Abstract
Background
This study investigated the oxidative burst function of peripheral phagocytic cells (granulocytes and monocytes) and assessed the relation between oxidative burst and periodontal status in adult individuals with Down syndrome (DS) versus other groups.
Methods
55 DS individuals (18–56 years old), 74 mentally retarded (MR) individuals and 88 medically healthy controls (HC) participated in the study. The MR and HC groups were age, race and gender matched with the DS group. Gingival index, plaque index, probing depth, attachment level (AL) and bleeding on probing (BOP) were recorded for each subject. Whole blood was collected for granulocyte/monocyte oxidative burst tests. Oxidative burst was determined by flow cytometry in terms of percentage of cells actively involved in oxidative burst, and oxidative intensity (magnitude of reactive oxygen intermediates per cell).
Results
the basal oxidative burst intensity of DS granulocytes was higher than that of HC and MR granulocytes, p=0.05. The Escherichia coli (E. coli) stimulated oxidative burst intensity of DS monocytes was higher than that of HC and MR monocytes, p=0.05. Regression analysis controlling for age, sex race and plaque levels showed a significant association between monocyte oxidative burst intensity and loss of periodontal attachment in the DS subjects, p<0.01. Regression analysis also showed a significant association between granulocyte oxidative burst intensity and BOP in all subjects, p< 0.05.
Conclusions
Oxidative burst activity of peripheral monocytes and granulocytes is elevated in DS affected individuals and may contributes to periodontal tissue inflammation and loss of periodontal attachment in this susceptible group.
Keywords: Down syndrome, periodontitis, granulocytes, monocytes, oxidative burst
Introduction
Down syndrome (DS) results from the presence of an extra copy of the 21st chromosome and occurs with an incidence estimated at 1 per 800 births, making it the most common of all genetic disorders (1, 2). Common manifestations of the syndrome include characteristic physical appearance and varied mental and physical disorders (3). Several physical disorders are also associated DS, including congenital heart disease, thyroid dysfunction, Alzheimers disease and immune system abnormalities including granulocyte/monocyte cell dysfunction (3–5).
Individuals with DS also have a high prevalence of periodontal disease (6, 7), which is attributed, in part, to impairments in phagocytic cell function (8–10). Granulocytes (polymorphonuclear neutrophils, PMNs) and monocytes represent the first line of defense against microbial plaque and are involved in the pathogenesis of periodontitis (11–15). Several studies have shown that granulocyte chemotaxis is impaired in DS children with gingivitis (16–19).
Previously we investigated the relation between granulocyte/monocyte phagocytic cell activities in DS individuals. We reported an association between loss of periodontal attachment and peripheral monocyte phagocytosis in DS individuals (20). Phagocytosis is followed by an oxidative burst and production of oxygen radicals to kill the engulfed pathogens. An investigation of oxidative burst in phagocytic cells from adults with DS may broaden our understanding of the relation between periodontal disease and immune cell function.
We hypothesized that the noted association between phagocytic cell activity and periodontitis in DS individuals may be related to increased oxidative stress secondary to trisomy 21. Thus, the aims of this study were to investigate oxidative burst activity of peripheral granulocytes and monocytes from adult individuals with DS and non-DS, and to determine if the generation of reactive oxygen intermediates in these cells is associated with periodontitis.
Materials and Methods
Subject recruitment and clinical evaluations were previously described (21).
Study sites
The study was done in cooperation with the Georgia Department of Human Resources (GRH)/Georgia Regional Hospitals in Atlanta, Savannah and Augusta. The study protocol and consent forms were approved by the Georgia Regional Hospital Institutional Review Board and the Georgia Health Sciences University Human Assurance Committee. The study included three subject groups, Down Syndrome group (DS), mental retardation (disability) non-Down group (MR) and a mentally normal control group (HC). Both the DS and MR subjects were recruited from the GRH healthcare systems in Atlanta, Savannah and Augusta, Georgia. All DS and MR subjects were patients of record at the three hospital locations; some were institutionalized while others were outpatients living in group-homes or with their families. All DS and MR subjects were receiving periodic dental care at one of the three GRH locations.
Inclusion criteria
The study inclusion criteria implemented for DS subjects were: confirmed diagnosis of Trisomy 21, receiving periodic dental care, age 18 years or older, a minimum of 10 teeth present, no other medical conditions known to affect periodontal status (e.g. diabetes mellitus), no antibiotic treatment in the past 3 months prior to entry in the study, no history of cigarette smoking and being able to cooperate with the study examiners. Study inclusion criteria for the MR subjects were similar to the DS subjects except for a confirmed diagnosis of mental retardation without Trisomy 21. Study inclusion criteria for the HC subjects were also similar to the DS subjects except for absence of mental retardation.
