Abstract
Cardiovascular fitness is thought to offset declines in cognitive performance, but little is known about the cortical mechanisms that underlie these changes in humans. Research using animal models shows that aerobic training increases cortical capillary supplies, the number of synaptic connections, and the development of new neurons. The end result is a brain that is more efficient, plastic, and adaptive, which translates into better performance in aging animals. Here, in two separate experiments, we demonstrate for the first time to our knowledge, in humans that increases in cardiovascular fitness results in increased functioning of key aspects of the attentional network of the brain during a cognitively challenging task. Specifically, highly fit (Study 1) or aerobically trained (Study 2) persons show greater task-related activity in regions of the prefrontal and parietal cortices that are involved in spatial selection and inhibitory functioning, when compared with low-fit (Study 1) or nonaerobic control (Study 2) participants. Additionally, in both studies there exist groupwise differences in activation of the anterior cingulate cortex, which is thought to monitor for conflict in the attentional system, and signal the need for adaptation in the attentional network. These data suggest that increased cardiovascular fitness can affect improvements in the plasticity of the aging human brain, and may serve to reduce both biological and cognitive senescence in humans.
Several approaches to maintaining or improving cognitive performance in older adults have shown promise. It has long been known that older experts in a variety of domains can maintain high levels of performance into their 70s (1, 2). Also, in some cases, older adults have been shown to benefit as much or more than young adults from formal training of different cognitive abilities (3, 4). However, with few exceptions (4), the beneficial effects of these interventions tend to be limited to the tasks used in training. For example, expertise in typing has little or no effect on one's ability to drive a car, and training in visual search paradigms has little effect on working memory performance.
Since Spirudiso's seminal study of aging racquet sportsmen (5), there has been increasing interest in the utility of physical exercise as a more global moderator of age-related declines in cognition (6). A recent metaanalysis of the literature examining the effects of cardiovascular fitness training (CFT) on cognitive function has confirmed that CFT can play such a role (7). An analysis of 18 longitudinal fitness training studies demonstrated that regardless of the cognitive task type on which participants were tested, CFT participants showed a significantly greater improvement than control participants.
Although, to date, the neural mechanisms underlying cognitive improvements associated with cardiovascular fitness in aging adults have not been well studied in human populations, data collected from animal models suggests some possibilities. For example, aerobic training has been shown to increase levels of brain-derived neurotrophin factor (8) among other important neurochemicals (9), which, in turn increases neuronal survival (10), synaptic development, and plasticity (11), and the development of new neurons (12). The end result is a brain that is more efficient, plastic, and adaptive, which translates into better learning and performance in adult animals (12).
Additionally, recent neuroanatomical evidence from human populations shows that the same benefits in brain health seen in aging animals may, in fact, extend to aging humans (13). In a cross-sectional study of humans ranging in age from 55 to 79 years, the estimated trajectory of age-related declines in cortical tissue density were significantly reduced as a function of cardiovascular fitness. Moreover, these effects were greatest in the frontal, prefrontal, and parietal cortices. These regions of cortex also show the greatest age-related declines in humans (14). Interestingly, these regions are also thought to support executive cognitive functions; these executive functions also show the greatest behavioral improvement with CFT in aging humans (6, 7).
Data from animal models, human behavioral paradigms, and most recently, from human neuroanatomical models, all suggest that cardiovascular fitness should positively affect cortical functioning in aging humans. More specifically, increases in cardiovascular fitness should provide the neural substrates of the aging brain with a degree of flexibility and plasticity that is not present in less-fit counterparts.
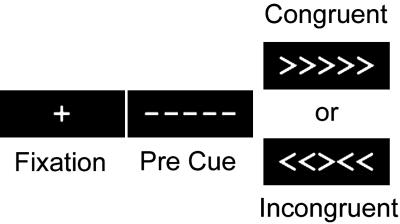
The task we chose to address this issue was a modified version of the Ericksen flanker paradigm (ref. 15 and Fig. 1). To make a correct response in the incongruent trials, participants were required to inhibit or filter misleading information provided by incongruent flanking cues. Our primary interest in this study was in the cortical circuitry invoked when generating a response in the incongruent condition, when the misleading flankers must be filtered to make a response, compared with the congruent condition, in which no such filtering is required.
