Abstract
Immune cells and their secreted growth factors play major roles in tumor growth and metastasis. Interplay between the growing tumor and infiltrating immune cells decides the nature of immune response and ultimately, tumor fate. Increased infiltration of pro-tumorigenic immune cells promotes tumor growth as well as dissemination to distant sites. These cells induce immunosuppression that inhibits proliferation and functions of cells of anti-tumor immune response. One population of immunosuppressive cells that is increasingly gaining attention is myeloid-derived suppressor cells (MDSCs). MDSCs are immature myeloid progenitors that suppress T cell effector functions and promote angiogenesis. MDSC numbers are elevated at both the primary tumor and metastatic sites including bone. In addition to immunosuppressive functions of MDSCs, we and other have recently discovered a novel function for MDSCs as osteoclast progenitors. Osteolysis is a common complication in the carcinomas of breast, lung, prostate and multiple myeloma with poor prognosis. Therefore, targeting MDSCs functions may exert dual therapeutic effects on immunosuppression and bone pathology.
Keywords: Breast cancer, Bone metastasis, Osteoclasts, MDSC
Introduction
One of the hallmarks of tumor progression is evading immunosurveillance, which allows tumor cells to escape anti-tumor immune response and/or to actively suppress it (1). This contributes to the establishment of primary tumors and subsequent metastasis. Innate and adaptive immune cell often infiltrate tumor sites. Innate and adaptive immune cells often infiltrate at the tumor sites. Different types of these immune cells and the products secreted by them determine the fate of tumor progression. Immune cells also play a major role in establishing metastasis of the primary tumor to various organs including the bone (2, 3). A protective immune response against tumor is often prevailed by the pro-tumor immune response and eventually, it is the balance or the lack of it between these two processes that determines the fate of tumor growth and metastasis. One of the immuosuppressive populations that are rapidly gaining interest and attention in the tumor biology is myeloid-derived suppressor cells (MDSCs). These cells infiltrate various cancers and are often associated with poor prognosis (4). Several reports have demonstrated increased infiltration of MDSCs in breast cancer, lung cancer and multiple myeloma, both in the primary tumor and metastatic sites including bone (5–7), which necessitates further understanding of the roles played by this population in cancer progression.
MDSCs as immunosuppressive cells
MDSCs are a heterogeneous population comprising of immature myeloid cells (IMC). Under normal conditions, the IMC differentiate into mature macrophages, dendritic cells and granulocytes. But in the pathological conditions including cancer, IMC differentiation is inhibited resulting in the accumulation of immunosuppressive MDSCs (4). In mice MDSCs are identified mainly by presence of CD11b and Gr-1. Two main subsets of MDSCs are monocytic MDSCs (M-MDSCs) (CD11bhiGr-1midLy6ChiLy6Glo) and granulocytic MDSCs (G-MDSCs) (CD11bhiGr-1midLy6CloLy6Ghi). In humans, MDSCs are characterized mainly as CD11b+CD33+HLA-DR- cells. MDSCs expressing CD14 are M-MDSCs whereas CD14- cells are G-MDSCs. In both mice and humans, M-MDSCs have high levels of nitric oxide (NO) and very low levels of reactive oxygen species (ROS) whereas the reverse is true for G-MDSCs. However, both these subtypes have high arginase activity (4, 7, 8).
MDSCs play a pivotal role in cancer progression by suppressing both innate and adaptive immune response. Accumulation of MDSCs has been reported in almost all cancers, both in the pre-clinical models and human patients. MDSCs are present in abundance within primary and metastatic solid tumors (8). Tumor progression is associated with gradual accumulation of this immunosuppressive population in bone marrow, spleen, peripheral blood and lymph nodes. MDSCs suppress T cell effector functions in several ways. MDSCs deplete L-arginine through arginase-1 (ARG)-dependent consumption and by sequestering L-cysteine and thus suppress the proliferation of T cells. ROS and NO generated by MDSCs suppress T cell functions by loss of TCR-ζ chain expression, nitration and desensitization of the T-cell receptor (TCR) and interference with IL-2 receptor signaling. MDSCs express certain surface proteins such as ADAM 17 (a disintegrin and metalloproteinase domain-containing 17) and Galectin 9 that interfere with trafficking of T cells and induce their apoptosis, respectively. Besides suppressing effector T cell populations, MDSCs promote the activation and expansion of Treg and thus mediate immunosuppression. MDSCs also produce factors that promote tumor growth by inducing angiogenesis and lymphangiogenesis. MDSCs secrete several pro-angiogenic markers and can directly incorporate into tumor endothelium (4, 7).