Subject recruitment
The attending dentist in charge of the dental clinic at each of the three GRH sites reviewed the available records and identified dentulous DS patients who would meet the study criteria and would be able to participate in a dental examination. Then MR patients matched to the previously identified DS patients on age, race and gender were identified from the same hospital records. The matched MR patients were selected based on their ability to cooperate and sit for the dental examination without need for sedation. Their mental retardation was secondary to head trauma at birth. The HC subjects were recruited from the general population living in the vicinity of the GRH locations used. All HC subjects were under care of private dentists and they also were matched on gender, race and age to the DS subjects.
Subject screening and enrollment
A total of 289 subjects were screened for the study, 26 were disqualified for medical reasons, 46 completed portions of the study evaluations and were not able to return to complete the remaining portions, and 217 completed most of the study evaluations. This report will focus on the 217 subjects who completed most of the study evaluations, including immunological testing. None of the subjects ever smoked cigarettes, had diabetes, or was on a medication known to influence periodontal status.
Ethical issues
The GRH dentist contacted the family or caretaker of each potential DS or MR subject, explained the study protocol and obtained their consent to enroll the subject in the study. All subjects (including DS and MR) signed the consent form.
Oral/periodontal Assessments
Two experienced dental hygienists blinded to the objectives of the study performed all exams. The examiners were calibrated and standardized in the use of the clinical evaluation measures employed in the study (21). The examiners recorded the Loe and Silness gingival index (GI) (22), the Quigley Hein plaque index (23). Probing depth (PD), attachment levels (AL) and bleeding on probing (BOP) were recorded on six sites per tooth. Third molars were excluded. Periodontitis was defined as 5% or higher of teeth scored exhibiting attachment loss =>5 mm (20, 21, 24–26).
Blood sampling
Whole blood was collected from the subjects for granulocyte/monocyte phagocytosis and other tests. All blood draws were done in the morning. A heparinized tube of blood was drawn, and immediately placed in a cooler box for transportation to the laboratory. Upon arrival at the laboratory, samples were stored at room temperature for processing the following morning. All samples were processed within a range of 16–20 hours from collection. An EDTA blood sample was also collected and processed for complete blood cell count and differential.
Oxidative burst
The production of reactive oxygen intermediates (ROIs) was monitored by the oxidation of a fluorgenic substrate using the Bursttest kit (Orpegen, Heidelberg, Germany), which allows quantification of the percentage of cells producing ROIs and their mean enzymatic activity (fluorescence intensity) by flow cytometry. One hundred microliters of cooled whole blood was mixed with 20 µl pre-cooled unlabeled opsonized E. coli, resulting in a bacteria/leukocyte ratio of ~25:1. As a negative control, whole blood was incubated with 20 µl of wash solution. All tubes were mixed at once, then incubated at 37°C for 10 minutes in a water bath. Dihydrorhodamine (DHR) 123 was added, the samples were vortexed and then incubated at 37°C for 10 more minutes. This allowed the nonfluorescent DHR to convert to fluorescent rhodamine 123 upon the production of ROIs. At the end of the incubation period the tubes were removed from the water bath and lysing solution was added and allowed to incubate for 20 min at room temperature. The tubes were then centrifuged and the supernatant decanted. The remaining 100 µl of cells were washed and then 200 µl of DNA staining solution (propidium iodide) was added to stain the DNA of the bacteria and the cells.
Flow cytometry
Between 10,000 and 15,000 leukocytes were counted from each sample on a FACSort flow cytometer. The instrument was calibrated and standardized by using Calibrite beads (Becton Dickinson, Erembodegen- Aalst, Belgium). All sample analyses were performed with Cellquest software (Becton Dickinson). The granulocyte and monocyte populations were gated by using their forward- and side-scatter dot plots. During fluorescence-activated cell sorter analysis, free bacteria and aggregates of bacteria were separated from leukocytes based on their much lower DNA content compared to that of eukaryotic cells. Oxidative burst was monitored by determining both the proportion of cells fluorescing and the relative fluorescence intensities of the gated granulocytes/monocytes.
Statistical analysis
For the purposes of this report, mean AL per subject was used as the measure of periodontal tissue loss, and percentage of sites with BOP per subject was used as the measure of periodontal tissue inflammation. Analysis of variance (ANOVA) or chi-square test was used for means or proportions respectively to examine the differences between the three groups on demographics, clinical data, white cell counts and granulocyte/monocyte oxidative burst activity. Tukey HSD (Honestly Significant Difference) test was used for post hoc mean comparisons. Multiple regression analysis was used to examine the association between oxidative burst measures and periodontal measures while controlling for known demographic risk factors for periodontitis including age, sex, race and plaque levels.
