Abstract
Introduction
The role of aminophylline in the treatment of severe acute asthma in the pediatric critical care unit (PCCU) is not clear. We sought to examine the association of aminophylline treatment with PCCU length of stay and time to symptom improvement.
Material and Methods
Patients with severe acute asthma who were admitted to our PCCU and received aminophylline infusion were retrospectively compared with similar patients who did not receive aminophylline. The primary outcome measure was functional length of stay (i.e. time to which patients could be transferred to a general pediatric ward bed). A secondary outcome was time to symptom improvement.
Results
Adjusted functional length of stay was longer for subjects who received aminophylline (n=49) than for the patients who did not (n=47) (hazard ratio 0.396, p<0.001), as well as the time for symptom improvement (hazard ratio 0.359, p<0.001). In the group of subjects receiving aminophylline, those with a serum theophylline level ≥10mcg/ml (therapeutic) (n=31) had longer functional length of stay (hazard ratio 0.457, p=0.0225) and time to symptom improvement (hazard ratio 0.403, p=0.0085) than those with levels <10mcg/ml (sub-therapeutic) (n=18).
Conclusions
The addition of aminophylline to therapy with corticosteroids and inhaled β-agonists was associated with statistically and clinically significant increases in functional length of stay and time to symptom improvement in the PCCU. This potential morbidity supports the National Asthma Education and Prevention Program guideline proscribing aminophylline use in acute asthma.
1. Introduction
Asthma is the most prevalent chronic disease in children and the most frequent reason for childhood hospitalization in the United States. The economic impact of pediatric asthma is substantial, exceeding $15 billion annually in both direct and indirect costs[1–6]. The management of acute severe asthma exacerbations varies widely between pediatric critical care providers and frequently does not conform to expert consensus guidelines[7–8]. In the critical care setting, the treatment of asthma is directed towards rapid relief of inflammation and airflow obstruction. This is accomplished through a variety of medical interventions, including systemic corticosteroids; continuous inhaled and intravenous Beta-2 agonists; intravenous magnesium sulfate and inhaled and sub-cutaneous epinephrine. Methylxanthines, including intravenous aminophylline, are one of the oldest classes of medications used to treat asthma. The therapeutic benefit of aminophylline is hypothesized to result from multiple mechanisms. These includes direct bronchodilatory effects through non-selective inhibition of phosphodiesterase, antagonism of the adenosine receptor, modulation of intracellular calcium release through agonism of the ryanodine receptor as well as stimulation of endogenous catecholamine release. It is further believed that methylxanthines have significant immunomodulatory effects[9].
Contemporary use of aminophylline is typically limited to severe exacerbations after other treatments have failed[10]. Although not recommended by current American guidelines[7], aminophylline is still used by many institutions and remains part of the current British guidelines[10–12] for the treatment of status asthmaticus. A recent meta-analysis examined the use of aminophylline[13]. The result of the aggregation of 7 studies including 380 patients showed improved pulmonary function in the first 6 hours, but no significant improvement in symptoms, need for pediatric critical care unit (PCCU) admission, PCCU length of stay (LOS), or necessity for mechanical ventilation[13–15]. In a most recent adult review study the risk benefit balance of amniophylline was considered unfavorable by the authors[16]. These studies did not, however, focus on patients requiring critical care The role of aminophylline in the treatment of patients with severe acute asthma in the PCCU has not been clarified. We sought to examine the association of aminophylline treatment with LOS and symptom improvement in patients with severe acute asthma managed in a pediatric critical care unit.
2.Materials and Methods
We performed a retrospective review of all patients admitted to the PCCU at the Monroe Carell Jr. Children's Hospital at Vanderbilt University (Nashville, Tennessee) with the diagnosis of severe asthma (ICD-9 493) during a three-year period (January, 2007 to January, 2010). Using an established electronic medical record (EMR), we identified all patients who received aminophylline infusion as well as those patients who did not.
Each asthma patient in our PCCU is routinely evaluated at admission and hourly by a respiratory therapist with a “Respiratory Distress Score” (RDS) (Table 1). Our institution has modified this evaluation tool based on an asthma scoring system suggested by Qureshi et al.[17]. This modification added a category for normal signs and symptoms. This addition helps separate subjects who have mild symptoms from those with more severe distress.
Table-1.
