Abstract
Alpha7 nicotinic acetylcholine receptor (α7 nAChR) agonists may be valuable treatments for negative symptoms and cognitive impairment in schizophrenia. Unfortunately, chronic exposure to an agonist may reduce the receptor’s sensitivity. Therefore, we combined CDP-choline, a dietary source of the direct agonist choline, with galantamine, a positive allosteric modulator (PAM) of nicotinic acetylcholine receptors, to improve the efficiency of transducing the choline signal and, possibly, preserve the receptor in a sensitive state. We conducted a single-site, double-blind randomized clinical trial comparing galantamine/CDP-choline to placebos in schizophrenia patients with negative symptoms who were receiving second generation antipsychotics. Forty-three subjects received galantamine and CDP-choline or matching placebos for 16 weeks. The primary outcome measure was the 5-item Marder negative-symptoms factor of the Positive and Negative Syndrome Scale (PANSS). Cognition and functioning were also assessed. Trial completion was high; 79%. There was no significant treatment effect on negative symptoms, other PANSS symptom factors, or the MATRICS Cognitive Consensus Battery. There were significant treatment effects in overall functioning and a test of free verbal recall. Three subjects discontinued treatment in the active treatment group for gastro-intestinal adverse events (AE). The most common AE for galantamine/CDP-choline was abdominal pain; for placebo it was headache and sweating. Although there was no significant treatment effect on negative symptoms, the direction of effect mirrored the effects on a cognitive measure and overall functioning. Further study of α7 nAChR agonist/PAMs are warranted in larger studies that will have greater power.
Keywords: acetylcholine, alpha7 nicotinic receptor, schizophrenia, positive allosteric modulator, negative symptoms, neurocognition
I. Introduction
Current medications for the treatment of schizophrenia are only partially effective; therefore, many people living with schizophrenia do not achieve full functional recovery. In particular, negative symptoms such as social withdrawal and blunted affect, as well as disturbances of memory and attention still persist. These signs and symptoms contribute to poor social and vocational outcomes leading to chronic disability (Green et al., 2000). In this study, we examined whether a selective α7 nicotinic agonist intervention would improve negative symptoms and cognitive impairment associated with schizophrenia.
Preclinical and clinical findings support the hypothesis of deficient α7 nicotinic acetylcholine receptor (α7 nAChR)-mediated neurotransmission in schizophrenia (Deutsch et al., 2005; Jones et al., 2011; Martin and Freedman, 2007). Schizophrenia patients and their biological relatives display a sensory gating deficit that shows genetic linkage to the locus on chromosome 15, which codes the gene for the α7 nAChR subunit (i.e., CHRNA7) (Freedman et al., 1997). Moreover, decreased expression of the α7 nAChR in the frontal cortex (Guan et al., 1999), interneurons of the hippocampus (Freedman et al., 1995), and reticular nucleus of the thalamus (Court et al., 1999) of postmortem brains obtained from patients with schizophrenia may reflect single nucleotide changes within promoter regions of the gene (Leonard et al., 2002). Thus, promoter variants could account for diminished rates and amount of expression of structurally intact α7 nAChRs, which would support strategies for improving the efficiency of transduction of the acetylcholine signal by functional receptors.
The hypothesis of α7 nAChR “hypofunction” in schizophrenia has stimulated the development of selective α7 nicotinic receptor agonists as putative treatments for negative symptoms and cognitive dysfunction. An early study showed that a partial α7 nicotinic agonist, 3-(2,4-dimethoxy-benzylidene) anabaseine (DMXB-A), co-administered with neuroleptics, reduced auditory sensory gating deficits and improved cognition in the Repeatable Battery of Assessment of Neuropsychological Status (RBANS) in 12 schizophrenia patients in a single-day administration trial (Olincy et al., 2006). In another study, DMXB-A administered over four weeks to 31 patients reduced negative symptoms on the Scale for the Assessment of Negative Symptoms, but did not improve performance on the MATRICS Consensus Cognitive Battery, the primary outcome in the study (Freedman et al., 2008). More recently, another α7 nicotinic receptor partial agonist, TC-5619, was tested in a 12-week randomized, placebo-controlled trial in 185 persons with schizophrenia, and significantly reduced negative symptoms and improved a cognitive measure of executive functioning (Lieberman et al., 2013). Together, these findings suggest that selective α7 nicotinic receptor partial agonists can both reduce residual negative symptoms and improve cognition in trials of up to 12-weeks in duration.
We have proposed a parallel avenue of facilitating α7 nAChR-mediated neurotransmission in schizophrenia (Deutsch et al., 2008). Positive allosteric modulators (PAM) – agents that act at sites distinct from the orthosteric or agonist binding sites – have the capacity to improve the efficiency of coupling the binding of agonists to their biological effects while maintaining the receptor in a responsive, as opposed to refractory, state. Theoretically, allosteric modulatory strategies are very attractive because they preserve the spatial and temporal characteristics of endogenous neurotransmitter release; that is, although they lack intrinsic activity of their own, they are effective where and when neurotransmitters are released in the brain (Dani et al., 2007; Gregory et al., 2011; Lightfoot et al., 2008).
