcomes measures are needed.
Keywords: GFR, glomerular filtration rate, creatinine clearance, geriatrics, BLSA, drug safety
Age-related decline in kidney function is seen in a substantial portion of the older population.1 Nearly 40% of adults aged 70 years or older have an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2.2 Most of these older adults have no obvious source of loss of kidney function other than physiologic aging. Accurate estimation of kidney function is especially important in this population (13% of the US population), as these older adults consume nearly 34% of all prescription drugs,3 and many of these drugs have elimination that is dependent on the kidneys. Estimating kidney function by using an equation that is based on serum creatinine concentration (Scr) instead of directly measuring kidney function can lead to substantial dosing errors in some populations. Older adults can have normal or minimally increased Scr in the presence of significantly reduced renal function due to their reduced muscle mass. Failure to account for reduced glomerular filtration rate (GFR) can lead to excessive drug doses due to prolongation of the drug’s half-life, especially in older adults.4,5 Hanlon et al. recently reported that nearly 12% of the residents in a Veterans Affairs nursing home were prescribed at least one incorrect dosage based on kidney function; excessive drug doses were the most common medication error reported in this study.6
Collecting urine for determination of measured 24-hour creatinine clearance (mClcr) is the gold standard measurement of kidney function in pharmacokinetic studies conducted during drug development. However, this method is time consuming and logistically difficult, and is rarely done in clinical settings. For almost 50 years, kidney function has been estimated using the Cockcroft-Gault (CG) equation, which estimates creatinine clearance based on Scr, age, sex, and weight.7 A recent survey of new drug applications submitted to the FDA from 1998–2007 showed that the CG equation was specifically mentioned as the basis for calculating dosage adjustments in patients with renal impairment for 25% of the drugs reviewed.8 Other equations have been proposed for estimating renal function, including the Modification of Diet in Renal Disease (MDRD) equation9 and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.10 It is important to note that the CG equation was designed to estimate creatinine clearance, whereas the MDRD and CKD-EPI equations estimate GFR. The GFR is the volume of blood delivered to Bowman’s capsule per unit of time and is regulated by afferent and efferent tone in the vessels before and after the capsule. The GFR can be directly measured by inulin or iothalamate clearance. Creatinine clearance, on the other hand, is affected by GFR and postcapsule secretion and is directly measured in a 24-hour urine collection. Although the MDRD and CKD-EPI equations were not designed to estimate creatinine clearance, in clinical practice they are often used interchangeably with the CG equation. The values obtained from the three equations are used as if they all estimate creatinine clearance, and the numeric values obtained from the three equations are used without adjustment when considering changing the dosage of a medication to account for kidney function.
A recent study showed that using estimated GFR (eGFR) values obtained from the MDRD equation instead of the traditional CG equation led to higher doses and increased risk of bleeding for enoxaparin and eptifibatide11, as well as excessive doses of dofetilide, which has been associated with cardiac conduction abnormalities such as changes in the QTc interval and the life-threatening arrhythmia, torsade de pointes.12 A recent review of FDA-approved drug dose labels showed that the most common estimating equation used in renal drug dose algorithms is the CG equation.8 Clinicians often use the MDRD and CKD-EPI equations to calculate drug dosages since clinical laboratories routinely report eGFR values obtained when Scr tests are ordered. The National Kidney Disease Education Program (NKDEP) recently recommended that eGFR (MDRD equation) and the CG equation can be used interchangeably for the purpose of drug dosing13, a recommendation that is controversial and has not been rigorously evaluated in older patients. In older individuals with very low Scr values (<1.0 mg/dL) and reduced muscle mass, a common practice of replacing Scr with an arbitrary value, such as 1.0 mg/dL, for use in the CG equation, has been reported but not fully evaluated.14–16
The objectives of the current study were to evaluate performance of kidney function estimation equations and to determine the frequency of drug dose discordance in an older population. Specifically, we identified the bias and precision of the CG, MDRD, and CKD-EPI equations relative to mClcr in older subjects, evaluated differences in dose calculations between the CG and MDRD equations for commonly prescribed drugs, and evaluated the use of an arbitrary Scr value (1.0 mg/dL) in the CG equation in patients with very low Scr values (< 1.0 mg/dl).
Methods
Study Population
Study subjects were randomly selected from the Baltimore Longitudinal Study of Aging (BLSA) database.16 The BLSA, started in 1958, is a continuing observational study of normative aging in community-dwelling volunteers conducted at, and sponsored by, the U.S. National Institute on Aging (NIA). Subjects undergo comprehensive medical, physiological, and psychological examinations at regular intervals. The data used in our study are from a cross-sectional evaluation of subjects who participated in the BLSA from January 1, 2005–December 31, 2010. Subjects were included if they were at least 70 years of age and had an mClcr less than 70 ml/min. Subjects were excluded if they had overt signs of renal failure or were receiving any form of dialysis. Our study population therefore consisted of individuals at high risk for taking medications, and because of their reduced kidney function, they were likely to require drug dosage adjustments. Our study protocol was approved by the institutional review boards at both the NIA and the University of Maryland.