The Respiratory Distress Score; a clinical tool used to assess the severity of asthma and guide the treatment plan in the pediatric critical care unit.
| Asthma Severity | Normal | Mild | Moderate | severe |
|---|---|---|---|---|
| RDS | 0 – 4 | 5 – 7 | 8 – 11 | 12– 15 |
| Scoring Factors | 0 | 1 | 2 | 3 |
| Respiratory Rate | ||||
| 2–3 years | 18–26 | 27–34 | 35–39 | >40 |
| 4–5 years | 16–24 | 25–30 | 31–35 | >36 |
| 6–12 years | 14–20 | 21–26 | 27–30 | >31 |
| >12 years | 12–18 | 19–23 | 24–27 | >28 |
| Oxygen Saturation (SpO2) | >98% on room air | 95% to 97% on room air | 90% to 94% on room air | < 90% on room air or on any oxygen |
| Auscultation | Normal Breath sounds with good aeration throughout | End expiratory wheezes only | Expiratory wheezing | Inspiratory and expiratory wheezing to diminished breath sounds |
| Retractions | None | Intercostal | Intercostal & substernal | Intercostal, substernal and supraclavicular |
| Dyspnea | Speaks in complete sentences | Speaks in short sentences, coos & babbles | Speaks in partial sentences, short cry | Speaks in single words. Short phrases/grunting |
Adapted with modification from Qureshi F, Pestian J, Davis P et al. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. N Engl J Med 1998; 339(15):1030–1035.
We use the RDS to determine the need for albuterol administration and dosing according to an established protocol implemented at the bedside by the respiratory therapists. RDS and administered treatments were entered into the EMR. The study protocol was reviewed and approved by our Institutional Review Board (protocol # 100151)
We included children ages 3–18 years admitted to the PCCU with severe acute asthma who were receiving continuous nebulized albuterol on admission. We defined “severe acute asthma” as an RDS at admission to the PCCU of 12 to 15 or endotracheal intubation for asthma (in which cases RDS could not be determined). All subjects received initial treatment in our emergency department. We excluded patients younger than 3 years of ages in order to exclude those with bronchiolitis, those with RDS <12, and those who received an aminophylline bolus but did not receive a continuous infusion.
Our primary outcome measure was functional length of stay (FLOS). We defined FLOS as the time from PCCU admission to the time the subject met criteria for transfer to a general pediatric floor bed. To meet these criteria, the subject must have continued to tolerate tolerated the spacing of albuterol treatments to an interval of two hours without relapse of symptoms. FLOS was calculated using the time that the second of these two treatments was administered. Our secondary outcome was time to symptom improvement, defined as time to achieve an RDS of 7 or less.
We used Cox proportional hazards regression to model the time-dependent association of aminophylline treatment with FLOS in one model and time to symptom relief in a second model, each adjusted for baseline RDS, age, race (White/Hispanic, Black), and sex. All predictors except for age were regarded as categorical variables. Using adjusted hazard ratios we assessed the associations of aminophylline treatment versus no treatment with FLOS or time to symptom relief and calculated corresponding 95% confidence intervals. A value less than one was indicative of less risk of being discharged from the PCCU and hence greater FLOS, or in the time to symptom relief model, less risk of having relief of symptoms.
Among the subjects who received aminophylline (n=49) we additionally sought to assess whether there was a dose-response association between aminophylline treatment and the outcomes of interest. For this subgroup who received aminophylline (n=49), we employed a Cox regression to model FLOS and time to symptom improvement separately as a function of serum theophylline level (≥10 mcg/ml vs. <10 mcg/ml), baseline RDS and race.
Finally, we examined the dose response to aminophylline as a continuous variable using the absolute serum theophylline level value (in mcg/ml). For those subjects who received aminophylline (n=49), using a Cox proportional hazards model we examined the relation between theophylline levels as absolute values and the outcome variables FLOS and time to symptom relief.
In our unit, the decision to start aminophylline was based on the physician judgment and preference, We initiate aminophylline treatment by a loading dose of 6mg/kg over 30 minutes followed by a maintenance dose of 1–1.2mg/kg/hr. Drug levels are obtained every 6 hours during aminophylline infusion. We consider a therapeutic theophylline level greater than or equal to 10 mcg/ml. Subjects with one or more therapeutic levels were categorized as “therapeutic” regardless of the number of levels obtained. When evaluating subjects with therapeutic levels using the drug level as a continuous variable, we used the highest recorded level.