Thus, we combined galantamine, a PAM at the α7 nAChR and a cholinesterase inhibitor, and CDP-choline, a dietary source of exogenous choline; choline mimics electrophysiological and pharmacological effects of ACh at the α7 nAChR (Albuquerque et al., 1997; Albuquerque et al., 1998; Alkondon et al., 1997). We hypothesized that galantamine would improve the efficiency of coupling between the binding of choline and channel opening and, perhaps, maintain the receptor in a sensitive configuration over time. The dose of CDP-choline used in this trial was adopted from published literature in healthy volunteers, patients with acute ischemic stroke, and those with cognitive impairment due to chronic cerebral disorders (Clark et al., 1997; Clark et al., 1999; Davalos et al., 2002; Fioravanti and Yanagi, 2004; Wurtman et al., 2000; Wurtman et al., 2001). Results show that CDP-choline is safe and well tolerated in dosages of up to 2000 mg/day. In addition, the persistence of elevated plasma levels for up to 8 hours after the administration of the 2000 mg oral dose suggested that, at steady state, daily administration of 2000 mg, in two divided doses, would maintain elevated levels throughout much of the day (Wurtman et al., 2000). Theoretically, combining CDP-choline with galantamine might mitigate the potential of α7 nAChRs, like nAChRs in general, to desensitize rapidly upon exposure to an agonist, whereby a full selective agonist, such as choline derived from dietary CDP-choline, becomes a functional antagonist. We predicted that the effect of the combination treatment would be long-term and therefore of potential clinical value.
This trial was designed as a “proof of concept” that selective and sustained stimulation of α7 nAChRs, using a combination of galantamine and CDP-choline, would provide therapeutic advantages to schizophrenia patients maintained on their stable regimens of second-generation antipsychotic medications. The study was a 16-week randomized, double-blind trial, comparing the combination of galantamine/CDP-choline to matching placebos for both agents (“double-dummy”) in schizophrenia patients with predominantly negative symptoms. The primary hypothesis was that galantamine/CDP-choline would reduce negative symptoms measured by the Positive and Negative Syndrome Scale (Kay et al., 1989). Secondary hypotheses were that combination treatment would improve overall functioning assessed by the Scale of Functioning (Rapaport et al., 1996), cognition, and clinical symptoms (e.g., PANSS total).
The α7 nAChR has been identified by the MATRICS working group as a promising target for medication development to improve cognitive impairment in schizophrenia (Geyer et al., 2004). We expected that the domains of attention and memory in particular would be sensitive to the effects of α7 nicotinic stimulation based on prior research on nicotinic receptor function and cognition (Jones et al., 2011; Martin and Freedman, 2007, for review). Therefore, the neurocognitive battery in this study included selected measures of attention and memory from the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein et al., 2008) and additional measures of verbal learning, free recall, and recognition memory from the University of Southern California-Repeatable Episodic Memory Test (USC-REMT; Parker et al., 2004; Schwartz et al., 2009).
2. Methods
2.1 Summary of design and study procedures
This study was conducted at the Washington DC VA Medical Center and was approved by the Institutional Review Boards of the VA Medical Center and Georgetown University Medical Center. Interested veterans provided written informed consent for study participation and were randomly assigned to either galantamine/CDP-choline or placebo condition. Consenting participants who met inclusion exclusion criteria and completed baseline assessment entered the 16-week trial. Study participants were reimbursed for participation in assessments.
2.2 Subjects
Subjects met DSM-IV criteria for a diagnosis of schizophrenia or schizoaffective disorder using the Structured Clinical Interview for DSM-IV (First et al., 1996), were on a stable dose of a second-generation antipsychotic medication for at least four weeks prior to enrollment, and had a score of at least 4 (moderate) on at least one of the following five PANSS negative symptom items: blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, and lack of spontaneity and flow of conversation. Men and women between 18 and 70 years of age, both smokers and non-smokers, were included. Exclusion criteria were inpatient admission within two months of enrollment, antipsychotic medication change within 4 weeks, PANSS positive symptom score for conceptual disorganization, hallucinations, suspiciousness, and delusions that exceeded 18, co-morbid alcohol and/or substance abuse diagnosis within three months of enrollment or significant medical or neurological disorder.
2.3 Study medication and titration
Participants who met inclusion exclusion criteria began the double-blind, double-dummy dose titration phase. In the galantamine/CDP-choline condition, galantamine was titrated to 24 mg/day over two weeks. Subjects received 8 mg/day in two divided doses for one week, 16 mg/day in two divided doses for one week, and 24 mg/day in two divided doses beginning in week 3. CDP-choline was titrated to 2000 mg/day over one week. Subjects received 500 mg/day for three days; on day four, the dose of CDP-choline was increased to 1,000 mg/day in two divided doses for four days. At the beginning of week 2, patients received the maximum fixed dose of 2000 mg/day in two divided doses, which was held constant throughout the duration of the trial (end of week 16). The schedule of dose titration of placebo galantamine and placebo CDP-choline followed the schedule of active medication. Placebos were identical in appearance to galantamine and CDP-choline. Study participants in each condition were maintained on their dose of adjuvant medication (or placebo) and their stable regimen of antipsychotic medication through the end of week 16 to complete the trial. Dose titration was slowed for two participants in the galantamine/CDP-choline group due to the emergence of adverse events. One dropped out of the study and the other reached the target dose for both agents by week 4.