These diverse mechanisms used by MDSCs allow tumor growth and metastasis to multiple organs including the bone (4, 9). MDSCs numbers are elevated in multiple myeloma and as breast cancer disseminates to the bone (10, 11). Bone is one of the major organs for breast, lung and prostate metastasis whereas MM originates in the bone. Bone metastasis is often associated with poor prognosis and high morbidity. Nearly 80–90% of all breast cancer patients with advanced disease have osteolytic pathology as characterized by increased bone damage resulting from enhanced osteoclast activity (12). Bone undergoes a constant remodeling through osteoclast-mediated bone resorption and osteoblast-mediated bone regeneration in a tightly coupled manner to maintain homeostasis. But during tumor growth in the bone, dysregulation of this process leads to either osteolytic or osteoblastic phenotypes (13). Bone metastases are often associated with increase in osteoclast activation and osteolysis.
MDSCs being progenitors of macrophages, which are osteoclast precursor cells, together with the fact that MDSCs numbers greatly increase during cancer bone metastasis, their role in enhanced osteoclastogenesis is of significant interest.
MDSCs as novel osteoclast progenitors
Osteoclasts are giant, multi-nucleated, bone degrading cells. They are characterized by high expression of tartarate resistant acid phosphatase (TRAP) and cathepsin K. Two critical factors for osteoclast formation are receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). Interactions of RANKL with the receptor RANK and that of M-CSF with its receptor colony-stimulating factor 1 receptor (c-fms) trigger a series of signaling pathways that lead to osteoclastogenesis (13). Stimulation of macrophages in vitro with M-CSF and RANKL induces their differentiation into multi-nucleated osteoclasts (14). Various signaling mechanisms such as ROS and NO production are involved in osteoclastogenesis (15).
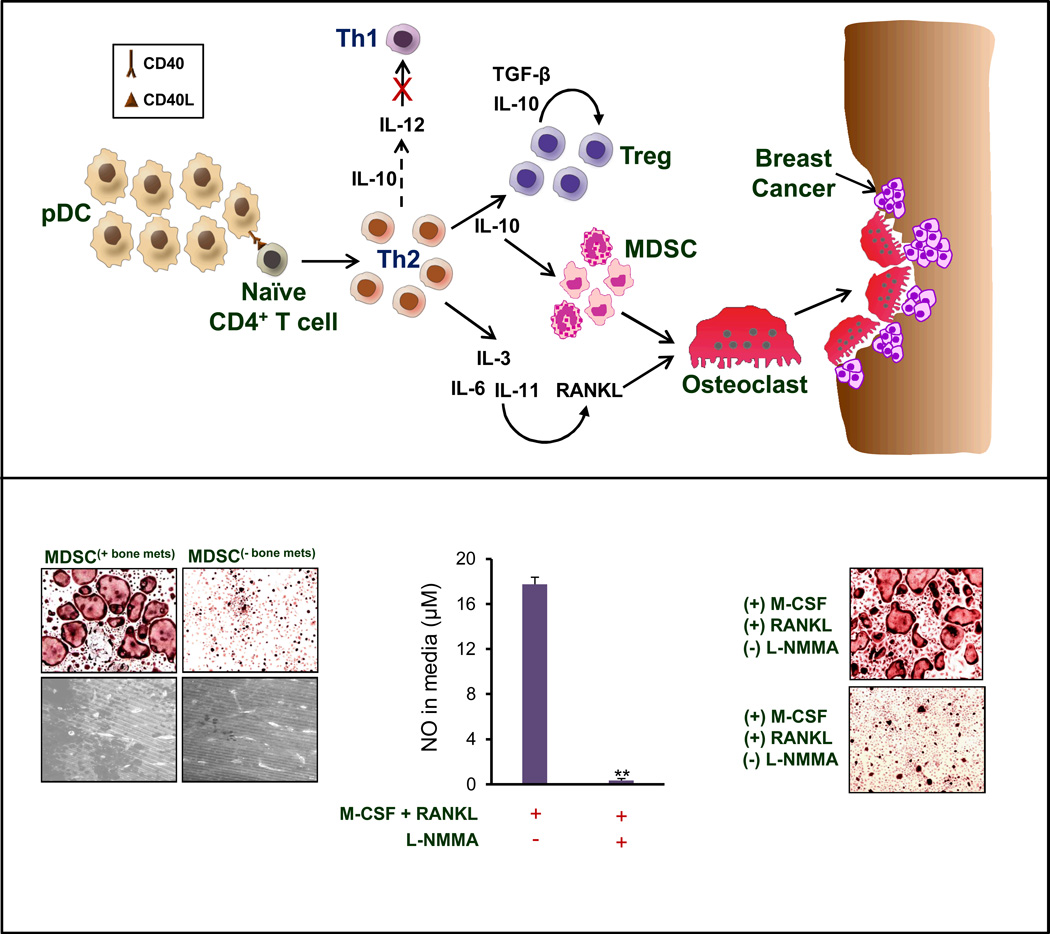
Besides MDSCs being progenitors of macrophages, which differentiate into osteoclasts, we and others have recently identified that MDSCs in the bone-tumor microenvironment directly undergo osteoclast differentiation and contribute to enhanced bone destruction and tumor growth. Our data demonstrated that MDSCs isolated from the tumor-bearing mice with bone metastasis differentiated into functional bone-resorbing osteoclasts in vitro and in vivo (11). This