Primary Outcome Measures
Our primary outcome variables were mClcr, estimation of creatinine clearance calculated by using the CG equation, estimations of GFR based on the MDRD and CKD-EPI equations, and estimation of creatinine clearance calculated by using the CG equation where Scr is replaced with 1.0 mg/dL (r-CG) in individuals with Scr < 1.0 mg/dl. The equations we used are provided in Appendix S-1. Twenty-four hour creatinine clearance was measured as part of the comprehensive medical evaluations that BLSA subjects receive during a 2–3 day stay at the clinical research unit of the BLSA at Harbor Hospital (Baltimore, MD). Creatinine concentrations in serum and urine were determined by using the enzymatic Vitros CREA method performed on the Ortho Fusion 5.1 Analyzer (Ortho-Clinical Diagnostics, Rochester, NY). The isotope dilution mass spectrometry (IDMS)-traceable serum creatinine assay was used for BLSA samples acquired after September 2009.
Statistical Analysis
Data for the CG, MDRD, and CKD-EPI equations are expressed in mL/min. Values for the MDRD and CKD-EPI equations (in mL/min/1.73m2) were multiplied by each subject’s body surface area (BSA) and divided by 1.73 (i.e., BSA/1.73) to yield units of mL/min. The accuracy (and reliability) of the three equations was computed as the average (and standard deviation) of the within-person difference between the value returned by each of the three equations and the mClcr. Similarly, the accuracy (and reliability) of the MDRD and CKD-EPI equations was also computed as the average (and standard deviation) of the within-person difference between the value returned by each of the two equations and CG equation. The variance of the three estimating equations and the mClcr was compared using the Fisher F test. Agreement between the CG, CKD-EPI and MDRD equations with mClcr was inspected visually by using Bland-Altman plots and quantified as the 95% limits of agreement between estimates. The limits of agreement represent a range of values within which the true difference between two methods can be said to lie with 95% confidence.
For ten drugs (Table 1) and for each of the three equations estimating kidney function, we identified a dose discordance when there was a disagreement in recommended dose based on the CG equation (and the drug packaging insert) and one or more of the equations used to estimate GFR. For example, if for a given subject the CG value was 30 mL/min and the MDRD 50 mL/min, and the package insert for one of the ten drugs studied (Table 1) called for a reduction in drug dose for Clcr below 40 mL/min, we would identify a dose discordance (CG indicating need for dosage adjustment, MDRD indicating no need for adjustment). For each drug, we quantified percent dose discordance as 100 times the total number of subjects with a dose discordance divided by the total number of subjects studied. Unless otherwise specified, values are given as mean ± SD. A two-tailed p value less than 0.05 was considered significant. The R statistical package, version 2.15 (available from http://www.r-project.org/) was used for all statistical analyses.
Table 1.
Drugs Requiring Renal Dosage Adjustment According to U.S. Food and Drug
| Drug
|
Creatinine Clearanc
|
Dose Reduction* (%)
|
|---|---|---|
| Cefepime | 30 – 60 | 50 |
| 11 – 29 | 50 | |
| < 11 | 75 | |
|
| ||
| Ciprofloxacin | 30 – 50 | 50 |
| < 30 | 67 | |
|
| ||
| Dabigatran | 15 – 30 | 50 |
| < 15 | NR | |
|
| ||
| Dalfampridine | ≤ 50 | CI |
|
| ||
| Daptomycin | < 30 | 50 |
|
| ||
| Enoxaparin | < 30 | 50 |
|
| ||
| Famotidine | < 50 | 50 |
|
| ||
| Gabapentin* | 30 – 59 | 40 |
| 15 – 29 | 80 | |
| < 15 | 90 | |
|
| ||
| Metoclopramide | < 40 | 50 |
|
| ||
| Piperacillin-Tazobactam | 20 – 40 | 33 |
| < 20 | 50 | |
CI = Contraindicated
NR = Not recommended
Dose reductions relative to effective dose
Results
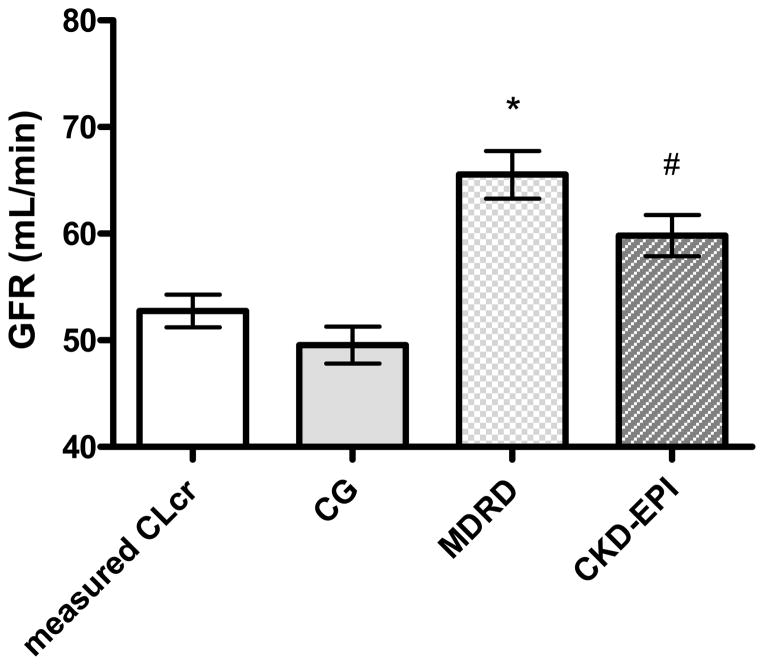
A total of 269 subjects were included in the analysis, 129 men and 140 women. The mean age of the subjects was 80.7±6.0 (mean±SD) years, mean Scr was 1.12±0.37 mg/dL, and mean body surface area was 1.76±0.20 m2. Women had significantly lower Scr, height, weight, BSA, and body mass index (BMI) than did men (Table 2). There were very few obese participants in the cohort; only 13 men (10%) and 15 women (11%) had a BMI > 30 kg/m2, and none of the subjects had a BMI ≥40 kg/m2. The mean mClcr of the cohort was 52.8±12.6 mL/min. The estimated creatinine clearance obtained by using the CG equation was 49.6±14.3 mL/min, estimated GFR from the MDRD equation was 65.5±18.5 mL/min, and estimated GFR from the CKD-EPI equation was 59.9±16.1 mL/min. Using the MDRD equation and mClcr to determine CKD categories, the numbers of subjects with various stages of chronic kidney disease were as follows: 0 vs. 1 (0% vs. 0.4%) stage 5, 6 vs. 16 (2% vs. 6%) stage 4, 105 vs. 154 (39% vs. 57%) stage 3, 141 vs. 98 (52% vs. 36%) stage 2, and 17 vs. 0 (6% vs. 0%) stage 1, for MDRD vs. mClcr.