2.4 Assessments
Clinical symptoms were assessed at baseline and weeks 4, 8, 12, and 16 using the PANSS, Clinical Global Impression (CGI; NIMH, 1970), and Scale of Functioning (Rapaport et al., 1996). The primary measure of efficacy was the PANSS five-item negative symptoms factor (i.e., blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, and lack of spontaneity and flow of conversation), which is based on factorial structures of negative symptoms (Marder et al., 1997). The positive symptoms factor (i.e., delusions, hallucinatory behavior, grandiosity, suspiciousness, stereotyped thinking, somatic concern, unusual thought content, lack of judgment and insight) was used to assess positive symptoms (Marder et al., 1997). CGI Severity was rated at all time points; CGI improvement was rated at the post-baseline time points. The Scale of Functioning was used to assess impairment in everyday functioning The scale includes 14 specific items and a rating of overall functioning, all rated on a four point scale where 1 = severe impairment and 4 = full independence. The sum of the 14 specific items and the overall functioning item were analyzed separately. In addition, vital signs and adverse events (AEs) were assessed (by systematic inquiry) weekly throughout the 16 week trial. Table 3 includes those events that were rated as moderate or severe for at least 5% of subjects in the galantamine/CDP-choline group. All AEs were assessed on a scale from 1 = absent to 4 = severe.
Table 3.
Adverse Events
| Adverse Event | Drug | Placebo |
|---|---|---|
| % | % | |
| Any Emergent Symptoms | 53 | 58 |
| Abdominal Pain | 32 | 13 |
| Drowsiness | 32 | 13 |
| Headache | 26 | 21 |
| Malaise | 26 | 8 |
| Restlessness | 26 | 8 |
| Dry Mouth | 21 | 17 |
| Indigestion | 21 | 4 |
| Nausea | 21 | 13 |
| Other Sleep Problems | 21 | 13 |
| Excess Salivation | 16 | 17 |
| Insomnia | 16 | 8 |
| Nasal Congestion | 16 | 17 |
| Sweating | 16 | 21 |
| Syncope | 16 | 8 |
| Decrease in Appetite | 11 | 8 |
| Diarrhea | 11 | 17 |
| Fever | 11 | 4 |
| Vomiting | 11 | 17 |
Only treatment emergent adverse events (AEs) that occurred in more than 5% of subjects in the galantamine/CDP-choline group are reported.
Cognition was assessed at baseline and weeks 8 and 16 to minimize practice effects. MCCB tests that assess the domains of processing speed [Trail Making Test: Part A; Brief Assessment of Cognition in Schizophrenia (BACS): Symbol Coding], attention/vigilance [Continuous Performance Test-Identical Pairs (CPT-IP)], working memory [Letter-Number Span; Wechsler Memory Scale (WMS-III): Spatial Span), verbal learning [Hopkins Verbal Learning Test-Revised (HVLT-R)], and visuospatial learning [Brief Visuospatial Memory Test-Revised (BVMT-R)] were included. USC-REMT tests of auditory verbal learning and memory were also assessed at the three time points. The USC-REMT is a brief memory test with seven equivalent forms that was designed for repeat testing of episodic memory. Each form contains a list of 15 unrelated words, a three-trial multi-trial free recall, a yes-no recognition test, and a three-alternative forced-choice recognition test (see Parker et al., 2004 for details). A different form of the test was employed at each time point. The National Adult Reading Test- Revised (NART; Blair and Spreen, 1989) was administered only at baseline to estimate premorbid IQ.
2.5 Data Analysis
The sample of interest for analysis was the intent-to-treat sample. Subjects who had at least one post-baseline follow-up assessment were included in the sample. Baseline differences between the two conditions on demographic characteristics and clinical and cognitive variables were assessed using chi-square tests for categorical variables and t-tests for continuous variables. Mixed effects models with a time fixed effect, a group by time interaction, and random intercepts and slopes were used to analyze treatment effects. Based on the design of the study, i.e. randomization to the 2 groups, mean baseline responses were assumed to vary about a single mean, hence the group indicator (galantamine/CDP-choline vs. placebo) was not included in the model (Fitzmaurice et al., 2011). Treatment effects were defined by a significant group by time interaction. For continuous scales, effect sizes are calculated as model-based mean difference at the end of treatment (week 16) divided by pooled baseline standard deviation (Feingold, 2009). We calculate and report means and standard deviations for the global item from the Scale of Functioning, which is on a 4-point scale; however, for the primary analysis we used an ordinal rather than a linear mixed effects model because this outcome better satisfies the assumptions of the ordinal model. The effect size analog for this outcome is the odds ratio of improvement at trial end comparing treatment versus control. All tests were 2-sided with alpha level set at p < .05.