Results
Demographic and clinical data of the entire subject population was previously reported (21). Demographic, clinical and white blood cell count data of the subset of subjects included in this report are summarized in Table 1 and were discussed in detail previously (20). In summary the HC group showed less mean PI and % BOP than the DS and MR groups (Tukey’s test, p=0.05), and the DS group showed greater mean AL than the HC and MR groups (Tukey’s test, p=0.05). Granulocyte blood counts were comparable between the three groups however monocyte blood counts were higher in the MR group than the HC group (Tukey’s test, p=0.05).
Table 1.
summary of demographic, clinical and white cell count data of the subset of subjects with oxidative burst data presented in this report.
| Variable | HC N = 88 |
DS N = 55 |
MR N = 74 |
p-value |
|---|---|---|---|---|
| Demographics: | ||||
| Age | 40.79 (1.28) | 36.18 (1.63) | 45.87 (1.40) | 0.0001* |
| White Percent | 70.45% | 78.18% | 81.08% | 0.26 |
| Male Percent | 42.05% | 52.73% | 58.11% | 0.11 |
| Institution | NA | 41.82% | 82.43% | 0.001** |
| Clinical: | ||||
| Plaque Index | 1.25 (0.08) | 1.56 (0.01) | 1.78 (0.08) | 0.0001# |
| Gingival Index | 0.68 (0.03) | 0.92 (0.04) | 1 (0.03) | 0.0001# |
| Bleeding on probing | 24.81 (2.98) | 40.60 (3.78) | 43.79 (3.25) | 0.0001# |
| Attachment Level | 2.24 (0.08) | 2.70 (0.10) | 2.24 (0.08) | 0.0006¶ |
| Missing teeth | 1.80 (0.41) | 4.61 (0.52) | 4.56 (0.45) | 0.0001# |
| Cell Counts: | ||||
| WBC | 6.3 (0.19) | 5.7 (0.24) | 6.3 (0.20) | 0.12 |
| % Granulocytes | 56.9 (1.08) | 58.5 (1.36) | 57.5 (1.18) | 0.65 |
| % Monocytes | 8.6 (0.31) | 9.4 (0.39) | 9.8 (0.34) | 0.03§ |
Data presented as mean (SE) or percentage. P-value gives the probability that the groups differ in either an ANOVA test or chi-square test for means or proportions respectively.
Post-hoc analysis (Tukey’s test) showed:
MR>DS and HC;
MR>DS;
HC<MR and DS;
DS> MR and HC;
MR>HC.
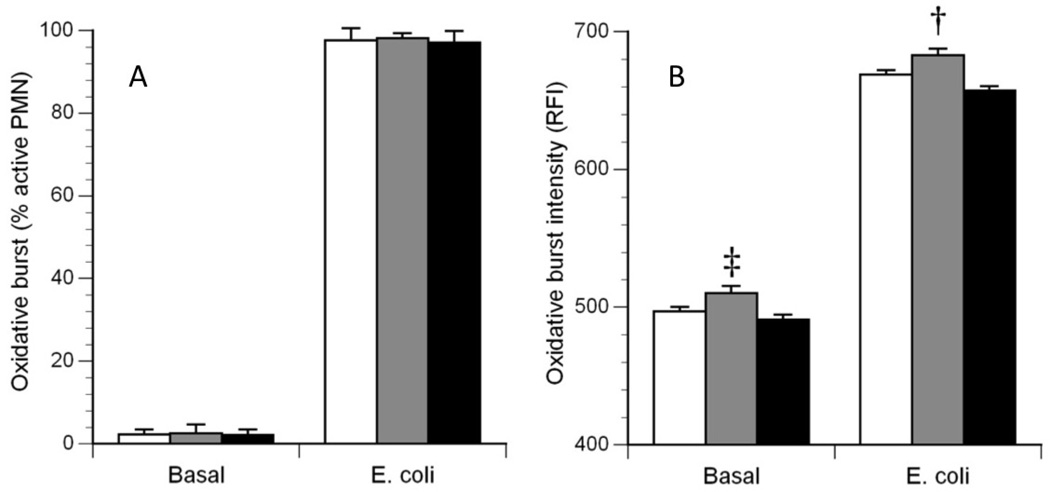
ANOVA showed no significant differences between the three groups on the percentage of granulocytes producing an oxidative burst either unstimulated or stimulated by E. coli (Figure 1A, Basal and E. coli). By contrast, ANOVA showed significant differences between the three groups for oxidative burst intensity per unstimulated granulocytes (p=0.006). Follow-up analysis with the Tukey HSD test showed that unstimulated granulocytes from the DS group had significantly higher oxidative burst intensity than granulocytes from HC and MR groups (p=0.05) (Figure 1B, Basal). ANOVA also showed significant differences between the three groups for oxidative burst intensity per E. coli stimulated granulocytes (p=0.02), with the DS group showing higher intensity than the MR group, Tukey’s HSD (p<0.05) (Figure 1B, E. coli).