Table 2.
Characteristics of the Study Subjects
| Characteristic
|
Women* (n=140)
|
Men* (n=129)
|
p-value
|
|---|---|---|---|
| Age (y) | 80.6 ± 5.9 | 80.9 ± 6.1 | 0.67 |
|
| |||
| Race | |||
| White | 124 (89) | 104 (81) | |
| Black | 14(10) | 19 (15) | |
| Other | 2 (1) | 6 (5) | 0.14† |
|
| |||
| Serum Creatinine (mg/dL) | 0.95 ± 0.31 | 1.30 ± 0.34 | <0.0001 |
|
| |||
| Measured 24-hr creatinine clearance (ml/min) | 53.0 ± 12.4 | 52.5 ± 12.9 | 0.76 |
|
| |||
| Height (cm) | 158.0 ± 6.4 | 172.0 ± 6.1 | <0.0001 |
|
| |||
| Weight (kg) | 61.7 ± 10.7 | 75.7 ± 10.1 | <0.0001 |
|
| |||
| BSA (m2) | 1.64 ± 0.16 | 1.89 ± 0.14 | <0.0001 |
|
| |||
| BMI (kg/m2) | 24.7 ±.0 | 25.7 ± 3.3 | 0.03 |
|
| |||
| BMI ≥ 30 kg/m2 | |||
| Yes | 15 (11) | 13 (10) | 0.86¶ |
| No | 125 (89) | 116 (90) | |
Data are mean ± SD or no. (%) of subjects.
Fisher exact test
Chi square test
All three estimating equations, CG, MDRD, and CKD-EPI, provided a biased estimate of mClcr, but the bias was smallest for CG. CKD-EPI and MDRD overestimated mClcr, whereas CG underestimated mClcr (Figure 1). The mean within-subject differences relative to mClcr were as follows: 7.1±15.1 ml/minute for CKD-EPI (p<0.001), 12.8±17.5 ml/minute for MDRD (p<0.001), and −3.2±14.2 ml/minute for CG (p<0.001). The limits of agreement of each method with mClcr were as follows: 7.1 ± 29.6 ml/minute for CKD-EPI, 12.8 ± 34.5 ml/min for MDRD, and −3.2 ± 27.8 ml/min for CG (Figure 2). Both CKD-EPI and MDRD were significantly higher than CG (10.3 ± 6.9 ml/min for CKD-EPI, p<0.001, and 16.0 ± 9.2 ml/min for MDRD, p<0.0001 [Figure S-1]). All three estimates had less precision (larger variance) than did mClcr. The ratios of the variances were as follows: CKD-EPI/mClcr 1.63 (p<0.001), MDRD/mClcr 2.2 (p<0.001), and CG/mClcr 1.3 (p<0.04). The MDRD had less precision than CG, with a ratio of variances of MDRD/CG 1.67 (p<0.0001). The CKD-EPI had a marginally larger variance than CG, with a ratio of variances of 1.26 (p<0.06). MDRD had less precision than CKP-EPI, with a variances of 1.32 (p<0.03).
Figure 1.
Comparison of kidney function estimation methods. Data are mean ± 95% confidence interval. Measured CLcr = creatinine clearance obtained from a 24-hour urine collection; CG = creatinine clearance estimated by using the Cockcroft-Gault equation; MDRD = glomerular filtration rate estimated by using the Modification of Diet in Renal Disease equation; CKD-EPI = glomerular filtration rate estimated using the Chronic Kidney Disease Epidemiology Collaboration equation. *p<0.001 vs measured CLcr, CG, and CKD-EPI; #p<0.001 vs. measured CLcr, CG, and MDRD, using paired analyses.
Figure 2.
Bland and Altman plots showing the within-person differences between the estimated creatinine clearance obtained by using the Cockcroft-Gault equation (CG) and measured 24-hour creatinine clearance (mCLcr) (panel A), estimated glomerular filtration rate obtained by using the Modification of Diet in Renal Disease equation (MDRD) and mCLcr (panel B), estimated glomerular filtration rate obtained by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and mCLcr (panel C). The solid line indicates mean difference, and the dashed line indicates limits of agreement.
There were 103 subjects with Scr values <1.0 mg/dl (0.80 ± 0.10 mg/dl, range 0.44–0.94 mg/dl). In these subjects, creatinine clearance estimated by using CG with Scr replaced by 1.0 mg/dl (r-CG) was 44.1±10.2 ml/minute vs. 55.8±15.0 ml/minute for the CG calculated by using the observed Scr, and 56.2±11.5 ml/minute for mClcr. The r-CG was significantly lower than either mClcr or CG for both comparisons (p<0.0001 [Figure S-2]).