Fig. 1.
Studies 1 and 2 used a slow-event-related fMRI design. Participants were presented with a 13.5-sec fixation cross, which was followed by a 500-ms pre cue that informed participants that the critical stimulus was about to appear. Finally, an array of five arrows appeared on the screen. Participants were asked to respond to the orientation of the central arrow by pressing a button with their left hand if the arrow pointed to the left, and with their right hand if the arrow pointed to the right. On half of the trials, the flanking arrows faced in the same direction as the central cue (congruent trials), and on the other half, they pointed in the opposite direction (incongruent trials). These stimuli remained on the screen for 2,000 ms.
Neuroimaging results in similar tasks have identified several regions involved in selective attention and the resolution of response competition engendered by conflicting response cues. Although a full discussion of this literature is beyond the scope of this paper, it is important to note that regions of the frontal and parietal cortices, particularly the middle frontal gyrus (MFG), superior frontal gyrus (SFG), and the superior and inferior parietal lobules (SPL and IPL), are consistently implicated in attentional selection and the resolution of response conflict elicited by incongruent response cues (16). Albeit somewhat oversimplified, successful task completion in the presence of incongruent flanking cues as described above would require older adults to invoke selective spatial attention through the frontal and parietal circuitry, which in turn should bias regions of visual cortex to isolate the central (target) cue, and inhibit the peripheral flanking cues (17, 18). To the degree that this circuitry is successfully invoked, the information provided by the peripheral cues is reduced, which then leads to reduction in conflict at the response stage, and a facilitation of the correct response. The reduction in conflict at the response stage can be indexed both behaviorally and neuroanatomically. Behaviorally, a reduction in conflict is signified by a relative reduction in reaction time to the incongruent stimuli. Additionally, the anterior cingulate cortex (ACC), a region of cortex in the medial wall of the brain, is known to be sensitive to response conflict (15, 19, 20), and is often thought to signal the need for adaptation in control processes (19, 21). As a result, successful invocation of the attentional network in the presence of conflicting response cues should result in a relative decrease in reaction time to inconsistent trials, as well as a reduction of task-related activity in the ACC.
Based on the human behavioral work on aging, CFT, and cognition, the animal work on CFT and the aging brain, and the human neuroimaging literatures surrounding the resolution of response competition, we can make the following predictions about the effects of cardiovascular fitness on older adults' functional brain activity in the flanker paradigm. Increases in cardiovascular fitness should be associated with more flexible (plastic) allocation of cortical resources. As such highly fit older adults (Study 1), and CFT older adults (Study 2), should show greater task-related activity in the attentional circuitry described above. Additionally, the enhanced performance of this network should result in a reduction in behavioral conflict, as measured by reaction times, and a reduction in task-related activity in the ACC.
Methods and Procedure
Study 1: Cross-Sectional Assessment. Participants. Participants were 41 high-functioning, community dwelling older adults, with no psychiatric disability. Participants were prescreened for psychiatric disability and dementia, as well as appropriateness for testing in the MRI environment. All participants were administered a preliminary near-vision acuity screening calibrated for a viewing distance of 18 in (1 in = 2.54 cm). Those whose vision was poorer than 20/20 were provided with appropriate corrective lenses to achieve visual acuity of at least 20/40. Written informed consent was granted by all participants. The study was approved by the University of Illinois Human Subjects Board and the Carle Hospital Foundation Medical Review Committee. See Tables 2 and 3 for population demographics.
Table 2. Demographic information for participants in Study 1.
| Demographic factor
|
High fit
|
Low fit
|
P value (two-tailed)
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t statistic | |||
| Study 1, cross-sectional | K-bit (composite) | 115.91 | 8.32 | 116.55 | 10.37 | −0.16 | 0.88 |
| Education | 13.50 | 2.58 | 13.14 | 2.97 | 1.29 | 0.21 | |
| Hypertension | 0.15 | 0.38 | 0.23 | 0.49 | −0.48 | 0.64 | |
| Age | 66.23 | 8.17 | 67.86 | 7.79 | −0.53 | 0.6 | |
K-bit, group average IQ score; education, years of education; hypertension, proportion of participants who had been diagnosed with hypertensive disorder (high blood pressure); age, average age in years.