Figure 1.
Oxidative burst characteristics of granulocytes. The left panel (A) illustrates the percentage of granulocytes actively producing an oxidative burst in the Basal (non-stimulated) condition and in the E. coli (stimulated) condition. The right panel (B) illustrates the average burst intensity of the active granulocytes in each condition. White bars: healthy control (HC) subjects; Gray bars: Down syndrome (DS) subjects; Black bars: Mentally disabled (MR) subjects. ‡: DS > HC and MR, P = 0.006; †: DS > MR only, P = 0.02.
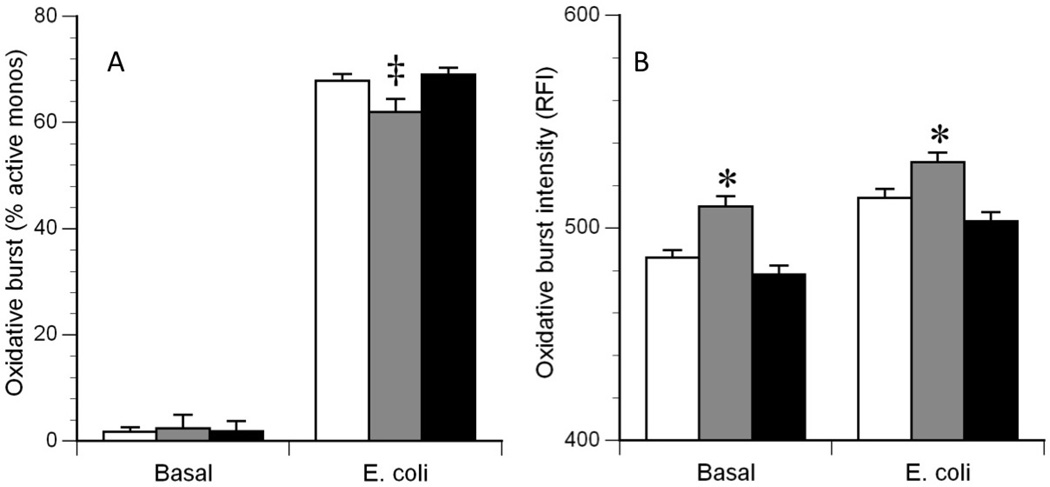
ANOVA showed no significant differences between the three groups on the percentage of unstimulated monocytes producing an oxidative burst (Figure 2A, Basal). On the other hand, ANOVA showed significant differences between the three groups on the percentage of E. coli stimulated monocytes producing an oxidative burst (p=0.01), with the percentage of monocytes from DS subjects being less than the percentage of monocytes from HC and MR subjects, Tukey HSD (p<0.05) (Figure 2A, E. coli). ANOVA also showed significant differences between the three groups for oxidative burst intensity per unstimulated (p<0.001) and E. coli stimulated monocytes (p<0.001), with the DS group showing higher intensity than the HC and MR groups, Tukey’s HSD (p<0.05) (Figure 2B, Basal and E. coli).
Figure 2.
Oxidative burst characteristics of monocytes. The left panel (A) illustrates the percentage of monocytes actively producing an oxidative burst in the Basal (non-stimulated) condition and in the E. coli (stimulated) condition. The right panel (B) illustrates the average burst intensity of the active monocytes in each condition. White bars: healthy control (HC) subjects; Gray bars: Down syndrome (DS) subjects; Black bars: Mentally disabled (MR) subjects. ‡: DS < HC and MR, P = 0.01;
*: DS > HC and MR, P < 0.001.
Analysis of covariance controlling for age, sex, race and periodontal status confirmed the univariate comparisons of phagocytic cell functions between groups.
Of all the oxidative burst measures recorded, stepwise regression analysis indicated that E. coli stimulated monocyte oxidative burst intensity was the only necessary predictor of AL (p=0.01) in all subjects and E. coli stimulated granulocyte oxidative burst intensity was the only necessary predictor of BOP in all subjects (p=0.001). These two measures of oxidative burst were used in the subsequent regression models investigating the relation between oxidative burst activity and periodontal measures.