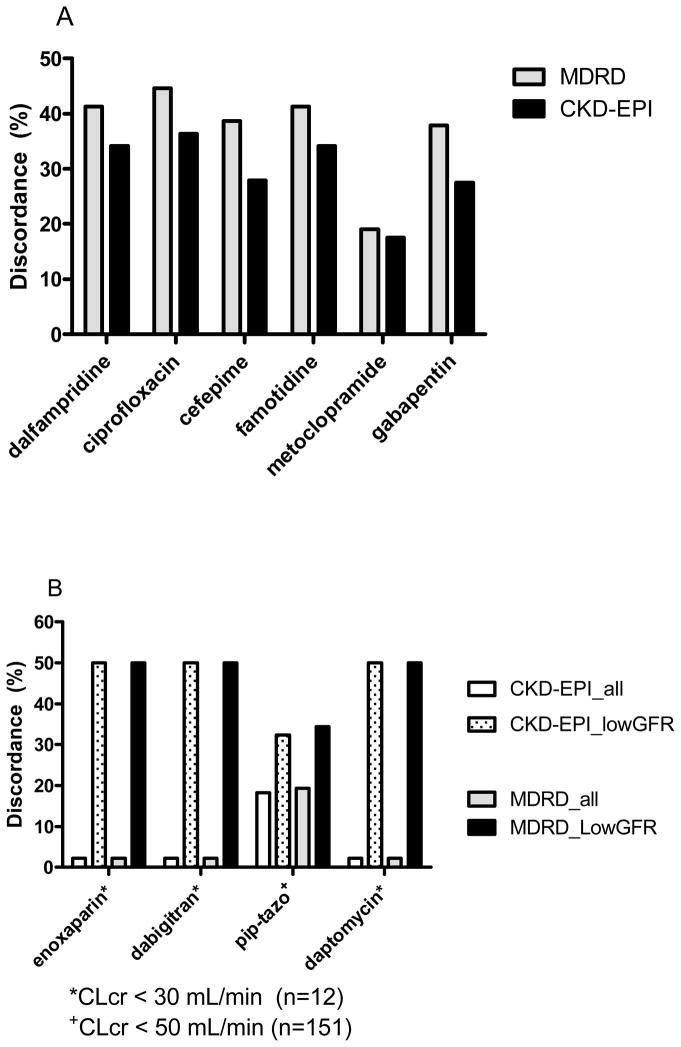
The percent discordance for 10 commonly prescribed drugs was calculated to quantify the implication that use the different methods for estimating kidney function could have on drug therapy (Figure 3). Median discordances relative to CG among the drugs tested were 28.6% (range 2.2 – 44.6%) for MDRD and 22.9% (range 2.2 – 36.4%) for CKD-EPI. The highest discordance was observed for drugs requiring dosage adjustment in patients with moderate-to-severe renal impairment (Clcr < 50 mL/min). For example, enoxaparin, dabigatran, and daptomycin had discordances for MDRD and CKD-EPI that increased from 2.2% in the entire cohort to 50% in those with mClcr < 30 mL/min, with all cases resulting in higher doses being given compared to using the CG equation. For piperacillin-tazobactam, the discordance rate increased from 19.3% in the entire cohort to 34.4% in patients with mClcr < 50 mL/min.
Figure 3.
Drug dose discordance rates. Panel A shows the discordance rates for the Modification of Diet in Renal Disease equation (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations compared with manufacturer-recommended dosing based on estimated creatinine clearance. Panel B illustrates the median discordance rates for four drugs requiring dosage adjustment at the lower range of creatinine clearance for MDRD and CKD-EPI.
Discussion
Our results show that in older adults with mild-to-moderate renal dysfunction but without any overt signs or symptoms of kidney impairment, MDRD and CKD-EPI should not be used in making decisions regarding drug dosage. Both MDRD and CKD-EPI values were consistently higher than CG, whereas the CG slightly underestimated mClcr. Our results also show that substituting 1.0 mg/dl for Scr when the concentration is <1.0 mg/dl should be avoided, as it leads to underestimation of renal function and can lead to subtherapeutic doses of critical medications. Discordance rates of approximately 25% for the MDRD and CKD-EPI equations were associated with higher drug doses calculated in all cases when compared to the CG equation.
An important role of health care practitioners is to maximize drug safety and ensure that the correct dose of a drug is given based on kidney function. The FDA-approved drug dose label provides dosing algorithms based on creatinine clearance, often estimated by the CG equation.8 Newer equations that estimate GFR, such as the MDRD and CKD-EPI equations, were originally developed for the purpose of epidemiologic research and CKD staging, not for calculating dosages in patients with altered renal function. Substitution of MDRD or CKD-EPI in place of the CG equation for calculating drug doses in patients with renal impairment is widely discouraged17–20, and a recent report by the FDA has cautioned against this practice.21 Surprisingly, in 2010, the NKDEP recommended that the eGFR (MDRD) and CG can be used interchangeably for the purpose of drug dosing13.