3. Results
3.1 Sample description
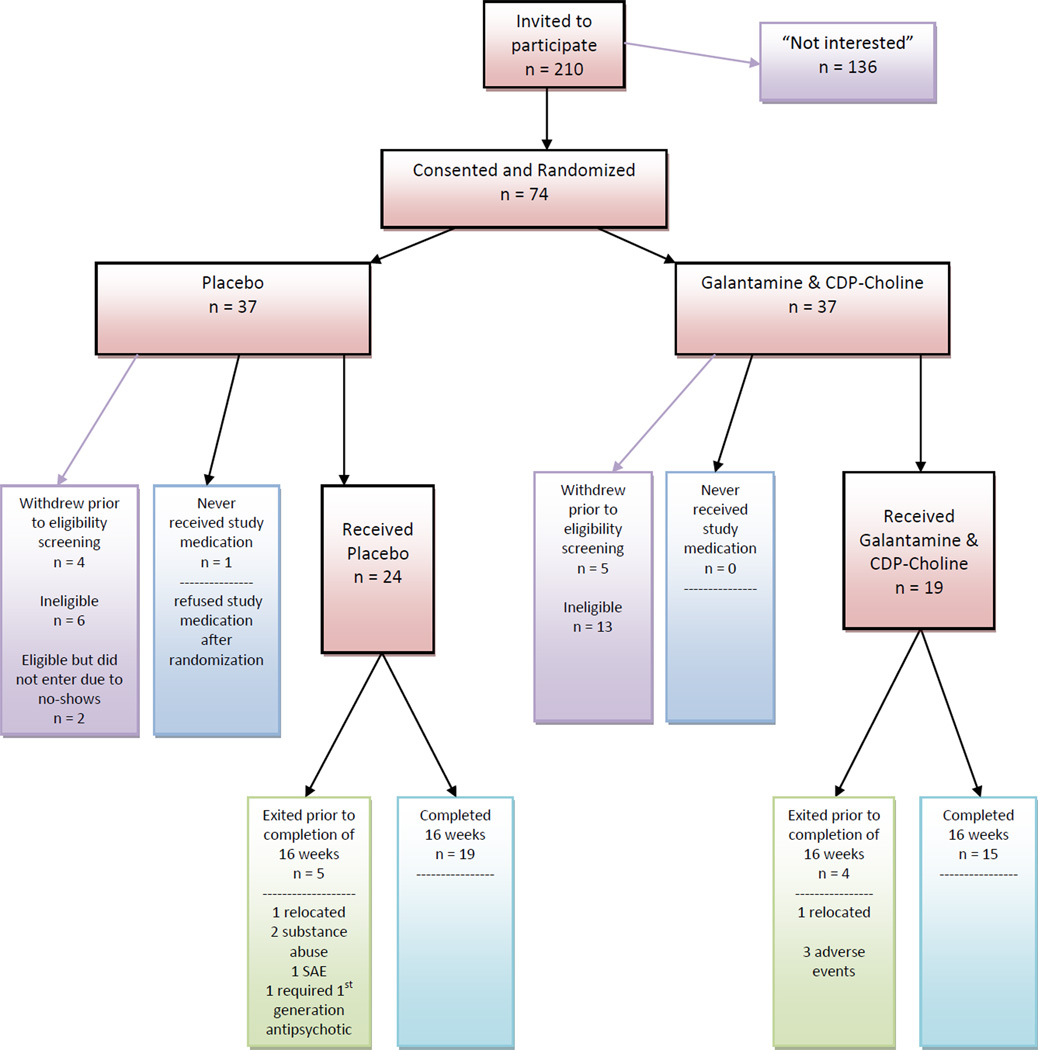
As shown in Figure 1, 74 participants consented and were randomized to treatment. Figure 1 also shows the numbers who did not meet inclusion exclusion criteria or who failed to validate consent by completing baseline assessments or taking at least one dose of medicine. Forty-three participants received medication; 19 (17 M, 2 F) received galantamine/CDP-choline and 24 (22 M, 2 F) received placebos. Of those 79% in both groups completed the 16-week trial. The mean age of the sample was 53.3 years, 91% were male, and 79% were African American (79% in each group). The mean age at illness onset was 27.3 years. There were 11 smokers in the placebo group (46%) and 12 in the galantamine/CDP-choline group (63%). As shown in Table 1, the groups were comparable in demographic and clinical characteristics. There were no statistically significant baseline differences between the groups, all p values > .05. By design, the sample had residual negative symptoms and less severe positive symptoms: Mean baseline score for the PANSS five-item negative symptoms factor was 17.8 and mean baseline score for the eight-item PANSS positive symptoms factor was 22.4. On average, 2.91 of the five items in the negative symptoms factor had a rating of moderate severity (4) or higher severity (5, 6, or 7). Mean pre-morbid IQ of the sample was 101.4. Supplemental Table A shows antipsychotic medications received by participants at baseline; risperidone was most commonly prescribed. During the trial, dose of antipsychotic medication was changed for two subjects in the galantamine/CDP-choline group (1 increase/1 decrease) and two in the placebo group (1 increase/1 decrease) during the 16-week trial.
Figure 1.
Consort chart displays the flow of subjects through the clinical trial from recruitment to completion, including the reasons why subjects did not complete the entire 16-week trial.
Table 1.