3. Results
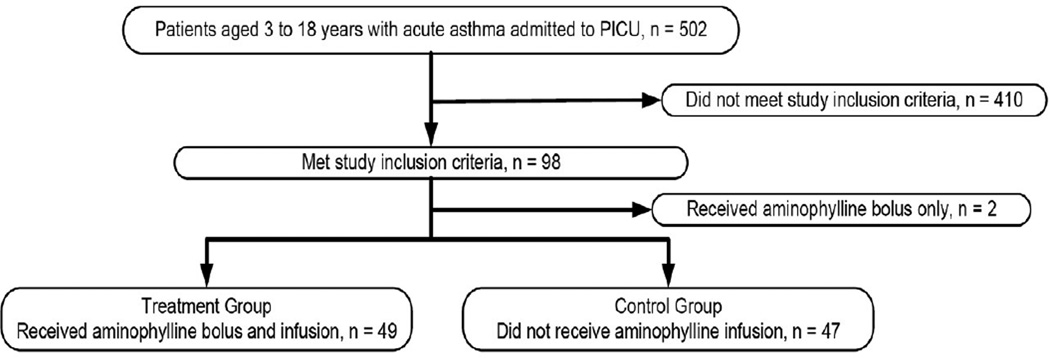
We examined the records of all patients admitted to our PCCU with the diagnosis of acute asthma exacerbation for the period January, 2007 to January, 2010. Five-hundred-two had this diagnosis and 96 met our inclusion criteria. Of these, 49 received aminophylline and 47 did not (Figure 1). Thirty-one of 49 subjects who received aminophylline had therapeutic theophylline levels (≥10mcg/ml), while 18 had sub-therapeutic levels (< 10mcg/ml). Demographic characteristics of the study subjects are shown in Table 2. The two cohorts differed significantly only by age with the aminophylline group being slightly older (p=0.017).
Figure 1.
Recruitment flow chart
Table-2.
The Demographics of the subjects included in the study
| Demographics | Aminophylline | No Aminophylline | p-Value | |
|---|---|---|---|---|
| Age (Years, mean) | 8 | 6 | 0.017 | |
| Gender% | 0.962 | |||
| Male | 61 | 62 | ||
| Female | 39 | 38 | ||
| Race% | 0.067 | |||
| Black | 69 | 51 | ||
| White + Hispanic | 31 | 49 | ||
| Severity% | 0.977 | |||
| RDS-12 | 35 | 36 | ||
| RDS-13 | 31 | 32 | ||
| RDS-14 | 8 | 9 | ||
| RDS-15 | 20 | 19 | ||
| Intubated | 6 | 4 | ||
| Pneumonia on Chest X-Ray | 11 | 14 | 0.352 | |
| Non-Invasive ventilation | 30 | 24 | 0.410 | |
| Terbutaline received | 15 | 13 | 0.678 | |
| Magnesium Sulfate received | 37 | 40 | 0.304 | |
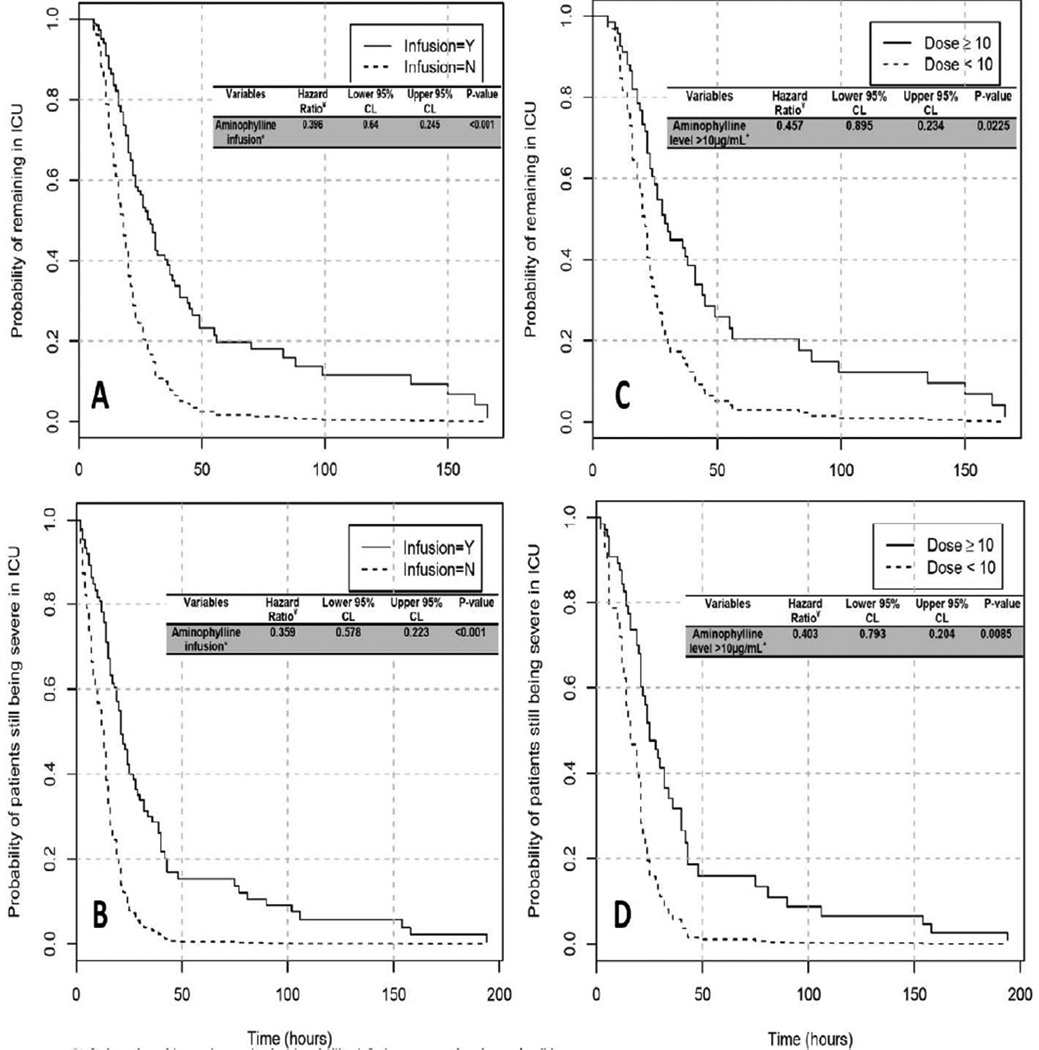
Those receiving aminophylline had an adjusted hazard ratio for discharge from the PCCU in a given time period of 0.396 (95% CI [0.245, 0.64]; p<0.001) (Figure 2-A). White subjects (including Hispanics) were more likely to remain in the PCCU (HR 0.605; 95% CI [0.369, 0.992]; p=0.046). Among subjects who received aminophylline, FLOS was significantly longer in those with therapeutic serum levels (HR 0.457; 95% CI [0.234, 0.895]; p=0.023) (Figure 2-C).