The CG equation has gained international acceptance as the primary index of kidney function in prospective, longitudinal studies of aging and renal function in Italy23 (InCHIANTI [Aging in the Chianti Area]) and Brazil23 (EPIDOSO [Epidemiology of the Elderly]), and is endorsed by the French Drug Agency24,25. In our study, we observed that both the MDRD and CKD-EPI equations were significantly positively biased compared to mClcr and CG, with the CG equation providing the least biased estimate of mClcr in this older population. These findings were not likely impacted by use of the non-IDMS assay, which showed < 4% bias when compared to the IDMS calibrators used on the Ortho-Diagnostics system employed here. This is also consistent with a previous study evaluating the performance of the MDRD and CG equations relative to mClcr in 122 older hospitalized patients in France.26 In the 122 older patients, the MDRD equation overestimated mClcr by 46 ± 64%, resulting in misclassification of nearly 50% of patients into lower CKD categories when compared to mClcr. The higher values for kidney function yielded by the MDRD and CKD-EPI equations in older patients is particularly worrisome for drugs with narrow therapeutic indexes or dose-dependent toxicities, in which FDA-approved drug dose algorithms are based on creatinine clearance, either using mClcr or the CG equation.8
The positive bias of the MDRD equation translated into significant dosing discordances for medications that require renal dosing based on creatinine clearance. For example, use of the MDRD equation resulted in a 41% discordance rate for famotidine, in which all discordant cases would have resulted in higher doses being given to patients if the MDRD equation was used instead of the CG equation. Failure to reduce doses of famotidine in patients with severe renal impairment is known to be associated with mental status changes including confusion, agitation, delirium, irritability, and hallucinations.27,28
Our results are consistent with several retrospective studies in over 20,000 patients with CKD reporting that use of the MDRD equation overestimates Clcr, leading to significantly higher drug doses compared to doses calculated by using CG.29–32 Our median discordance of 28% for the MDRD equation is consistent with data reported by Wargo et al.29 in 409 patients with stages 3–5 CKD, Wargo et al. showed that use of the MDRD equation resulted in kidney function estimates that were 14–28% higher (p<0.001) than CG, leading to discordant dosage adjustments in 20–36% of patients for eight antibiotics including cefazolin, cefepime, and meropenem. Similar findings were reported by Golik et al. in 207 hospitalized patients with stable kidney function.30 In their study, Golik et al. showed that the median MDRD eGFR values overestimated CG by nearly 40%, resulting in discordance rates of 54% and 57% for patients with CG values in the ranges of 11–30 and 31–60 mL/min, respectively. Similar discordance rates were also reported for levofloxacin, meropenem, and piperacillin-tazobactam. Discordance was also reported in a cross-sectional study of 180 older patients, aged 85 ± 8 years, with a CG of 49 ± 22 mL/min, residing in a long-term care facility in Canada.31 Gil et al. showed that the MDRD equation consistently overestimated CG by 40%, and provided discordant estimations of CKD category in over 60% of patients. This translated into significant dose discordance, in which the MDRD equation yielded 35% higher doses for amantadine compared to CG, and 32% of patients would have received higher initial doses of digoxin when using the MDRD equation, as compared to the CG equation. Taken together, the results of the current study and others are not consistent with the findings reported by Stevens et al.33 As pointed out previously34, Stevens et al. used a standard dose for their drug subset that was calculated based on “measured GFR”, which was then compared to doses calculated using two estimation methods (MDRD and CG Clcr). This analysis inherently favors the MDRD equation, since MDRD was derived from iothalamate-measured GFR. However, use of measured GFR as the index by which to calculate drug doses is neither consistent with the FDA-approved package label nor included in the FDA’s Guidance on Pharmacokinetic Studies in Patients with Renal Impairment.35 The lack of studies with findings similar to Stevens et al. may also be explained by use of a pooled dataset obtained from studies that used an abbreviated GFR measurement with short-term urine collections. Use of his method has recently been shown to be imprecise, with high intrasubject variability, and is not recommended when evaluating kidney estimation equations.36
Lack of appropriate renal dosage adjustments for oral anticoagulants such as enoxaparin and dabigatran could lead to serious adverse safety events. Our finding that both enoxaparin and dabigatran had 50% discordance rates when either the MDRD or CKD-EPI equations were used in subjects with mClcr < 30 mL/min is concerning and is consistent with other studies with these agents.11, 37,38 For example, Moranville et al. reported that use of the MDRD equation, when compared to Clcr, resulted in a failure to make manufacturer-recommended dosage reductions of enoxaparin in up to 11% of 4,698 hospitalized patients with stage 3 or 4 CKD.37 Higher doses of enoxaparin, when using the MDRD equation, were also reported by Melloni et al.11 and Nutescu et al.,38 in nearly 20,000 patients, resulting in fewer patients with dose reductions of enoxaparin when compared to the CG equation. To our knowledge, our study is the first to evaluate dabigatran dose discordance in an older population with reduced kidney function. This is important since few patients with renal impairment were enrolled in dabigatran pivotal clinical trials, and the renal dosing recommendation provided in the FDA-approved label is based on a pharmacokinetic study that used mClcr to stratify patients.39,40 It is further concerning that the largest randomized study with dabigatran (RE-LY [Randomized Evaluation of Long-Term Anticoagulation Therapy] trial) excluded subjects with a creatinine clearance lower than 30 mL/min/1.73 m2, and only 19% of subjects had a creatinine clearance of 30 to 49 mL/min/1.73 m2.41 A recent analysis of the RE-LY trial revealed that a subset of older subjects (> 75 years) without renal impairment had an increased risk of bleeding (hazard ratio 1.22 [95% confidence interval 0.65–2.266]).42 Coupled with recent case reports of serious bleeding with dabigatran in older adults with decreased renal function43–45, this further suggests that the higher doses of oral anticoagulants that are calculated using the MDRD and CKD-EPI equations can be problematic in older adults with reduced renal function.