Table 3. Demographic information for participants in Study 2.
| Demographic factor
|
Exercise
|
Control
|
P value (two-tailed)
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t statistic | |||
| Study 2, randomized trial | K-bit (composite) | 115.09 | 6.95 | 113.07 | 7.08 | −0.37 | 0.76 |
| Education | 15.39 | 2.79 | 14.7 | 2.18 | −0.61 | 0.66 | |
| Hypertension | 0.35 | 0.49 | 0.27 | 0.92 | −0.43 | 0.72 | |
| Age | 67.85 | 6.74 | 66.72 | 4.56 | −0.38 | 0.74 | |
K-bit, group average IQ score; education, years of education; hypertension, proportion of participants who had been diagnosed with hypertensive disorder (high blood pressure); age, average age in years.
Fitness assessment. After obtaining physician approval for a cardiovascular stress test, participants were asked to complete the Rockport 1-mile walk test (22), a measure of cardiovascular fitness that is appropriate for older adults and shows high concordance with other estimates of maximal oxygen uptake (VO2). Final estimates of VO2 were based on sex-specific formulae that combined height, weight, heart rate (HR), and time to complete the 1-mile walk. A subsample of these participants (n = 15) also performed a traditional treadmill-based exhaustion VO2 assessment (see Assessment of cardiorespiratory fitness in Study 2 for a more complete description). As in previous studies (13), the correlation between the Rockport and treadmill VO2 scores was high (r = 0.88, P < 0.01), confirming the validity of the fitness assessment used in this study.
Cognitive testing and functional MRI (fMRI) parameters. In a second session, participants performed a flanker task in which they were asked to respond to a central arrow cue embedded in an array of five arrows that pointed either to the left or right. In half of the trials, the flanking arrows point in the same direction as the central cue (e.g., < < < < <), and in the other half, the flanking arrows point in the opposite direction (e.g., > > < > >; see Fig. 1). Each participant underwent six successive 5-min blocks in which they were presented with 17 trials, which were first-order counterbalanced such that consistent and inconsistent trials followed each other equally as often. While performing the task, participants were scanned with an echo planar imaging protocol (20 slices, 5-mm thick, 3.75- × 3.75-mm in-plane resolution, flip angle = 90, TR = 2,000 ms) in a 1.5 Tesla General Electric Signa clinical magnetic resonance imager. A total of 145 echo planar imaging repetitions were collected for each block for each participant.
Behavioral analyses. The primary behavioral outcome was computed as the percent increase in reaction time (RT) to incongruent stimuli, over and above the average RT to congruent stimuli {[(incongruent-congruent)/congruent]·100}. The percent increase measure was derived to reflect interference unbiased by differences in base reaction time. Only correct responses were included in the outcome measure.
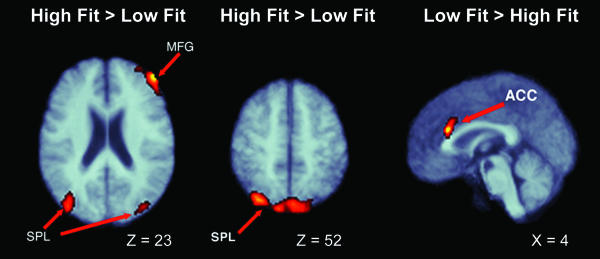
fMRI data processing and analyses. Primary fMRI data analysis was conducted with statistical parametric mapping (23) under SPM99. The data for each participant was corrected for slice time asynchrony, was realigned to a common image, was registered into stereotaxic space (24), and was spatially smoothed with a 7-mm three-dimensional Gaussian kernel. The resulting time series at each voxel was modeled against an expected time series derived by convolving the onset of each event type (consistent and inconsistent) with a double-γ-function, representing the expected time course of the hemodynamic response function (25). The resulting parameter estimates for the consistent < inconsistent contrast for each participant was entered into a second-level analysis, in which intersubject variability was treated as a random variable. These parameter estimates were tested in a two-sample t test at each voxel for groupwise differences in task-correlated activity. The t values were converted to a normalized Z distribution and were thresholded at a minimal voxel entry value of Z > 2.33 and a corrected cluster probability of P < 0.01 (24). The results of this analysis are displayed graphically in Fig. 2 and are described in Table 1.