Figure 2.
Kaplan-Meier curves showing the cumulative discharge from the pediatric critical care unit according to aminophylline infusion and therapeutic serum levels. Cox proportional hazards models were used to assess whether the time to these events data were statistically significant (Hazard ratios and 95% confidence intervals provided on individual graphics). A) Associations of aminophylline infusion with probability of being discharged from pediatric critical care unit. B) Associations of aminophylline infusion with probability of symptom relief in the pediatric critical care unit. C) Associations of therapeutic aminophylline level with probability of being discharged from pediatric critical care unit. D) Associations of therapeutic aminophylline level with probability of symptom relief in the pediatric critical care unit.
*Infusion= the subjects who received aminophylline infusion compared to those who did not.
¥ Cox proportional hazards models were used to provide estimated hazard ratios and 95% confidence intervals for each graph, adjusted for age, gender, and respiratory distress score.
Time to symptom improvement (i.e. time needed to reach a RDS ≤7 “mild”) was longer in subjects who received aminophylline compared to those who did not (HR 0.359; 95% CI [0.223, 0.578]; p<0.001) (Figure 2-B). Time to symptom relief was also significantly longer in those with therapeutic serum levels (HR 0.403; 95% CI [0.204, 0.793]; p=0.008) (Figure 2-D). Finally, when examining the association between the FLOS and drug level as a continuous variable, we found a trend toward longer FLOS with higher drug levels (hazard ratio 0.9490, 95% CI [0.899, 1.003]; P=0.06). A similar model for time to symptom relief with drug level as a continuous variable did not show evidence of an association (hazard ratio 0.966, 95% CI [0.892,1.046]; p=0.395).