Dalfampridine is contraindicated for use in patients with Clcr ≤50 mL/min due to its narrow therapeutic range and risk of dose-dependent seizures.46 A previous pharmacokinetic study conducted during drug development for dalfampridine showed significant accumulation in patients with moderate renal impairment (Clcr 30 to 50 mL/min using the CG equation), where area under the concentration-time curve (AUC) and maximum concentration (Cmax) values were 1.6 to 2.0-fold higher than in subjects with normal renal function.47 In our study, use of the CKD-EPI and MDRD equations resulted in discordance rates of 34% and 41.3%, respectively, for dalfampridine. In these discordance cases, nearly 100 patients would have received dalfampridine when it was contraindicated, but it is uknown whether these patients would have experienced adverse events. However, the FDA recently published a Drug Safety Communication warning about the risk of seizures when dalfampridine is used in patients with renal impairment and recommends that the CG equation should be used to calculate creatinine clearance before initiating therapy.48
Metoclopramide is associated with drug-induced Parkinsonism and tardive dyskinesia and requires dosage adjustment based on creatinine clearance. We found dosing discordances of approximately 18% for both CKD-EPI and MDRD equations, which resulted in higher doses being given in all cases (n=57) relative to doses calculated using the CG equation. This is important because dose-dependent toxicities related to extrapyramidal symptoms and QT-prolongation syndrome and torsade de pointes in renal impairment have been reported.49,50 In older patients with low Scr values, the practice of replacing Scr values with an arbritrary value is often performed by pharmacists in hospital settings; however, there is little evidence in the literature to support this practice. In a prior study conducted in 23 hospitalized patients over the age of 60 years with Scr values <1.0 mg/dL (mean ±SD 0.7 ± 0.1 mg/dL), Smythe et al. rounded Scr values up to 1.0 mg/dL and compared the rounded CG result to mClcr.14 Their study showed that rounded CG values were 27% lower than mClcr, leading to dose calculation errors for aminoglycosides that were confirmed by serum drug concentrations. Our results in a larger, community-based older population confirm that replacing low Scr values with an arbitrary value of 1.0 mg/dL leads to rounded CG values that were significantly lower than both mClcr (−17%) and unrounded CG values (−20%). Thus, the practice of rounding up or replacing Scr with an arbitrary value should be avoided and may lead to subtherapeutic doses of medications.
Understanding the limitations of using newer eGFR equations, such as CKD-EPI and MDRD, is particularly important in older patients. Our study provides strong support that both of the newer eGFR equations significantly overestimate creatinine clearance. The fact that eGFR calculated by using either CKD-EPI or MDRD is greater than mClcr suggests that the eGFR obtained from these equations is too high. This finding is independent of the creatinine assay employed (legacy vs. IDMS) because the measured creatinine clearance calculation requires both urine (numerator) and serum (denominator) to be analyzed using the same assay. Because creatinine is both filtered and secreted in the tubule, creatinine clearance should be 10–20% higher than true GFR 51. Although true GFR was not measured in the BLSA study, it is very likely that the MDRD and CKD-EPI equations would have overestimated true GFR in our population since these equations exceeded both measured and estimated creatinine clearance. An important question that remains unanswered by our study is the within-person reliability of mClcr (i.e., the day-to-day variation in mClcr).
Although our results indicate that use of the MDRD and CKD-EPI equations leads to dose calculation errors for drugs requiring renal dosage adjustments, dosing discordance would be best determined by measurement of serum drug concentrations, which we did not perform. In older patients, newer GFR estimation equations, such as the MDRD equation (which is now automatically reported in many electronic medical records) should not be used as a substitute for CG when adjusting drug dosage for renal function.
Conclusion
The MDRD and CKD-EPI equations significantly overestimated creatinine clearance (mClcr and CG) in elderly individuals. This leads to dose calculation errors for many drugs, particularly in individuals with severe renal impairment. Thus, GFR-estimating equations should not be substituted in place of the CG equation in older adults for the purpose of renal dosage adjustments. Our results also indicate that the common practice among pharmacists of rounding or replacing low Scr values (< 1.0 mg/dl) with an arbitrary value of 1.0 mg/dL for use in the CG equation should be avoided. Additional studies that evaluate alternative eGFR equations in the older populations that incorporate pharmacokinetic and pharmacodynamic outcomes measures are needed.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant P30 AG028747-07 and the Baltimore Veterans Administration Geriatric Research Education and Clinical Center (GRECC).
Sponsor’s Role: None of the sponsors participated in data collection, analysis or interpretation of data, or writing the manuscript.
Footnotes
Presented at the annual meeting of the American College of Clinical Pharmacy, Pittsburgh, Pennsylvania, October 17, 2011.
Conflict of Interest:
The authors declare that they have no conflict of interest as regards the work reported herein.
Author Contributions: Dowling conceived the research questions addressed by this study, directed analyses, wrote the manuscript, and takes full responsibility for this work. Sorkin was responsible for analyses and interpretation of data, helped write and revise the manuscript. Wang contributed to interpretation of data and reviewed and revised the manuscript. Ferrucci was the principal investigator for BLSA, and critically revised the manuscript.