Fig. 2.
Regional differences in cortical recruitment as a function of cardiovascular fitness. See Table 1 for cluster descriptions.
Table 1. Regional differences in cortical recruitment as a function of fitness.
| Study no./type | Region | Cluster size | Max Z | X | Y | Z |
|---|---|---|---|---|---|---|
| Study 1, cross-sectional | Right MFG1 | 2,045 | 3.75 | 32 | 52 | 27 |
| SPL1 | 3,003 | 4.49 | 7.3 | −70 | 56.7 | |
| VC1 | 247 | 2.55 | −28 | −92 | 8 | |
| ACC2 | 163 | 3.41 | 3.4 | 25.5 | 23.7 | |
| Study 2, randomized trial | Right MFG1 | 368 | 3.28 | 27 | 63 | 19 |
| SPL1 | 381 | 3.07 | −8 | −71 | 65.8 | |
| ACC1 | 60 | 2.87 | 2.3 | 30.1 | 18.8 |
This table describes the regions that differed in task-related activity as a function of fitness, including the size of the cluster, the maximum Z statistic for the cluster, and the location of the maximal Z statistic in Taliarach coordinates. In Study 1, (i) the large right MFG cluster had its peak in the right MFG and extended through the SFG, and (ii) the SPL cluster had its peak in the right SPL, and spread bilaterally to the left SPL. In Study 2, (i) the right MFG cluster showed peak activation close to the right MFG cluster in Study 1, but did not subsume the SFG, (ii) the SPL cluster peaked in the left SPL, and extended bilaterally into the right SPL. VC, visual cortex. Study 1: 1 = high fit > low fit; 2 = high fit < low fit. Study 2: 1 = exercise > control; 2 = exercise < control.
Study 2: Randomized Clinical Trial. Participants. A separate sample of 29 high-functioning, community dwelling older adults, ranging in age from 58 to 77 years (mean = 65.60, SD = 5.66; 11 males) participated in the longitudinal study. These participants were randomly assigned to participate in either a CFT (aerobic) group, or a stretching and toning (control) group. Participants were prescreened and vision-corrected in the same protocols used in Study 1. The study was approved by the University of Illinois Human Subjects Board and the Carle Hospital Foundation Medical Review Committee. See Tables 2 and 3 for population demographics.
Cognitive testing and fMRI parameters and analyses. Participants underwent the same cognitive testing and fMRI scanning as in Study 1, 1 week before beginning the 6-month intervention, and again 1 week after completion of the intervention; see Fitness training intervention and Assessment of cardiorespiratory fitness. Preprocessing and statistical analyses for the fMRI and RT data at the pre- and postintervention sessions were identical to those in Study 1. However, to test for changes in network recruitment from pre- to postintervention, we subtracted the preinterventional contrast maps from the postinterventional parameter maps, and forwarded these to a second-level analysis. In this analysis, we again tested for groupwise differences in activity by using a two-sample t test at each voxel. The t values were converted to a unit-normal Z score, which were thresholded with a minimum voxel entry criterion of Z > 2.33, and a minimal contiguous voxel criterion of n > 30 (25-27). As an additional hedge against false-positive results, we compared the surviving clusters from this analysis against those identified in study 1 in a region of interest approach. Specifically, we identified surviving clusters from this study that spatially intersected the clusters identified in Study 1. These results are reported in Table 1.
Exercise intervention protocol. The exercise intervention was implemented by trained exercise personnel and both the walking and stretching and toning groups met three times per week for 6 months. All participants engaged in warm-up and cool-down activities before and after each session. Given the sedentary nature of participants at the trial outset, the intensity component of the exercise program began at a light to moderate level for the first 2 months and increased to a moderately vigorous intensity for the duration of the intervention.