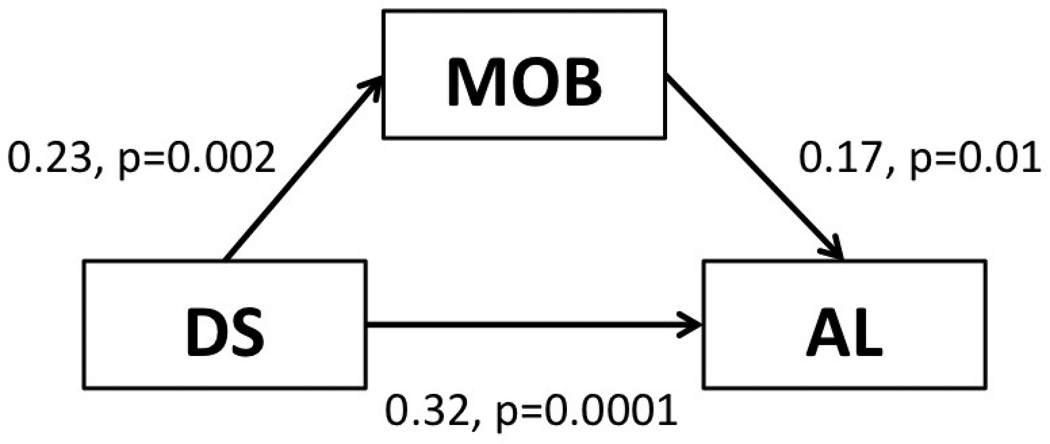
The association between monocyte oxidative burst and AL was investigated in a sequential series of regression models (table 2). In model (1) we examined the association between monocyte oxidative burst and AL without any adjustments. Monocyte oxidative burst significantly associated with AL (p<0.01). In model (2) known risk factors associated with periodontitis (age, gender, race and plaque levels) were added. Introducing these variables improved model fit (R2=0.14). Monocyte oxidative burst intensity continued to show an independent association with AL (p<0.01). In model (3) DS and MR were added. Adding DS and MR to the model further improved model fit (R2=0.23) and significantly reduced the association between monocyte oxidative burst intensity and AL. MR showed no association with AL however DS showed a direct independent association with AL (p<0.01). The reduction in the association between monocyte oxidative burst intensity and AL upon introducing DS into the model indicates that this association depends on the presence of the DS group and suggests, more importantly, that increased AL in the DS individuals may be partially explained by mechanisms related to monocyte oxidative stress (Figure 3).
Table 2.
Linear regression models relating AL to monocyte oxidative burst while controlling for demographic variables, plaque levels, DS status, and MR. Data presented as beta weights.
| Model 1 N=217 |
Model 2 N=217 |
Model 3 N=217 |
|
|---|---|---|---|
| Monocyte oxidative burst intensity | 0.17** | 0.19** | 0.11 |
| Age | 0.27** | 0.34** | |
| Sex (male=1, female=0) | 0.19** | 0.19** | |
| Race (non-white=1, white=0) | 0.15** | 0.17** | |
| Plaque levels | 0.05 | 0.06 | |
| DS (yes=1, no=0) | 0.30** | ||
| MR (yes=1, no=0) | 0.05 | ||
| R square | 0.03 | 0.14 | 0.23 |
p < 0.01
p < 0.05
Figure 3.
Association between monocyte oxidative burst (MOB), Down syndrome (DS) and periodontal attachment loss (AL). Data adjusted for age, gender, race, plaque levels and MR status and presented as beta weight, p-value.
The association between granulocyte oxidative burst and BOP was also investigated in a sequential series of regression models (table 3). In model (1) we examined the association between granulocyte oxidative burst and BOP without any adjustments. Granulocyte oxidative burst significantly associated with BOP (p<0.01). In model (2) factors that might influence BOP (age, gender, race, plaque levels and periodontal probing depth) were added. Introducing these variables improved model fit (R2=0.15). The association between granulocyte oxidative burst intensity and BOP slightly diminished however it remained significant (p<0.05). In model (3) DS and MR were added. Adding DS and MR to the model further improved model fit (R2=0.26). DS and MR both showed independent direct associations with BOP (p<0.01). The direct association between granulocyte oxidative burst intensity and BOP remained significant suggesting that its effect is independent from DS and MR status. Follow-up analysis investigating the interaction between granulocyte oxidative burst intensity and DS on BOP was insignificant.
Table 3.
Linear regression models relating BOP to granulocyte oxidative burst while controlling for demographic variables, periodontal variables, DS status, and MR. Data presented as beta weights.
| Model 1 N=217 |
Model 2 N=217 |
Model 3 N=217 |
|
|---|---|---|---|
| Granulocyte oxidative burst intensity | 0.21** | 0.15* | 0.16* |
| Age | 0.03 | −0.09 | |
| Sex (male=1, female=0) | 0.06 | −0.02 | |
| Race (non-white=1, white=0) | 0.14* | 0.14* | |
| Plaque levels | −0.03 | −0.14* | |
| Probing depth | 0.23** | 0.28** | |
| DS (yes=1, no=0) | 0.18** | ||
| MR (yes=1, no=0) | 0.42** | ||
| R square | 0.04 | 0.15 | 0.26 |
p < 0.01
p < 0.05
Discussion
Results from this study showed that in DS individuals the oxidative burst intensity of both granulocyte and monocyte cells were heightened. In all subjects the monocyte oxidative burst intensity was a stronger predictor of AL than granulocyte oxidative burst intensity and the granulocyte oxidative burst intensity was a stronger predictor of BOP than monocyte oxidative burst intensity. Furthermore, loss of periodontal attachment in DS individuals may be partially explained by their increased monocyte oxidative burst intensity.