4. Discussion
Our study of children admitted to the PCCU with severe asthma exacerbation suggests that the use of aminophylline is associated with a greater FLOS and time to symptom improvement after adjusting for baseline acute asthma severity, sex, race and age. We observed an average 8-hour greater functional length of PCCU stay and a 9.5-hour greater time to symptom improvement in univariate analysis. This significant difference persisted when adjusted for asthma severity, sex, race and age. When comparing therapeutic versus non-therapeutic drug levels in those subjects receiving aminophylline, we also observed significantly greater FLOS and time to symptom improvement in those groups with therapeutic drug levels. Finally, there was a trend toward longer FLOS when the dose response was examined as a continuous variable.
In a randomized controlled study, Ream and colleagues found that aminophylline administration added to an aggressive regimen of inhaled and intravenous (IV) β-agonist, inhaled ipratropium, and IV methylprednisolone led to a significant decrease in the time to resolution of symptoms (as measured by improvement in a clinical evaluation score). The authors did not find a significant decrease in either PCCU or hospital length of stay. They did, however, claim significantly shorter PCCU and hospital lengths of stay in a very small subset of intubated patients who had received aminophylline (n=3) compared to those intubated patients receiving placebo (n=3) [18]. In another randomized trial, Yung, et al, found no significant difference in PCCU length of stay between the group receiving aminophylline and that receiving placebo in the subset of patient’s admitted to the PCCU. In a most recent review of fifteen randomized controlled trails by Nair, et al, the authors concluded that there was no statistical significant bronchodilation effect of aminophyline when added to standard care with inhaled beta-2-agonists. They also concluded that the risk benefit balance of aminophylline was unfavorable due to high rate of complications [16].
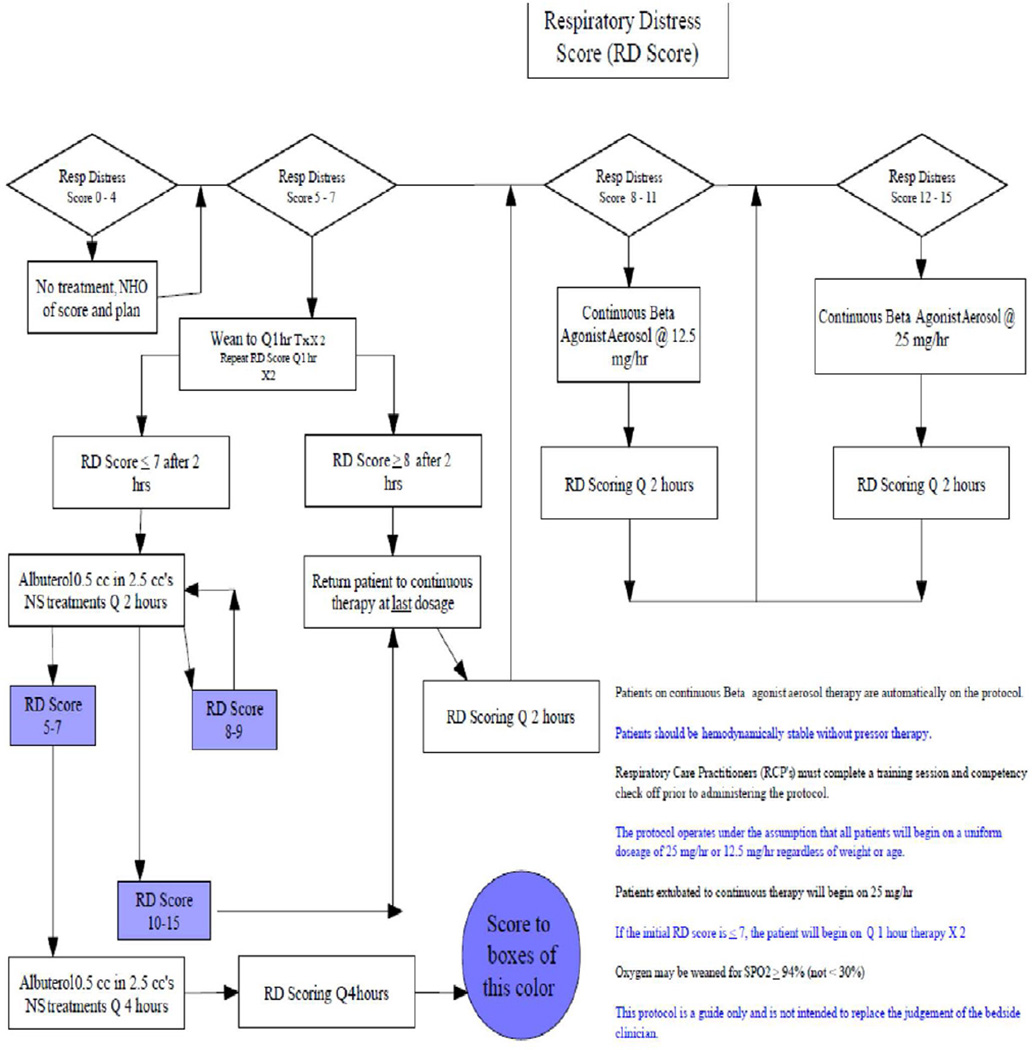
Our study is limited as a retrospective chart review using our hospital’s EMR system. As such, the decision to use aminophylline was determined by clinician preference rather than randomization. Thus, there may be unmeasured confounders in the associations we observed. We used the RDS as our severity index. Beyond this severity score we could not fully examine the particular factors which influenced the decision to use aminophylline. Further, rather than using the duration of PCCU stay as our primary outcome, we chose to use the functional length of stay (i.e. the time to the patient meeting hospital criteria for transfer from the PCCU to a general pediatric ward). We did this in an effort to minimize factors other than the patients’ clinical