Aerobic exercise group. The aerobic exercise intervention was designed to improve cardiorespiratory fitness with exercise intensity prescription based on peak HR responses to baseline-graded exercise testing. Intensity levels began at 40-50% HR reserve, increasing to 60-70% HR reserve over the course of the trial. Duration of walking activity began at 10-15 min per session and increased by 1 min per session until participants were walking for 40-45 min per session (≈3 months). This latter level of duration was maintained for the remainder of the study. Intensity levels were monitored on a daily basis by HR assessment and ratings of perceived exertion. These latter values and details of exercise duration were recorded on daily exercise logs and monitored by exercise leaders.
Stretching and toning control group. This group served as a control group against which to gauge the effects of aerobic conditioning on neurocognitive function. Participants in this group followed exactly the same schedule and format as the aerobic conditioning group, were led by an experienced exercise leader, and therefore received the same amount of attention as the treatment condition. A program of stretching, limbering, and toning for the whole body designed for individuals 60 years of age and older constituted the activity for this group. Activities focused on flexibility enhancement, and as the individual's level of flexibility increased, stretches with increasing levels of difficulty were incorporated into the program and included proprioneuromuscular facilitation stretches by using rubber tubing for added resistance
Assessment of cardiorespiratory fitness. Cardiorespiratory fitness was assessed by means of a Parvo Medics TrueMax 2400 integrated metabolic measurement system for maximal oxygen consumption and indirect calorimetry assessment. Participants completed a modified Balke protocol maximal graded exercise test on a Trackmaster TMX425 motor-driven treadmill with electrocardiography activity monitored by a 12-lead fully digital Cardio Perfect stress system. Participants began walking at a speed of 3 mph and the grade of the treadmill was increased 2% every 2 min. Measurements of oxygen uptake, HR, and blood pressure were continuously monitored. Peak oxygen uptake (VO2) was measured from expired air samples taken at 30-sec intervals until the highest VO2 was attained at the point of test termination due to symptom limitation and/or volitional exhaustion.
Results
Study 1: Cross-Sectional. Participants. The 41 participants who completed both the cardiovascular fitness testing and fMRI portions of the study were separated by a median split on a measure of their maximal oxygen uptake (VO2), estimated by means of the Rockport 1-mile walk protocol. Although these groups, of course, differed significantly in terms of VO2, they were statistically similar to each other on measures of age, education, hypertension, and IQ (see Tables 2 and 3).
Behavioral results. To investigate the amount of behavioral interference engendered by the inconsistent flanking items, we compared the RT to inconsistent trials with the RT to consistent trials, computing a percent increase score {[(inconsistent-consistent)/consistent]·100} to control for individual differences in base-response times. Both groups performed the task with a very low error rate, averaging 1.6% and 1.9% for high- and low-fit participants, respectively [(t(39)lt] 1, ns]. When comparing the high- and low-fit participants, high-fit older adults are reliably more efficient in dealing with the conflicting cues (18% interference) than are the lower-fit older adults [26% interference; t(39) = 2.63 P < 0.02]. This basic pattern of results is consistent with our predictions and earlier studies of CFT on behavioral measures of executive functioning (6, 7).
fMRI results. Consistent with our predictions derived from the human and animal literatures, older adults who tested high in cardiovascular fitness demonstrated significantly greater activation in several cortical regions associated with effective attentional control: the right MFG (Brodmann's area 46), SFG (Brodmann's area 8), SPL (Brodmann's area 40), and significantly less activity in the ACC (Brodmann's area 32; see Table 1 and Fig. 2).
Post hoc fMRI covariates. Recent evidence (13) has shown that individual differences in cardiovascular fitness affects regional cortical density, especially in regions of the frontal and parietal cortices. To assess any potential confounding influence of cortical density in these results, we computed maps of gray matter density from high-resolution structural scans of each of our participants, and extracted the average gray matter density within each of the five regions of interest identified in the initial groupwise comparison. We then used these values as covariates in a post hoc analysis of covariance for each of these regions of interest. In none of the cases did the gray matter density covariate negate the significance of the regions of activity identified in the groupwise analyses.
Study 2: Longitudinal Intervention. Participants. Older adults in Study 2 were randomly assigned to participate in a CFT (aerobic) group, or a stretching and toning (control) group, for a maximum of 40-45 min three times a week over a 6-month period. The exercise and control participants were statistically similar to one another on measures of age, education, hypertension, and IQ (see Tables 2 and 3).