It is interesting to note that even though plaque levels and gingival inflammation were elevated in both the MR and the DS subjects, the DS subjects showed greater loss of periodontal attachment than the MR subjects (Table 1). This suggests that the pathogenesis of periodontitis in the DS individuals is influenced by additional factors other than those traditionally associated with periodontitis in the general population. The heightened oxidative burst activity of DS phagocytic cells reported in this study may represent an additional factor influencing the pathogenesis of periodontitis in this susceptible group.
The heightened oxidative burst activity of DS phagocytic cells may be related to a gene dose effect associated with trisomy of chromosome 21. Chromosome 21 encodes several proteins that are associated with production of oxygen radicals such as nicotinamide adenine dinucleotide phosphate (NADPH) and superoxide dismutase (SOD) (27–29). Increased amounts of these products would lead to oxidative stress. The resulting reactive oxygen species (ROS) would enhance tissue damage and inflammation. Thus the extra chromosome 21 in granulocyte/monocytes of DS individuals would result in increased oxidative burst activity.
It is relevant to note that the average burst intensity of active cells rather than the percentage of cells actively producing an oxidative burst was more important on periodontal tissue damage in the DS subjects. This observation further support the notion that increased susceptibility to periodontitis in DS individuals is due in part to increased dosage of oxidative burst related products encoded by chromosome 21.
The flow cytometry methods used in the present study have been demonstrated to compare favorably with classical assays for oxidative burst, such as reduction of cytochrome C and radioactive iodine binding assays (30), and flow cytometry avoids cellular isolation procedures that can be a source of variability. Using flow cytometry methods, differences of ~200 MFI in the E. coli-stimulated oxidative burst have been reported for cells from burn patients versus controls (31). Thus, the ~20 MFI differences reported in the present study represent a relatively small abnormality. Nevertheless, such chronically elevated generation of reactive oxygen intermediates by cells from DS individuals may have a cumulative adverse effect on their health.
Granulocytes involved in periodontal infections tend to accumulate in the periodontal pocket in proximity to the microbial plaque in the dentogingival region (32). They directly interact with microbial plaque and their oxidative burst discharge may irritate and ulcerate the lining epithelium and would explain the association between their oxidative burst activity and gingival bleeding on probing. On the other hand, monocytes tend to infiltrate inflamed gingival connective tissues as tissue macrophages and play various important roles including phagocytosis of cellular debris and pathogens, production of oxygen radicals, production of inflammatory cytokines and tissue degrading enzymes (15, 33–35). Thus due to their deeper location inside the periodontal tissues and their multiple damaging products their activity may inflict greater damage on the periodontium. This may explain why monocyte oxidative burst intensity is a more reliable predictor of AL than granulocyte oxidative burst intensity.
Previous studies investigating phagocytic cell function in relation to periodontal disease in DS individuals mainly focused on chemotactic activity of granulocytes (16–18). All studies agreed that DS individuals show diminished granulocyte chemotactic activity. The increased ROS activity observed in the present study may provide a partial explanation for this reduced chemotaxis. Sakai et al. (36) have recently reported that elevated ROS production can inhibit actin polymerization and subsequently impair neutrophil chemotaxis. Thus granulocytes in DS individuals may fail to reach the dentogingival region and form a barrier between microbial plaque and the periodontium leaving the macrophages within the gingival tissues to provide the primary defense against the infection. Gingival tissue macrophages are predominantly present in inflamed gingival tissues obtained from DS individuals (37). They seem to be active in immune interactions and expression of HLA Class II antigens (38). The basal conditions shown in Figure 2 would be most representative of the native activation state of the cells. Although only a small percentage of the monocytes were active under basal conditions, the burst intensity of the active cells from DS subjects were significantly greater than the intensity for HC or MR subjects, thus indicating a chronically elevated state of inflammation. The predominant presence and activity of monocytes within the gingival tissues of DS individuals may further explain why attachment level in DS was associated with monocyte oxidative burst intensity.
In conclusion, the findings from this study provide additional evidence that deficits in phagocytic cell function in DS individuals are associated with periodontal tissue damage. This indicates that clinical procedures aimed at reducing microbial load would reduce host microbial interactions and would benefit individuals in this susceptible group.