indicates that these cells are primed to be osteoclast progenitors and the bone microenvironment in vivo triggers their differentiation into functional osteoclasts. More importantly, not all MDSCs populations were capable of osteoclast differentiation. In our study, we differentiated MDSCs from lung, spleen, blood, lymph nodes of tumor-challenged mice into osteoclasts. None of these MDSCs differentiated into osteoclasts, which suggested that the bone microenvironment is essential. However, MDSCs isolated from bone showing presence of metastasized tumor underwent osteoclast differentiation but not the ones which did not have any tumor or control bone marrow derived MDSCs (Figure 1). This clearly shows that for MDSCs to differentiate into osteoclasts, signals from both bone marrow cells and bone metastases. It remains to be determined which factors are involved in polarizing MDSCs for osteoclast differentiation. Breast cancer cells present in the bone secrete a variety of growth factors including MCP-1 and RANTES, both of which are osteoclastogenic. MDSCs express CCR2, receptor for MCP-1 and thus are responsive to this cytokine. Ongoing studies in our lab are focused on understanding interactive signals between cancer cells, MDSCs and the bone microenvironment.
Figure 1.
(A) With dissemination of breast cancer to the bone, there is a significant elevation in the numbers of plasmacytoid dendritic cells (pDCs). pDCs interact with naïve CD4+ T cells via CD40/CD40L and induce their differentiation in the immunosuppressive Th2 cells. Presence of Th2 cells results into suppression of Th1 cell response and further induces immunosuppression via regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Cytokines secreted by Th2 cells induce RANKL. MDSCs in the bone microenvironment undergo osteoclast differentiation and induce osteolysis. This allows growth and establishment of bone metastasis of breast cancer. (B) MDSCs isolated from tumor-bearing mice with bone metastasis (MDSC(+bone mets)) undergo osteoclast differentiation and resorb bone whereas those from tumor-bearing mice without bone metastasis (MDSC(-bone mets)) are incapable of differentiating into osteoclasts or induce osteolysis. Furthermore, nitric oxide (NO) is crucial for differentiation of MDSCs into osteoclasts. In the presence of L-NMMA, an inhibitor of NO synthesis, MDSC(+bone mets) do not differentiate into osteoclasts, as described earlier in Sawant et al, Can. Res. 2013.