Fitness training intervention. We found that after the 6-month intervention period, older adults in the aerobic group demonstrated a significant increase in cardiovascular fitness, as indexed by maximal VO2 uptake, compared with control participants [10.2% vs. 2.9%, t(26) = 2.66, P < 0.02].
Behavioral results. Participants in the aerobic group demonstrated an 11% reduction in behavioral conflict from time 1 to time 2 [(t(15) = 2.49, P < 0.04]. However, control participants demonstrated only a 2% reduction in interference, which was not statistically different from 0 [t(9) < 1, ns]. Again, these data are consistent with our predictions and previous analyses of CFT on executive functioning in older adults (6, 7).
fMRI results. After the 6-month intervention period, participants in the aerobic group, compared with nonaerobic control participants, showed a significantly greater level of task-related activity in attentional control areas (MFG, SFG, and SPL), and a significantly reduced level of activity in the ACC. Moreover, these regions overlap, in both spatial and directional terms, with those identified as differing between high- and low-fit participants in Study 1. As such, the data from this randomized interventional study represent a conceptual replication and extension of the effects of fitness in the cross-sectional population in Study 1 (see Fig. 2 and Table 1).
Post hoc fMRI covariates. As in Study 1, we performed a post hoc comparison of the regions described in Table 1, using the average regional cortical density score as a covariate. Again, none of the regions of interest were negated when controlling for individual differences in cortical density.
Discussion
Consistent with extant literatures on CFT, cognition, and aging, we find that in both cross-sectional and longitudinal samples, increased CFT is associated with better performance on a test of executive functioning in aging humans (6, 7). Moreover, we find that, consistent with predictions derived from the vast animal (8-12) and emerging human (13) neuroscientific literatures on aging and CFT, increased CFT is associated with increased task-related activity in the regions of cortex thought to be necessary for successful task completion. Additionally, this increased recruitment in frontal and parietal regions appears to be indexed by an increase in the ability of the frontal attentional circuitry to bias task-related activation in posterior regions of cortex. These results strongly suggest that participants who either entered the testing situation with a relatively high level of aerobic fitness (Study 1), or those who gained greater cardiovascular capacity through a training intervention (Study 2), show a reduced amount of activity in the ACC, a region associated with the presence of behavioral conflict and the need to adapt attentional control processes. The strikingly similar manner in which cardiovascular fitness impacts functional recruitment in the cross-sectional and longitudinal studies suggest that the neurocognitive benefits of cardiovascular fitness can begin to accrue in a relatively short amount of time (as short as 6 months).
The precise histological mechanisms responsible for these changes have yet to be clearly established in humans. However, the animal literatures on CFT, aging, and brain health suggest several possibilities. One of the most likely candidates is suggested by the known increase in brain-derived neurotrophin factor and other nerve growth factors associated with CFT (8, 9), which has been shown to increase the number of synapses, capillaries, and cell bodies in adult rats (8-12, 28). One possibility, then, is that increases in cardiovascular fitness increases the number of interconnections (synapses) in frontal and parietal gray matter, allowing for greater systematic recruitment of these areas under higher cognitive load. Another possibility is that increases in fitness leads to increases in blood (capillary) supply in these regions, which, in turn, provides the metabolic resources necessary to coherently respond during task performance. Finally, animal models have shown positive cholinergic effects in association with exercise, which translates into faster learning for these animals (29). Of course, these possibilities are not mutually exclusive, and could work in concert to produce the increased performance of the attentional network seen in these studies. Further research into this issue seems warranted.
It is worth noting that the fMRI measures used in these studies are sensitive to changes in blood flow. As such, one might question whether the results seen in this study are simply artefactual changes due to fitness-related differences in blood flow, and not due to changes in neural recruitment per se. However, it is clear that increased levels of fitness do not result in a global increase in task-related signals in this study. In these studies, higher-fit or CFT-trained participants showed a decrease in activity in the ACC. If increased fitness leads to artefactual increases in task-related blood flow, it should be seen in all areas invoked during the task. This finding is clearly not the case, and thus these data likely reflect low-level changes in cortical functioning.