condition which could delay transfer, such as availability of beds in the general pediatric units, competing nursing duties, and other logistical factors. One drawback to FLOS outcome measure, however, is that FLOS can be influenced by how a patient is weaned off various interventions. Generally, the providers in our unit wean interventions sequentially (Figure-3). Thus, the mere fact that a patient is on multiple interventions could result in a longer LOS. Our secondary outcome measure (i.e. symptom improvement), however is independent of this sequential weaning process.
Figure 3.
The Asthma management protocol for pediatric critical care unit at Monroe Carrol Jr. Children’s Hospital at Vanderbilt University Medical Center
To our knowledge this is the first study to suggest a significant correlation between the use of aminophylline for treatment of severe asthma in the PCCU setting and increased LOS and time to symptom improvement. Interestingly, several studies of the pediatric asthma population have shown a slight trend toward increased LOS or increased time to symptom improvement with the administration of aminophylline or theophylline [19–22]. These results suggest the use of aminophylline and other methylxanthines in children with severe asthma exacerbations may be associated with increased morbidity. More research is necessary to determine the causality of these adverse outcomes in order to define more clearly which subset of asthma patients, if any, would benefit from aminophylline.
5. Conclusion
Adding aminophylline to corticosteroid and inhaled albuterol treatment regimens may be associated with statistically and clinically significant increases in FLOS and time to symptom improvement in the PCCU, when adjusted for relevant subject characteristics (RDS, age, gender and race). This potential morbidity supports the NAEPP guideline proscribing against aminophylline use in patients with acute asthma. If this drug continues to be used for status asthmaticus, further study of efficacy and safety is warranted.
Acknowledgments
Funding: This research was supported by the National Institutes of Health [Grant K23 HL80005-01A2] (Dr. Arnold).
Abbreviations list
- EMR
Electronic Medical Record
- FLOS
Functional Length of Stay
- HR
Hazard ratio
- IV
intravenous
- LOS
Length of Stay
- mcg/ml
Microgram per milliliters
- n
Number
- PCCU
Pediatric Critical Care Unit
- RDS
Respiratory Distress Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institutional Review Board: Committee HS3 (protocol # 100151)
Disclaimer: None
Contributor Information
Abdallah R Dalabih, Email: dalabiha@missouri.edu.
Steven A Bondi, Email: steven.a.bondi@vanderbilt.edu.
Zena L Harris, Email: zena.harris@vanderbilt.edu.
Benjamin R Saville, Email: b.saville@vanderbilt.edu.
Wenli Wang, Email: steffi4444@gmail.com.
Donald H Arnold, Email: don.arnold@vanderbilt.edu.