References
- 1.Agrawal V, Jaar BG, Frisby XY, et al. Access to health care among adults evaluated for CKD: findings from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2012;59(3 Suppl 2):S5–15. doi: 10.1053/j.ajkd.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clase CM, Garg AX, Kiberd BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2002;13(5):1338–49. doi: 10.1097/01.asn.0000013291.78621.26. [DOI] [PubMed] [Google Scholar]
- 3.Families USA. [Accessed on 6/25/12];Cost overdose: Growth in drug spending for the elderly, 1992–2010. Available at: http://familiesusa2.org/assets/pdfs/drugod852b.pdf.
- 4.Papaioannou A, Clarke JA, Campbell G, et al. Assessment of adherence to renal dosing guidelines in long-term care facilities. J Am Geriatr Soc. 2000;48:1470–1473. doi: 10.1111/j.1532-5415.2000.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 5.Breton G, Froissart M, Janus N, et al. Inappropriate drug use and mortality in community-dwelling elderly with impaired kidney function--the Three-City population-based study. Nephrol Dial Transplant. 2011;26(9):2852–2859. doi: 10.1093/ndt/gfq827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanlon JT, Wang X, Handler SM, et al. Potentially inappropriate prescribing of primarily renally cleared medications for older veterans affairs nursing home patients. J Am Med Dir Assoc. 2011;12(5):377–383. doi: 10.1016/j.jamda.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Dowling TC, Matzke GR, Murphy JE, Burckart GJ. Evaluation of renal drug dosing: prescribing information and clinical pharmacist approaches. Pharmacotherapy. 2010;30(8):776–786. doi: 10.1592/phco.30.8.776. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melloni C, Peterson ED, Chen AY, et al. Cockcroft-Gault versus modification of diet in renal disease: importance of glomerular filtration rate formula for classification of chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2008;51(10):991–996. doi: 10.1016/j.jacc.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 12.Denetclaw TH, Oshima N, Dowling TC. Dofetilide dose calculation errors in elderly associated with use of the modification of diet in renal disease equation. Ann Pharmacother. 2011;45(7–8):e44. doi: 10.1345/aph.1Q159. [DOI] [PubMed] [Google Scholar]
- 13.NKDEP. [Accessed July 12, 2012];Chronic Kidney Disease and Drug Dosing: Information for Providers. 2010 http://www.nkdep.nih.gov/professionals/CKD_DrugDosing_508.pdf.
- 14.Smythe M, Hoffman J, Kizy K, Dmuchowski C. Estimating creatinine clearance in elderly patients with low serum creatinine concentrations. Am J Hosp Pharm. 1994;51(2):198–204. [PubMed] [Google Scholar]
- 15.Dooley MJ, Singh S, Rischin D. Rounding of low serum creatinine levels and consequent impact on accuracy of bedside estimates of renal function in cancer patients. Br J Cancer. 2004;90(5):991–995. doi: 10.1038/sj.bjc.6601641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shock NW, Greulich RC, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Govt. Printing Office; 1984. [Google Scholar]
- 17.Lalonde RL, Wagner JA. Drug development perspective on pharmacokinetic studies of new drugs in patients with renal impairment. Clin Pharmacol Ther. 2009;86(5):557–561. doi: 10.1038/clpt.2009.182. [DOI] [PubMed] [Google Scholar]
- 18.Spruill WJ, Wade WE, Cobb HH., 3rd Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother. 2008;6(3):153–160. doi: 10.1016/j.amjopharm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Wolowich WR, Raymo L, Rodriguez JC. Problems with the use of the modified diet in renal disease formula to estimate renal function. Pharmacotherapy. 2005;25(9):1283–1284. doi: 10.1592/phco.2005.25.9.1283. [DOI] [PubMed] [Google Scholar]
- 20.Dowling TC, Matzke GR, Murphy JE. Estimated GFR vs creatinine clearance for drug dosing. Am J Kidney Dis. 2009;54(5):984–985. doi: 10.1053/j.ajkd.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Park EJ, Wu K, Mi Z, et al. A systematic comparison of Cockcroft-gault and modification of diet in renal disease equations for classification of kidney dysfunction and dosage adjustment. Ann Pharmacother. 2012;46:1174–87. doi: 10.1345/aph.1Q757. [DOI] [PubMed] [Google Scholar]
- 22.Giannelli SV, Graf CE, Herrmann FR, et al. Natural history of older adults with impaired kidney function: the InCHIANTI study. Rejuvenation Res. 2011;14(5):513–523. doi: 10.1089/rej.2011.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sesso R, Prado F, Vicioso B, Ramos LR. Prospective study of progression of kidney dysfunction in community-dwelling older adults. Nephrology (Carlton) 2008;13(2):99–103. doi: 10.1111/j.1440-1797.2008.00919.x. [DOI] [PubMed] [Google Scholar]
- 24.Andro M, Estivin S, Comps E, Gentric A. Assessment of renal function in elderly after eighty years: Cockroft and Gault or Modification of diet in renal disease equation? Rev Med Interne. 2011;32(11):698–702. doi: 10.1016/j.revmed.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Roblin I, De Sobarnitsky S, Basselin C, et al. Estimated glomerular filtration rate for drug dose adjustment: Cockcroft and Gault or abbreviated MDRD equation? Clin Biochem. 2009;42(1–2):111–113. doi: 10.1016/j.clinbiochem.2008.09.110. [DOI] [PubMed] [Google Scholar]