Besides our report, Danilin et al reported similar observation using a human breast cancer cell line (9). They demonstrated that MDSCs from tumor-bearing mice upregulated parathyroid hormone-related protein (PTHrP) and Gli2 mRNA levels in cancer cells, both of which are involved in osteoclast activation. When mice were injected with breast cancer cells alongwith either immature myeloid precursors (iMC) from healthy donors or MDSCs from tumor-bearing mice, the latter showed significant bone metastasis and osteolysis, thus supporting our observation that MDSCs from bone, containing tumor, are primed to be osteoclast precursors. Although iMC underwent osteoclast differentiation, they generated much fewer osteoclasts and reduced bone resorption compared to MDSCs. It is possible that prolonged culture of iMC in RANKL and M-CSF may have induced their differentiation into macrophages and therefore, resulted into osteoclast differentiation. Clearly these findings can be also extended to other osteolytic malignancies such as lung metastases and multiple myeloma. A recent report by Zhuang et al demonstrated similar function of MDSCs as osteoclast progenitors in a syngeneic murine multiple myeloma model (10). MDSCs from this model could promote bone resorption both in vitro and in vivo. This study also demonstrated that MDSCs from tumor-bearing mice differentiated with greater potential into osteoclasts, thus bolstering observations reported in breast cancer. Studies have demonstrated osteoclast differentiation of macrophages can occur independently of RANK stimulation during certain pathological states (16, 17). It will be interesting to determine whether any such RANK-independent activation triggers MDSCs differentiation into osteoclasts.
Similar to bone metastasis, osteolysis is also observed under other pathological conditions such as rheumatoid arthritis (RA). Using a murine RA model, which is clinically similar to human RA, Charles et al demonstrated MDSCs as the primary osteoclast progenitor (OCP) that is capable of differentiating into functional osteoclasts involving NO signaling (18). Similar to our observation, in the RA model OCPs also express CD115, the M-CSF receptor. From these recent reports it is clear that MDSCs are novel osteoclast progenitors and thus targeting MDSC could help develop new therapeutic strategy for controlling osteolysis.
Role of NO in MDSCs mediated osteoclast differentiation
Both NO and ROS are well-known mediators of osteoclast differentiation. NO is known to induce osteoclast differentiation of macrophages. An inhibitor of inducible nitric oxide synthase (iNOS) and iNOS knock-out mice show reduced bone loss due to impaired osteoclast function. Stimulation of macrophages via RANKL transiently increases ROS production through TNF receptor-associated factor (TRAF) 6, Rac1 and NADPH oxidase (Nox) 1. Inhibitors that block Nox1 or a deficiency in TRAF6 inhibit response of macrophages to RANKL, thus resulting in reduced osteoclastogenesis. Pretreatment of osteoclasts with anti-oxidants also reduced RANKL signaling and therefore osteoclast differentiation (15). Since MDSCs mediate immunosuppression via ROS and NO, we investigated if any of these pathways are also involved in osteoclast differentiation. Data showed a critical role of NO synthesis in generation of MDSCs mediated osteoclastogenesis. Inhibition of NO production reduced MDSC-mediated osteolysis both in vitro and in vivo. Hence, NO production has a dual role in MDSCs function. Considering that monocytic MDSCs are major producers of NO, it will be interesting to determine if M-MDSCs are the major source of MDSC-generated osteoclasts.
The question is what leads to increased NO levels in the bone-tumor microenvironment. One of the important features of the bone microenvironment during metastasis is hypoxia, which makes it a fertile ground for homing of metastatic cancer cells. The most common transcription factor that is expressed under hypoxic conditions is hypoxia inducible factor (HIF)-1α. Breast cancer that has metastasized to the bone expresses higher levels of HIF-1α than the primary tumor and it was shown to in-turn increase bone metastatic potential of these cells. On the other hand, reduced bone metastasis was observed in tumor cells lacking HIF-1α. Overexpression of HIF-1α increased osteoclast formation while inhibiting osteoblast differentiation, thus demonstrating a direct role of this transcription factor in osteoclastogenesis (19).
One of the signaling pathways regulated by HIF-1α is NO production via iNOS (20). Evidence also shows that NO can induce HIF-1α by signaling through phosphotidyl inositol-3-kinase (PI3kinase) and mitogen-activated protein kinase (MAPK) (21). Hence, this positive feedback response allows for osteoclast differentiation.