These results are scientifically important, because they provide glimpses into the neural bases of cognitive enhancement through CFT in aging humans, and establish a direct link between the extant animal and human literatures on aging, brain health, and cardiovascular fitness. These data also carry significant public health implications. Even moderate cardiovascular activity of the sort that is within reach of most healthy older adults results in improved neural functioning, and may help to extend or enhance independent living in older adult populations. Recent estimates suggest that even a 10% reduction of hospital, nursing home, and home care costs associated with aging would have saved $50.4 billion in the year 2000, a figure that grows to over $133 billion by the year 2020 (30). Additionally, although the populations examined in these studies were highly functioning individuals, a recent metaanalysis of the behavioral literature on CFT and aging humans found that CFT provides similar benefits for both clinical and nonclinical populations (7). The efficacy of CFT on the cerebral health of lower-functioning individuals has yet to be investigated, but these data suggest the import of such endeavors.
The degree to which these findings are consistent with those in animal literatures on brain health and aging is clearly encouraging. It also begs the question of what other types of interventions might be taken from the animal literatures and applied to humans, and how these interventions might be combined with more traditional cognitive training regimens. For example, dietary interventions that include increased levels of antioxidants can improve brain health in animal models (31, 32). Also, increases in estrogen in ovariectomized animals has been shown to increase mRNA transcription of brain-derived neurotrophin factor (33). Future research that investigates the applicability of these results to humans, as well as a multitiered combinatorial application of these interventions may prove valuable.
Acknowledgments
This work was supported by National Institute on Aging Grants AG14966 and AG21887 and the Institute for the Study of Aging.
Abbreviations: CFT, cardiovascular fitness training; MFG, middle frontal gyrus; SFG, superior frontal gyrus; SPL, superior parietal lobule; IPL, inferior parietal lobule; ACC, anterior cingulate cortex; fMRI, functional MRI; RT, reaction time; HR, heart rate.
References
- 1.Salthouse, T. A. (1984) J. Exp. Psychol. Gen. 113, 345-371. [DOI] [PubMed] [Google Scholar]
- 2.Masunaga, H. & Horn, J. (2002) Psychol. Aging 16, 293-311. [PubMed] [Google Scholar]
- 3.Scialfa, C. T., Jenkins, L., Hamaluk, E. & Skaloud, P. (2000) J. Gerontol. B Psychol. Sci. Soc. Sci. 55, 27-46. [DOI] [PubMed] [Google Scholar]
- 4.Kramer, A. F., Larish, J., Weber, T. & Bardell, L. (1999) In Attention and Performance XVII, eds. Gopher, D. & Koriat, A. (MIT Press, Cambridge, MA).
- 5.Spirduso, W. W. (1975) J. Gerontol. 30, 18-23. [DOI] [PubMed] [Google Scholar]
- 6.Kramer, A. F., Hahn, S., Cohen, N. J., Banich, M. T., McAuley, E., Harrison, C. R., Chason, J., Vakil, E., Bardell, L., Boileau, R. A. & Colcombe, A. (1999) Nature 400, 418-419. [DOI] [PubMed] [Google Scholar]
- 7.Colcombe, S. J. & Kramer, A. F. (2002) Psychol. Sci. 14, 125-130. [DOI] [PubMed] [Google Scholar]
- 8.Neeper, S. A., Gomez-Pinilla, F., Choi, J. & Cotman, C. (1995) Nature 373, 109. [DOI] [PubMed] [Google Scholar]
- 9.Churchill, J. D., Galvez, R., Colcombe, S. J., Swain, R. A., Kramer, A. F. & Greenough, W. T. (2002) Neurobiol. Aging 23, 941-955. [DOI] [PubMed] [Google Scholar]
- 10.Barde, Y.-A. (1994) Prog. Clin. Biol. Res. 390, 45-56. [PubMed] [Google Scholar]
- 11.Lu, B. & Chow, A. (1999) J. Neurosci. Res., 58, 76-87. [PubMed] [Google Scholar]
- 12.van Praag, H., Christie, B. M., Sejnowski, T. J. & Gage, F. H. (1999) Proc. Natl. Acad. Sci.USA 96, 13427-13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colcombe, S. J., Erickson, K. I., Raz, N., Webb, A. G., Cohen, N. J., McAuley, E. & Kramer, A. F. (2003) J. Gerontol. A Biol. Sci. Med. Sci. 58, 176-180. [DOI] [PubMed] [Google Scholar]
- 14.Raz N. (2000) In The Handbook of Aging and Cognition, eds. Craik, F. I. M. & Salthouse, T. A. (Lawrence Erlbaum Associates, Mahwah, NJ), pp. 1-90.