- 26.Péquignot R, Belmin J, Chauvelier S, et al. Renal function in older hospital patients is more accurately estimated using the Cockcroft-Gault formula than the modification diet in renal disease formula. J Am Geriatr Soc. 2009;57(9):1638–43. doi: 10.1111/j.1532-5415.2009.02385.x. [DOI] [PubMed] [Google Scholar]
- 27.Henann NE, Carpenter DU, Janda SM. Famotidine-associated mental confusion in elderly patients. Drug Intell Clin Pharm. 1988;22:976–978. doi: 10.1177/106002808802201209. [DOI] [PubMed] [Google Scholar]
- 28.Odeh M, Oliven A. Central nervous system reactions associated with famotidine: report of five cases. J Clin Gastroenterol. 1998;27:253–254. doi: 10.1097/00004836-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Wargo KA, Eiland EH, 3rd, Hamm W, et al. Comparison of the modification of diet in renal disease and Cockcroft-Gault equations for antimicrobial dosage adjustments. Ann Pharmacother. 2006;40(7–8):1248–1253. doi: 10.1345/aph.1G635. [DOI] [PubMed] [Google Scholar]
- 30.Golik MV, Lawrence KR. Comparison of dosing recommendations for antimicrobial drugs based on two methods for assessing kidney function: cockcroft-gault and modification of diet in renal disease. Pharmacotherapy. 2008;28(9):1125–1132. doi: 10.1592/phco.28.9.1125. [DOI] [PubMed] [Google Scholar]
- 31.Gill J, Malyuk R, Djurdjev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group –a cautionary tale. Nephrol Dial Transplant. 2007;22:2894–2899. doi: 10.1093/ndt/gfm289. [DOI] [PubMed] [Google Scholar]
- 32.Hermsen ED, Maiefski M, Florescu MC, et al. Comparison of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for dosing antimicrobials. Pharmacotherapy. 2009;29(6):649–655. doi: 10.1592/phco.29.6.649. [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Nolin TD, Richardson MM. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009 Jul;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowling TC, Matzke GR, Murphy JE. Estimated GFR vs. creatinine clearance for drug dosing. Am J Kidney Dis. 2009;54(5):984–5. doi: 10.1053/j.ajkd.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 35.FDA; Bethesda, MD: [Accessed Dec 26, 2012]. 2010 Renal impairment guidance: guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis and impact on dosing and labeling. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf. [Google Scholar]
- 36.Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56:39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moranville MP, Jennings HR. Implications of using modification of diet in renal disease versus Cockcroft-Gault equations for renal dosing adjustments. Am J Health Syst Pharm. 2009;66(2):154–161. doi: 10.2146/ajhp080071. [DOI] [PubMed] [Google Scholar]
- 38.Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43(6):1064–1083. doi: 10.1345/aph.1L194. [DOI] [PubMed] [Google Scholar]
- 39.Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49(4):259–68. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Boehringer Ingelheim Pharmaceuticals, Inc. Pradaxa (dabigatran etexilate) Prescribing Information. Ridgefield, CT: 2012. [Google Scholar]
- 41.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 42.Boehringer Ingelheim. [Accessed June 25, 2012];Pradaxa (dabigatran etexilate): advisory committee briefing document. 2010 Aug 27; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM247244.pdf.
- 43.Legrand M, Mateo J, Aribaud A, et al. The use of dabigatran in elderly patients. Arch Intern Med. 2011;171(14):1285–1286. doi: 10.1001/archinternmed.2011.314. [DOI] [PubMed] [Google Scholar]
- 44.Freshour JE, Hudson JQ, Stevens AB, Franks AS. Epistaxis associated with dabigatran in an elderly patient with reduced creatinine clearance. Am J Health Syst Pharm. 2012;69(14):1184–1186. doi: 10.2146/ajhp110644. [DOI] [PubMed] [Google Scholar]
- 45.Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother. 2012;46(4):e10. doi: 10.1345/aph.1Q747. [DOI] [PubMed] [Google Scholar]
- 46.Acorda Therapeutics, Inc. Ampyra (dalfampridine) Prescribing Information. Hawthorne, NY: 2010. [Google Scholar]
- 47.Smith W, Swan S, Marbury T, et al. Single-dose pharmacokinetics of sustained-release fampridine (Fampridine-SR) in healthy volunteers and adults with renal impairment. J Clin Pharmacol. 2010;50:151–159. doi: 10.1177/0091270009344857. [DOI] [PubMed] [Google Scholar]
- 48.Ampyra (dalfampridine): Drug Safety Communication –Seizure Risk for Multiple Sclerosis Patients. US FDA; Rockville, MD: [Accessed January 3, 2013]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm313055.htm. [Google Scholar]
- 49.Siddique SM, Shariff N, Vesuwala N, Hafiz T. Metoclopramide as a possible cause of prolonged QT syndrome and torsade de pointes in a patient with heart failure and renal insufficiency. Ann Intern Med. 2009;150(7):502–504. doi: 10.7326/0003-4819-150-7-200904070-00016. [DOI] [PubMed] [Google Scholar]
- 50.Shaffer D, Butterfield M, Pamer C, Mackey AC. Tardive dyskinesia risks and metoclopramide use before and after U.S. market withdrawal of cisapride. J Am Pharm Assoc. 2004;44(6):661–665. doi: 10.1331/1544345042467191. [DOI] [PubMed] [Google Scholar]
- 51.Bauer JH, Brooks CS, Burch RN. Renal function studies in man with advanced renal insufficiency. Am J Kidney Dis. 1982;2(1):30–5. doi: 10.1016/s0272-6386(82)80040-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.