Acknowledgement
This study was supported by the National Institute of Dental and Craniofacial Research, Bethesda, Maryland (NIDCR: DE15012-02).
Footnotes
There are no perceived or actual conflicts of interest to be reported.
References
- 1.Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:221–227. doi: 10.1002/mrdd.20157. [Review] [93 refs]. [DOI] [PubMed] [Google Scholar]
- 2.Shin M, Besser LM, Kucik JE, et al. Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009;124:1565–1571. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- 3.Megarbane A, Ravel A, Mircher C, et al. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genetics in Medicine. 2009;11:611–616. doi: 10.1097/GIM.0b013e3181b2e34c. [Review] [58 refs]. [DOI] [PubMed] [Google Scholar]
- 4.Bloemers BL, van Bleek GM, Kimpen JL, Bont L. Distinct abnormalities in the innate immune system of children with Down syndrome. Journal of Pediatrics. 2010;156:804–809. doi: 10.1016/j.jpeds.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Kusters MA, Verstegen RH, Gemen EF, de Vries E. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol. 2009;156:189–193. doi: 10.1111/j.1365-2249.2009.03890.x. [Review] [74 refs]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakellari D, Arapostathis KN, Konstantinidis A. Periodontal conditions and subgingival microflora in Down syndrome patients. A case-control study. J Clin Periodontol. 2005;32:684–690. doi: 10.1111/j.1600-051X.2005.00737.x. [see comment]. [DOI] [PubMed] [Google Scholar]
- 7.Reuland-Bosma W, van Dijk J. Periodontal disease in Down's syndrome: a review. J Clin Periodontol. 1986;13:64–73. doi: 10.1111/j.1600-051x.1986.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 8.Novo E, Garcia MI, Lavergne J. Nonspecific immunity in Down syndrome: a study of chemotaxis, phagocytosis, oxidative metabolism, and cell surface marker expression of polymorphonuclear cells. Am J Med Genet. 1993;46:384–391. doi: 10.1002/ajmg.1320460408. [DOI] [PubMed] [Google Scholar]
- 9.Rascon Trincado MV, Lorente Toledano F, Villalobos VS. A study of the functions of polymorphonuclear neutrophil in patients with Down's syndrome. Allergol Immunopathol (Madr) 1988;16:339–345. [PubMed] [Google Scholar]
- 10.Wysocki H, Wysocki J, Wierusz-Wysocka B. The influence of thymus extract on the phagocytosis and the bactericidal capacity of polymorphonuclear neutrophils from children with Down's syndrome. Ann N Y Acad Sci. 1987;496:740–742. doi: 10.1111/j.1749-6632.1987.tb35839.x. [DOI] [PubMed] [Google Scholar]
- 11.Buchmann R, Hasilik A, Nunn ME, Van Dyke TE, Lange DE. PMN responses in chronic periodontal disease: evaluation by gingival crevicular fluid enzymes and elastase-alpha-1-proteinase inhibitor complex. J Clin Periodontol. 2002;29:563–572. doi: 10.1034/j.1600-051x.2002.290613.x. [DOI] [PubMed] [Google Scholar]
- 12.Figueredo CMS, Fischer RG, Gustafsson A. Aberrant neutrophil reactions in periodontitis. J Periodontol. 2005;76:951–955. doi: 10.1902/jop.2005.76.6.951. [DOI] [PubMed] [Google Scholar]
- 13.Gronert K, Kantarci A, Levy BD, et al. A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. J Immunol. 2004;172:1856–1861. doi: 10.4049/jimmunol.172.3.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone AM, Koh A, Goldberg MB, Glogauer M. A hyperactive neutrophil phenotype in patients with refractory periodontitis. J Periodontol. 2007;78:1788–1794. doi: 10.1902/jop.2007.070107. [DOI] [PubMed] [Google Scholar]
- 15.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 16.Izumi Y, Sugiyama S, Shinozuka O, Yamazaki T, Ohyama T, Ishikawa I. Defective neutrophil chemotaxis in Down's syndrome patients and its relationship to periodontal destruction. J Periodontol. 1989;60:238–242. doi: 10.1902/jop.1989.60.5.238. [DOI] [PubMed] [Google Scholar]
- 17.Reuland-Bosma W, van den Barselaar MT, van de Gevel JS, Leijh PC, de Vries-Huiges H, The HT. Nonspecific and specific immune responses in a child with Down's syndrome and her sibling. A case report. J Periodontol. 1988;59:249–253. doi: 10.1902/jop.1988.59.4.249. [DOI] [PubMed] [Google Scholar]
- 18.Sreedevi H, Munshi AK. Neutrophil chemotaxis in Down syndrome and normal children to Actinobacillus actinomycetemcomitans. J Clin Pediatr Dent. 1998;22:141–146. [PubMed] [Google Scholar]
- 19.Yavuzyilmaz E, Ersoy F, Sanal O, Tezcan I, Ercal D. Neutrophil chemotaxis and periodontal status in Down's syndrome patients. J Nihon Univ Sch Dent. 1993;35:91–95. doi: 10.2334/josnusd1959.35.91. [DOI] [PubMed] [Google Scholar]