Demographic and Clinical Characteristics of Participants in the Galantamine/CDP-choline and Placebo Groups
| Placebo (n=24) | Drug (n =19) | Total sample (n=43) | ||||
|---|---|---|---|---|---|---|
| Variable | Mean | S.D | Mean | S.D | Mean | S.D |
| Age (years) | 52.38 | 11.04 | 54.37 | 8.50 | 53.28 | 9.94 |
| Age at first hospitalization (years) | 25.74 | 8.00 | 29.26 | 7.96 | 27.29 | 8.07 |
| NART-R Score | 102.28 | 7.91 | 100.25 | 9.52 | 101.38 | 8.61 |
| PANSS Negative Symptoms Factor | 18.29 | 4.53 | 17.63 | 3.48 | 17.84 | 4.61 |
| PANSS Positive Symptoms Factor | 22.42 | 6.70 | 22.05 | 5.79 | 22.26 | 6.25 |
| PANSS Total Score | 76.75 | 15.12 | 77.16 | 12.11 | 76.95 | 13.67 |
3.2 Treatment effects
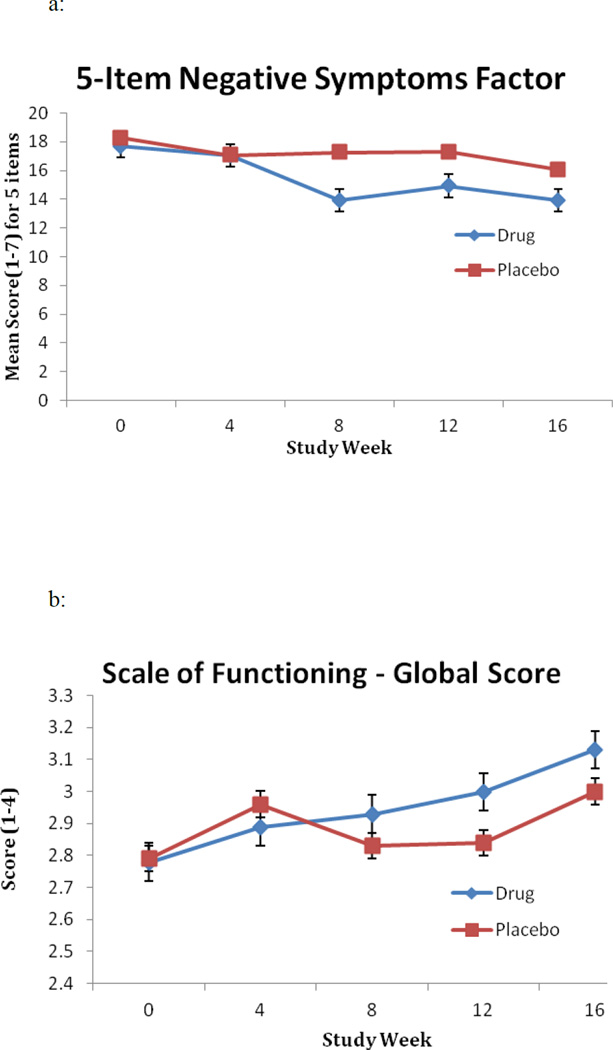
As shown in Figure 2a, both the galantamine/CDP-choline and the placebo groups had a reduction in the negative symptoms factor score, with the galantamine/CDP-choline group observed to have a greater reduction. However, the difference between the groups did not achieve statistical significance (Treatment X Time F = 2.47 [1, 106] p = .13, effect size = .46) and hence this small trial did not provide evidence of a treatment effect for negative symptoms. Further, there was no significant Treatment X Time effect for the PANSS positive symptoms factor, PANSS Total, CGI Severity or Improvement (all p values > .05). As with the negative symptoms factor, there were significant Time effects for all these variables. There was a significant Treatment X Time effect of galantamine/CDP-choline compared to placebo on the Scale of Functioning Overall Rating (t = 1.99 [df=42, p = .05] (Fig. 2b). The odds ratio (effect size) for improvement at trial end comparing the treatment group to the control group was 8.2. Supplemental Table B shows the results for clinical measures for all time points.
Figure 2.
(a) Mean score on the 5-item negative symptoms factor derived from the PANSS and (b) mean score on the global item of the Scale of Functioning at baseline (study week = 0) and weeks 4, 8, 12, and 16 for subjects in the galantamine/CDP-choline (drug) and placebo groups.
Table 2 shows performance on tests in the neurocognitive battery. No significant differences between groups were found on any of the scales at baseline, all p values > .05. There was no significant Treatment X Time effect for any measure in the MCCB. By contrast, there was a significant Treatment X Time effect for total recall across trials in the multi-trial free recall measure on the USC- REMT, (F = 3.95 [1, 98.1], p =.04, effect size = .48). There was a trend for a significant Treatment X Time effect for the forced-choice recognition memory measure of the USC-REMT, (p = .08). There was no effect of treatment condition in the yes-no recognition test for the measures of hits, false alarms, or recognition discrimination (calculated as the probability of hits minus the probability of false alarms), all p values > .05.
Table 2.