Clinical significance of MDSCs as an osteoclast precursor
Metastasis of cancer cells to the bone is a major clinical complication that is associated with pain, bone fracture, hypercalcemia, and decreased mobility and thus affects overall quality of life for the patient. Although there are current therapies for treating bone metastases, they are limited and are focused on symptomatic management, thus limiting progression of established disease. Hence, better understanding of the processes that play a role in therapy of bone metastases may offer new strategies for therapeutic intervention and extended survival. From recent evidence that MDSCs contribute to bone metastases in multiple ways, future treatment options may be developed that target this population of cells. Such approaches may be combined with existing therapies for adjuvant effect.
Gemcitabine is a chemotherapy agent used for carcinomas of lung, pancreas, bladder and breast. Interestingly, this drug also specifically inhibits MDSCs population in vivo (22). Therefore, gemcitabine may be used not only as an anti-tumor drug, but also for reducing bone destruction. More importantly, use of gemcitabine in patients with bone metastasis may be more impactful as it will not only reduce overall tumor burden, but will also reduce tumor growth in the bone and osteolysis. Our in vivo study showed that gemcitabine treated mice not only had less MDSCs but the breast cancer growth in the bone was also reduced (11). Elevation in MDSCs is also observed in lung cancer. Similar approach may be used for treatment of lung cancer patients, to control both the tumor growth and bone dissemination. As MDSCs function as osteoclast precursors in RA, gemcitabine could be used for controlling MDSCs mediated osteolysis under this pathological condition. Besides gemcitabine, doxorubicin-cyclophosphamide also has inhibitory effects on MDSCs proliferation. Docetaxel, a common chemotherapy drug for breast and prostate cancer patients also suppresses MDSCs by polarizing their differentiation into M1 macrophages (23). Docetaxel is commonly given to patients with bone metastasis where it delays the onset of osteolytic lesions, which may be the result of docetaxel-mediated inhibition of MDSCs (24). A therapeutic approach using combinations of these chemotherapy drugs may help in controlling bone destruction and tumor growth.
Studies have identified that plasmacytoid dendritic cells (pDCs) are elevated in patients with carcinomas of breast and lung, and multiple myeloma, both in the primary cancer and at metastatic sites. Using a syngeneic mouse model of breast cancer, we reported recently that pDCs numbers are significantly elevated during progressive stages of cancer dissemination to the bone (Figure 1). Elevated pDCs correlated with increased MDSCs infiltration in bone.(6). Similar situation is noted in multiple myeloma, where immune dysfunction is caused partially by pDCs and results in high MDSCs numbers in the bone. Therefore, treatments that control pDCs function may help not only to control tumor growth and osteolysis, but may also reduce MDSC-mediated immunosuppression and osteolysis. Depletion of pDCs in the pre-clinical murine model, using a specific antibody, results in a reduction of breast cancer growth and bone metastasis (6). pDCs depletion also reduced MDSCs levels. Therefore, targeting pDCs has dual effect on tumor growth by a) resulting in anti-tumor immune response that reduces the tumor growth and b) decreasing bone dissemination of cancer by reducing MDSC numbers and osteolysis induced by MDSCs. Although, these anti-tumor effects of pDCs depletion are reported in breast cancer, they may be extended to multiple myeloma as well as lung cancer, which also show increased pDCs and MDSC numbers with tumor progression. Depletion of pDCs may be a useful therapeutic approach in combination with chemotherapy or bisphosphonate therapy for achieving better anti-tumor activity.
Most commonly used therapy for controlling bone metastasis of cancer are use of bisphosphonates. It is used for the treatment of breast cancer, multiple myeloma and prostate cancer. Bisphosphonates attach preferentially to calcium and thus are accumulated in the bone at high concentrations. Nitrogen containing bisphosphonates such as zoledronic acid are ingested by osteoclasts where they inhibit the enzyme farnesyl pyrophosphate synthase. The bisphosphonates induce osteoclast apoptosis that in turn leads to an anti-resorptive effect (25). Such bisphosphonates also reduce proliferation of MDSCs and thus can be used as a viable agent for controlling both MDSCs and MDSC-mediated osteolysis. Zhuang et al treated MDSCs from multiple myeloma-challenged mice with zoledronic acid. This resulted in a significant decrease in the number of osteoclasts formed in MDSCs culture and also inhibition of MDSCs and bone lesions in vivo (10). Therefore, a combination therapy using zoledronic acid alongwith gemcitabine may help to greatly control bone loss and tumor growth. In-fact, a recent report demonstrated that gemcitabine alongwith bisphosphonate was more effective in reducing the number and size of bone metastases compared to gemcitabine alone in an animal model of human breast cancer (26). In addition to drugs that decrease MDSC numbers, inhibitors that can arrest MDSC functions including ROS and NO inhibitors may provide significant therapy effects not only targeting MDSCs but also other pro-tumorigenic mechanisms in the tumor microenvironment.