- 20.Khocht A, Russell B, Cannon JG, Turner B, Janal M. Phagocytic cell activity and periodontitis in Down syndrome. Oral Dis. 2012;18:346–352. doi: 10.1111/j.1601-0825.2011.01877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khocht A, Janal M, Turner B. Periodontal health in Down syndrome: contributions of mental challenge, personal and professional dental care. Spec Care Dentist. 2010;30:118–123. doi: 10.1111/j.1754-4505.2010.00134.x. [DOI] [PubMed] [Google Scholar]
- 22.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 23.Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962;65:26–29. doi: 10.14219/jada.archive.1962.0184. [DOI] [PubMed] [Google Scholar]
- 24.Khocht A, Yaskell T, Janal M, et al. Subgingival microbiota in adult Down syndrome periodontitis. J Periodontal Res. 2012;47:500–507. doi: 10.1111/j.1600-0765.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teles FR, Teles RP, Siegelin Y, Paster B, Haffajee AD, Socransky SS. RNA-oligonucleotide quantification technique (ROQT) for the enumeration of uncultivated bacterial species in subgingival biofilms. Molecular oral microbiology. 2011;26:127–139. doi: 10.1111/j.2041-1014.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khocht A, Heaney K, Janal M, Turner B. Association of interleukin-1 polymorphisms with periodontitis in Down syndrome. J Oral Sci. 2011;53:193–202. doi: 10.2334/josnusd.53.193. [DOI] [PubMed] [Google Scholar]
- 27.Akinci O, Mihci E, Tacoy S, Kardelen F, Keser I, Aslan M. Neutrophil oxidative metabolism in Down syndrome patients with congenital heart defects. Environmental & Molecular Mutagenesis. 2010;51:57–63. doi: 10.1002/em.20511. [DOI] [PubMed] [Google Scholar]
- 28.Lemieux N, Malfoy B, Forrest GL. Human carbonyl reductase (CBR) localized to band 21q22.1 by high-resolution fluorescence in situ hybridization displays gene dosage effects in trisomy 21 cells. Genomics. 1993;15:169–172. doi: 10.1006/geno.1993.1024. [DOI] [PubMed] [Google Scholar]
- 29.Strydom A, Dickinson MJ, Shende S, Pratico D, Walker Z. Oxidative stress and cognitive ability in adults with Down syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:76–80. doi: 10.1016/j.pnpbp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Kampen AH, Tollersrud T, Lund A. Flow cytometric measurement of neutrophil respiratory burst in whole bovine blood using live Staphylococcus aureus. Journal of immunological methods. 2004;289:47–55. doi: 10.1016/j.jim.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Parment K, Zetterberg A, Ernerudh J, Bakteman K, Steinwall I, Sjoberg F. Long-term immunosuppression in burned patients assessed by in vitro neutrophil oxidative burst (Phagoburst) Burns. 2007;33:865–871. doi: 10.1016/j.burns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Iacono VJ, Singh S, Golub LM, Ramamurthy NS, Kaslick R. In vivo assay of crevicular leukocyte migration. Its development and potential applications. J Periodontol. 1985;56:56–62. doi: 10.1902/jop.1985.56.11s.56. [DOI] [PubMed] [Google Scholar]
- 33.Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;6:87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [Review] [83 refs]. [DOI] [PubMed] [Google Scholar]
- 34.Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, et al. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arteriosclerosis, Thrombosis & Vascular Biology. 2004;24:2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 35.Younes R, Ghorra C, Khalife S, et al. Pertinent cell population to characterize periodontal disease. Tissue & Cell. 2009;41:141–150. doi: 10.1016/j.tice.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Sakai J, Li J, Subramanian KK, et al. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37:1037–1049. doi: 10.1016/j.immuni.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohoel PD, Johannessen AC, Kristoffersen T, Haugstvedt Y, Nilsen R. In situ characterization of mononuclear cells in marginal periodontitis of patients with Down's syndrome. Acta Odontol Scand. 1992;50:141–149. doi: 10.3109/00016359209012757. [DOI] [PubMed] [Google Scholar]
- 38.Sohoel DC, Johannessen AC, Kristoffersen T, Nilsen R. Expression of HLA class II antigens in marginal periodontitis of patients with Down's syndrome. Eur J Oral Sci. 1995;103:207–213. doi: 10.1111/j.1600-0722.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]