Treatment Effects on Neurocognition in the Galantamine/CDP-choline and Placebo Groups
| Neurocognitive Task |
Placebo | Drug | Treatment x Time Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 0 | 8 | 16 | 0 | 8 | 16 | F value [df] | ||||||
| MCCB | Mean | S.D | Mean | S.D | Mean | S.D | Mean | S.D | Mean | S.D | Mean | S.D | |
| Trail Making Test Part A | 45.1 | 16.5 | 40.8 | 13.3 | 36.5 | 14.7 | 42.1 | 11.3 | 42.7 | 14.2 | 34.9 | 9.64 | 0.19 [1,98.7] |
| BACS: Symbol Coding | 40.6 | 9.7 | 40.7 | 9.9 | 42.2 | 9.7 | 38.8 | 11.1 | 41.5 | 14.3 | 44.3 | 12.8 | 1.60 [1,35.2] |
| CPT-IP | 1.9 | 0.9 | 2.1 | 0.7 | 2.1 | 0.6 | 1.6 | 0.73 | 2.1 | 0.8 | 2.1 | 1.0 | 1.46 [1, 35.8] |
| Letter Number Span | 11.5 | 3.6 | 12.2 | 3.6 | 13 | 3.9 | 12.3 | 3.7 | 13.1 | 4.4 | 13.2 | 4.2 | 1.88 [1, 32] |
| WMS-III: Spatial Span | 13.7 | 3.5 | 12.7 | 3.2 | 13.7 | 3.0 | 13.0 | 3.7 | 13.3 | 3.7 | 13.9 | 3.3 | 0.03 [1, 91.9] |
| HVLT-R | 20.6 | 5.8 | 21.2 | 5.9 | 20.4 | 5.4 | 20.5 | 5.1 | 21.8 | 4.6 | 23 | 4.7 | 1.48 [1, 32.8] |
| BVMT-R | 17.8 | 8.23 | 18.9 | 7.6 | 19.6 | 8.5 | 16.7 | 8.4 | 20.3 | 7.3 | 21.7 | 7.2 | 2.16 [1, 33.3] |
| USC-REMT | |||||||||||||
| Free Recall (total) | 17.9 | 6.3 | 18.8 | 4.9 | 17.0 | 5.7 | 18.3 | 4.0 | 19.1 | 6.1 | 21.3 | 4.3 | 3.95 [1, 98.1]* |
| Yes-No Recognition | |||||||||||||
| Hits | 12.9 | 2.6 | 12.7 | 2.2 | 12.9 | 2.8 | 13.2 | 2.3 | 14.2 | 0.9 | 13.5 | 1.8 | 0.46 [1, 33] |
| False Alarms | 2.0 | 3.6 | 1.3 | 2.2 | 2.1 | 3.8 | 2.2 | 3.2 | 2.9 | 3.5 | 3.1 | 5.1 | 0.11 [1, 33] |
| Forced Choice Recognition | 9.9 | 2.5 | 10.3 | 3.2 | 9.8 | 3.1 | 9.3 | 2.6 | 10.6 | 3.4 | 11.3 | 2.0 | 3.25 [1, 32]** |
Note:
P < .05;
P<.10
3.3 Adverse Events
Figure 1 shows that three participants were discontinued from galantamine/CDP-choline prior to study completion because of adverse events; one placebo participant was hospitalized with cardiac symptoms. This SAE resolved. Table 3 presents adverse events by group that were at least moderate at any time point. As can be seen from the table, 53% of galantamine/CDP-choline and 58% of placebo experienced at least one event that was moderate or severe. The most common AE for galantamine/CDP-choline was abdominal pain (32%); for placebo headache and sweating (21%).
4. Discussion
This study tested the feasibility of combining a PAM and a cholinergic agonist to treat negative symptoms and cognitive impairment in schizophrenia. We did not find a significant effect on the primary outcome of negative symptoms or on other secondary measures of clinical symptoms. However, there was a significant treatment effect on the secondary outcome of functioning measured by the Scale of Functioning global item. Given the high threshold of negative symptoms that we selected this may be a population with more severe and intractable negative symptoms. Indeed, on average, negative symptoms at baseline were rated as 4 (moderately severe) or higher on approximately three items of the PANSS five-item negative symptoms factor for this sample. It is possible that because participants had pronounced negative symptoms, the global functioning measure was more sensitive than the negative symptoms factor in detecting changes with treatment. Although the results showed “moderate” effect sizes for both outcomes of interest, statistically significant treatment effects were observed only for global functioning. Confirming the results of our open-label pilot study (Deutsch et al., 2008) we found that the combination treatment was acceptable to patients. Further, the side effect burden was comparable to placebo. In addition, we observed a powerful effect of time in the trial. Symptoms improved and once subjects engaged in treatment they were likely to complete the study in both treatment groups. Our clinical impression was that joining the study and meeting weekly for study visits was a positive experience for many of the patients, but may have contributed to placebo effects. Fewer study visits may have reduced these effects and increased the probability of observing significant effects of the treatment.