Reference List
- 1.Asthma prevalence and control characteristics by race/ethnicity--United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53:145–148. [PubMed] [Google Scholar]
- 2.Asthma mortality and hospitalization among children and young adults--United States, 1980–1993. MMWR Morb Mortal Wkly Rep. 1996;45:350–353. [PubMed] [Google Scholar]
- 3.Sly RM. Decreases in asthma mortality in the United States. Ann Allergy Asthma Immunol. 2000;85:121–127. doi: 10.1016/S1081-1206(10)62451-9. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Schoendorf KC, Parker J. US childhood asthma prevalence estimates: the Impact of the 1997 National Health Interview Survey redesign. Am J Epidemiol. 2003;158:99–104. doi: 10.1093/aje/kwg109. [DOI] [PubMed] [Google Scholar]
- 6.Yawn BP, Wollan P, Kurland M, Scanlon P. A longitudinal study of the prevalence of asthma in a community population of school-age children. J Pediatr. 2002;140:576–581. doi: 10.1067/mpd.2002.123764. [DOI] [PubMed] [Google Scholar]
- 7.National Heart LaBINAEaPP. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. NIH. 2007 Available from: URL: http://www.nhlbi.nih.gov/guidelines/asthma/01_front.pdf.
- 8.Bratton SL, Odetola FO, McCollegan J, Cabana MD, Levy FH, Keenan HT. Regional variation in ICU care for pediatric patients with asthma. J Pediatr. 2005;147:355–361. doi: 10.1016/j.jpeds.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Tilley SL. Methylxanthines in asthma. Handb Exp Pharmacol. 2011:439–456. doi: 10.1007/978-3-642-13443-2_17. [DOI] [PubMed] [Google Scholar]
- 10.Dalabih A, Harris ZL, Bondi SA, Arnold DH. Contemporary Aminophylline Use for Status Asthmaticus in Pediatric ICUs. Chest. 2012;141:1122–1123. doi: 10.1378/chest.11-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen SE, Hurd SS, Lemanske RF, Jr, Becker A, Zar HJ, Sly PD, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 12.British Guideline on the Management of Asthma. Thorax. 2008;63(Suppl 4):iv1–121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 13.Mitra A. The current role of intravenous aminophylline in acute paediatric asthma. Minerva Pediatr. 2003;55:369–375. [PubMed] [Google Scholar]
- 14.Mitra A, Bassler D, Goodman K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005:CD001276. doi: 10.1002/14651858.CD001276.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79:405–410. doi: 10.1136/adc.79.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair P, Milan SJ, Rowe BH. Addition of intravenous aminophylline to inhaled beta(2)-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2012;12:CD002742. doi: 10.1002/14651858.CD002742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. N Engl J Med. 1998;339:1030–1035. doi: 10.1056/NEJM199810083391503. [DOI] [PubMed] [Google Scholar]
- 18.Ream RS, Loftis LL, Albers GM, Becker BA, Lynch RE, Mink RB. Efficacy of IV theophylline in children with severe status asthmaticus. Chest. 2001;119:1480–1488. doi: 10.1378/chest.119.5.1480. [DOI] [PubMed] [Google Scholar]
- 19.Carter E, Cruz M, Chesrown S, Shieh G, Reilly K, Hendeles L. Efficacy of intravenously administered theophylline in children hospitalized with severe asthma. J Pediatr. 1993;122:470–476. doi: 10.1016/s0022-3476(05)83443-2. [DOI] [PubMed] [Google Scholar]
- 20.Needleman JP, Kaifer MC, Nold JT, Shuster PE, Redding MM, Gladstein J. Theophylline does not shorten hospital stay for children admitted for asthma. Arch Pediatr Adolesc Med. 1995;149:206–209. doi: 10.1001/archpedi.1995.02170140088016. [DOI] [PubMed] [Google Scholar]
- 21.DiGiulio GA, Kercsmar CM, Krug SE, Alpert SE, Marx CM. Hospital treatment of asthma: lack of benefit from theophylline given in addition to nebulized albuterol and intravenously administered corticosteroid. J Pediatr. 1993;122:464–469. doi: 10.1016/s0022-3476(05)83442-0. [DOI] [PubMed] [Google Scholar]
- 22.Strauss RE, Wertheim DL, Bonagura VR, Valacer DJ. Aminophylline therapy does not improve outcome and increases adverse effects in children hospitalized with acute asthmatic exacerbations. Pediatrics. 1994;93:205–210. [PubMed] [Google Scholar]