- 15.Botvinick, M., Nystrom, L. E., Fissel, K., Carter, C. & Cohen, J. D. (1999) Nature 402, 179-181. [DOI] [PubMed] [Google Scholar]
- 16.Casey, B. J., Thomas, K. M., Welsh, T. F., Badagaiyan, R. D., Eccard, C. H., Jennings, J. R. & Crone, E. A. (2000) Proc. Natl. Acad. Sci. USA 97, 8728-8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barcelo, F., Suwanzono, S. & Knight, R. T. (2000) Nat. Neurosci. 3, 399-403. [DOI] [PubMed] [Google Scholar]
- 18.van Veen, V., Cohen, J. D., Botvinick, M. M., Stenger, V. A. & Carter, C. (2001) NeuroImage 14, 1302-1308. [DOI] [PubMed] [Google Scholar]
- 19.Botvinick, M.M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. (2001) Psychol. Rev. 108, 624-652. [DOI] [PubMed] [Google Scholar]
- 20.Barch, D. M., Braver, T. S., Sabb, F. W. & Noll, D. C. (2000) J. Cognit. Neurosci. 12, 298-309. [DOI] [PubMed] [Google Scholar]
- 21.Posner, M. I. & Dehaene, S. (1994) Trends Neurosci. 17, 75-79. [DOI] [PubMed] [Google Scholar]
- 22.Kline, G. M., Pocari, J. P., Hintemeister, R., Freedson, P. S., Ward, A., McCarron, R. F., Ross, J. & Rippe, J. M. (1987) Med. Sci. Sports Exercise 19, 353-359. [PubMed] [Google Scholar]
- 23.Friston K. J., Holmes, A. P., Worsley, K. J., Poline, J. P., Frith, C. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 24.Friston, K. J., Ashburner, J, Poline, J. B., Frith, C. D., Heather, J. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 165-189. [Google Scholar]
- 25.Friston, K. J., Frith, C. D., Turner, R. & Frackowiak, R. S. J. (1995) NeuroImage, 2, 157-165. [DOI] [PubMed] [Google Scholar]
- 26.Friston, K. J., Worsley, K. J., Frackowiak, R. S. J., Mazziotta, J. C. & Evans, A. C. (1994) Hum. Brain Mapp. 1, 14-22. [DOI] [PubMed] [Google Scholar]
- 27.Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 28.Swain, R. A., Harris, A. B., Wiener, E. C., Dutka, M. V., Morris, H. D., Theien, B. E., Konda, S., Engberg, K., Lauterbur, P. C. & Greenough, W. T. (2003) Neuroscience 117, 1037-1046. [DOI] [PubMed] [Google Scholar]
- 29.Fordyce, D. E. & Farrar, R. P. (1991) Behav. Brain Res. 46, 123-133. [DOI] [PubMed] [Google Scholar]
- 30.Alliance for Aging Research. (2003) Medical Never-Never Land: Why America is not Ready for the Coming Aging Boom (The Alliance for Aging Research, Washington, DC).
- 31.Cotman, C. W., Head, E., Muggenburg, B. A., Zickler, S. & Milgram, N. W. (2000) Neurobiol. Aging 23, 809-818. [DOI] [PubMed] [Google Scholar]
- 32.Galli, R. L, Shukitt-Hale, B., Youdim, K. A. & Joseph, J. A. (2002) in Increasing Healthy Life Span: Conventional Measures and Slowing the Innate Aging Process. Annals of the New York Academy of Science, ed. Harman, D. (New York Academy of Sciences, New York), Vol. 959, pp. 128-132. [Google Scholar]
- 33.Berchtold, N.C., Kesslak, J. P., Pike, C. J., Aldard, P. A. & Cotman, C. W. (2001) Eur. J. Neurosci. 14, 1992-2002. [DOI] [PubMed] [Google Scholar]