The secondary outcome of cognition was assessed with a subset of measures from the MCCB and the USC-REMT. There were no significant treatment effects on MCCB measures (see also Freedman et al., 2008). In contrast, there was a significant treatment effect in the free recall test on the USC-REMT. We included the USC-REMT because we expected activation of α7 nicotinic-mediated neurotransmission to enhance learning and episodic memory. Studies in animals and humans have shown that nicotine and selective nAChR and mAChR agonists improve multiple aspects of cognition, including memory (Jones et al., 2011, for review). Further, α7nAChRs are found in the frontal cortex and hippocampus (Ji et al., 2001; Rousseau et al., 2005), structures critical to episodic memory functioning in schizophrenia (Heckers, 2001; Heckers et al., 1998; Ragland et al., 2009). Our positive treatment effect on memory is consistent with other studies showing that α7 nicotinic agonists reduce cognitive impairment in schizophrenia patients (Olincy et al., 2006; Lieberman et al., 2013).
In the current study, the combination of galantamine and CDP-choline was chosen to maximize and sustain the biological effect of α7 nAChR channel opening and calcium ion conductance. However, it is also possible that treatment effects resulted from galantamine alone, rather than the combination of galantamine and CDP-choline. A prior report showed improvement in alogia with galantamine administration after 12-weeks in schizophrenia patients (Conley et al., 2009). Because galantamine is also an acetylcholinesterase inhibitor, pharmacological effects of its administration may reflect nonspecific stimulation of diverse muscarinic and nicotinic acetylcholine receptors (i.e., mAChRs and nAChRs, respectively). In fact, deficient transduction of the acetylcholine (ACh) signal by M1, M4 and M5 mAChRs has been implicated in the pathophysiology of schizophrenia (Jones et al., 2011). Galantamine was the only suitable PAM approved for human administration when this clinical trial was approved and implemented. Thus, future studies targeting the α7 nAChR designed to improve the efficiency of signal transduction by this receptor should employ PAMs that are more selective than galantamine.
The study differed from other studies (Freedman et al., 2008; Lieberman et al., 2013), which may have contributed to our absence of findings on negative symptoms, and may represent limitations of the study. As a VA sample, subjects were almost exclusively male (91%), were on average 53 years old, and had been ill for 26 years. In addition, subjects were selected specifically for their prominence and persistence of negative symptoms. In doing so, we may have selected individuals whose negative symptoms reflect structural brain changes that are more resistant to improvement. Prior studies did not include a threshold for negative symptoms. Further, we included both smokers and non-smokers in the sample; however, the small sample sizes limited our ability to perform analyses on treatment differences between these groups. Smoking status has affected treatment effects in other studies of α7 nicotinic partial agonists in schizophrenia patients (Lieberman et al., 2013). These findings suggest that nicotine use is an important variable to consider in future studies of treatments designed to enhance α7 nicotinic receptor neurotransmission in schizophrenia.
This proof of concept trial administered a PAM in combination with a full agonist, which has theoretical appeal in neuropsychiatric disorders characterized by diminished density of specific functional receptors (Deutsch et al., 2005). In this circumstance, goals of treatment are to maximize and sustain the desired biological effect of receptor stimulation (e.g., receptor-gated calcium ion conductance). PAMs act selectively within only those synapses containing the specific receptors to which they bind to improve the efficiency of coupling the binding of agonists (e.g., ACh or choline) to their biological effects. Moreover, PAMs may oppose agonist-induced desensitization, which would be particularly problematic in disorders characterized by diminished expression of functional receptors, resulting in functional receptor antagonism. Whereas partial agonists may have lesser liability to cause agonist-induced receptor desensitization than full agonists, they are not capable of achieving the maximum biological effect of receptor stimulation. The safety and tolerability of the combination of galantamine and CDP-choline and the suggestion of efficacy signals on functionality and memory support future investigations of more selective PAMs administered alone and in combination with full agonists.
Supplementary Material
Acknowledgements
Janssen Scientific Affairs LLC donated the galantamine and galantamine placebo. LifeLink and Mr. David Blanco supplied the CDP-choline and CDP-choline placebo.
Funding body agreements and policies
The research was supported by NIMH grant R34MH077849 (SID, principal investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Stephen Deutsch developed the pharmacological model. Stephen Deutsch, Nina Schooler, and Barbara Schwartz designed the study and wrote the first draft of the manuscript. Clayton Brown developed the statistical approach and performed the statistical analysis. Richard Rosse managed adverse event assessment and analysis. Stephanie Rosse managed the literature search and helped with data analysis. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Stephen Deutsch, M.D., Ph.D. has served as Consultant to Merck and on the Speaker Bureau for AstraZeneca. Nina Schooler, Ph.D. has received grant/research support from the following companies over the past 24 months: Neurocrine and Otsuka and she has served as Consultant or on Advisory Boards for the following companies over the past 24 months: Eli Lilly & Company, Abbott Laboratories, Hoffman LaRoche, H. Lundbeck, Merck Inc, Janssen Psychiatry, Johnson & Johnson, Shire, NuPATHE, Amgen.Barbara Schwartz, Clayton Brown, Richard Rosse, and Stephanie Rosse do not have anything to disclose.
References
- Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CTF, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. 1997;280(3):1117–1136. [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Braga MFM, Alkondon M. Contribution of nicotinic receptors to the function of synapses in the central nervous system: The action of choline as a selective agonist of α7 receptors. J. Physiol. Paris. 1998;92:309–316. doi: 10.1016/s0928-4257(98)80039-9. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin. Neuropsychol. 1989;3:129–136. [Google Scholar]
- Clark WM, Warach SJ, Pettigrew LC, et al. Stroke Study Group. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Neurology. 1997;49:671–678. doi: 10.1212/wnl.49.3.671. [DOI] [PubMed] [Google Scholar]
- Clark WM, Williams BJ, Selzer KA, Zweifler RM, Sabounjian LA, Gammans RE. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke. 1999;30:2592–2597. doi: 10.1161/01.str.30.12.2592. [DOI] [PubMed] [Google Scholar]
- Conley RR, Boggs DL, Kelly DL, McMahon RP, Dickinson D, Feldman S, Ball MP, Buchanan RW. The effects of galantamine on psychopathology in chronic stable schizophrenia. Clin. Neuropharmacol. 2009;32(2):69–74. doi: 10.1097/WNF.0B013E31816F2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: α-bingarotoxin and nicotine binding in thalamus. J. Neurochem. 1999;73:1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davalos A, Castillo J, Alvarez-Sabin J, Secades JJ, Mercadal J, López S, Cobo E, Warach S, Shermanm D, Clark WM, Lozano R. Oral citicoline in acute ischemic stroke. An individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–2857. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Schwartz BL, Weizman A, Chilton M, Arnold DS, Mastropaolo J. Therapeutic implications of a selective α7 nicotinic receptor abnormality in schizophrenia. Isr. J. Psychiatry Relat. Sci. 2005;42(1):33–44. [PubMed] [Google Scholar]
- Deutsch SI, Schwartz BL, Schooler NR, Rosse RB, Mastropaolo J, Gaskins B. First administration of cytidine diphosphocholine and galantamine in schizophrenia: A sustained alpha7 nicotinic agonist strategy. Clin. Neuropharmacol. 2008;31(1):34–39. doi: 10.1097/wnf.0b013e31806462ba. [DOI] [PubMed] [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14(1):43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. The Cochrane Database of Systematic Reviews. 2004;4:CD000269. doi: 10.1002/14651858.CD000269.pub2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington (DC): American Psychiatric Press, Inc; 1996. [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed. Hoboken: John Wiley; 2011. pp. 128–134. [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl. Acad. Sci. USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Gusman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am. J. Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Tamminga CA. Measurement and treatment research to improve cognition in schizophrenia: Neuropharmacological aspects. Psychopharmacology. 2004;174:1–2. [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Dong EN, Meiler J, Conn PJ. Allosteric modulation of metabotropic glutamate receptors: Structural insights and therapeutic potential. Neuropharmacology. 2011;60(1):66–81. doi: 10.1016/j.neuropharm.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor [alpha]7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10(8):1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch S, Goff D, Savage C, Schacter D, Fischman A, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat. Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;231:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology Reviews. 2011:1–27. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. Br J Psychiatry. 1989;7:59–6. [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene are associated with an inhibitory deficit found in schiziophrenia. Arch. Gen. Psychiatry. 2002;59:1085–1090. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seonane F, Beaver JS, Duan N, Hosford DA. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;8:968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot AP, Kew JNC, Skidmore J. α7 nicotinic acetylcholine receptor agonists and positive allosteric modulators. Prog Med Chem. 2008;46:131–171. doi: 10.1016/S0079-6468(07)00003-3. [DOI] [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J. Clin. Psychiatry. 1997;58(12):538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the α7 nicotinic acetylcholine receptor. Int Rev. Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, III, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seiman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Guy W, Bonato RR, editors. National Institute of Mental Health. CGI: Clinical Global Impressions. Manual for the ECDEU Assessment Battery. 2nd ed. Chevy Chase; 1970. [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Arch. Gen. Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Parker ES, Landau SM, Whipple SC, Schwartz BL. Aging, recall, and recognition: A study on the sensitivity of the University of Southern California Repeatable Episodic Memory Test (USC-REMT) J. Clin. Exp. Neuropsychol. 2004;26(3):428–440. doi: 10.1080/13803390490510130. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am. J. Psychiatry. 2009;166(8):863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Bazzetta J, McAdams LA, Patterson T, Jeste DV. Validation of the scale of functioning in older outpatients with schizophrenia. Am. J. Geriatr. Psychiatry. 1996;4(3):218–228. doi: 10.1097/00019442-199622430-00005. [DOI] [PubMed] [Google Scholar]
- Rousseau SJ, Jones IW, Pullar IA, Wonnacott S. Presynaptic α7 and non-α7 nicotinic acetylcholine receptors modulate [3H]d-aspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Parker ES, Rosse RB, Deutsch SI. Recognition memory probes affect what is remembered in schizophrenia. Psychiatry Res. 2009;167:21–27. doi: 10.1016/j.psychres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem. Pharmacol. 2000;60(7):989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Schwamm LH, Kelly PJ, et al. Citicoline: Efficacy & Safety Review: Clinical Expert Report. Submission to the Regulatory Authorities of Belgium and Portugal for the European Economic Community. Barcelona, Espana: Ferrer Internacional, S.A; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.