Administration of all-trans retinoic acid (ATRA), a vitamin A metabolite, also results in substantial decreased in MDSC numbers in cancer patients and tumor-bearing mice. ATRA reduces numbers of MDSCs by inducing their differentiation into dendritic cells and macrophages (4). This function of ATRA will help reduce MDSC-mediated osteolysis when administered in patients with bone metastasis. So there are multiple therapy options that could be used to target MDSCs. These approaches will not only reduce MDSC- mediated osteolysis but also MDSCs mediated immunosuppression.
Conclusion
MDSCs besides being immunosuppressive, contribute actively to cancer-induced osteolysis by differentiating into functional, bone-resorbing osteoclasts. This displays the plasticity of the MDSC population in regards to their ability to differentiate into osteoclasts. This phenomenon is observed under pathological conditions of cancer and rheumatoid arthritis, both of which are associated with complications caused by bone destruction. Therefore, therapies that target MDSCs directly may not only reduce its immunosuppressive functions but also MDSC-mediated osteolysis and for controlling disease complications.
Acknowledgments
Grant support:
Financial support of the National Institutes of Health grants AR050251; AR560948, CA132077, CA133737, P30 AR046031 and P30 AR48311 and DoD grant DoD-BC101411 is gratefully appreciated.
Footnotes
Conflict of interest: There is no financial conflict of interest for any of the authors listed
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.D'Amelio P, Fornelli G, Roato I, Isaia GC. Interactions between the immune system and bone. World J Orthop. 2011;2:25–30. doi: 10.5312/wjo.v2.i3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DIaNS. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, Ponnazhagan S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2012;73:672–682. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–4265. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danilin S, Merkel AR, Johnson JR, Johnson RW, Edwards JR, Sterling JA. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. 2012;1:1484–1494. doi: 10.4161/onci.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang J, Zhang J, Lwin ST, Edwards JR, Edwards CM, Mundy GR, Yang X. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PLoS One. 2012;7:e48871. doi: 10.1371/journal.pone.0048871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, Ponnazhagan S. Myeloid-Derived Suppressor Cells Function as Novel Osteoclast Progenitors Enhancing Bone Loss in Breast Cancer. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippman ME. Breast Cancer. Harrison's Principles of Internal Medicine. 2005;5:516–523. [Google Scholar]
- 13.Boyce BF, Rosenberg E, de Papp AE, Duong le T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur J Clin Invest. 2012;42:1332–1341. doi: 10.1111/j.1365-2362.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 14.Boyce BF, Rosenberg E, de Papp AE, Duong LT. The osteoclast, bone remodeling and treatment of metabolic bone disease. European Journal of Clinical Investigation. 2012;42:1332–1341. doi: 10.1111/j.1365-2362.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima T, Takayanagi H. Osteoclasts and the immune system. J Bone Miner Metab. 2009;27:519–529. doi: 10.1007/s00774-009-0089-z. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 18.Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J Clin Invest. 2012;122:4592–4605. doi: 10.1172/JCI60920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: molecular mechanisms and novel therapeutic interventions. Med Res Rev. 2010;32:611–636. doi: 10.1002/med.20224. [DOI] [PubMed] [Google Scholar]
- 20.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheta EA, Trout H, Gildea JJ, Harding MA, Theodorescu D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene. 2001;20:7624–7634. doi: 10.1038/sj.onc.1204972. [DOI] [PubMed] [Google Scholar]
- 22.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 23.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RE, Roudier M, Jones J, Armstrong A, Canon J, Dougall WC. RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther. 2008;7:2160–2169. doi: 10.1158/1535-7163.MCT-08-0046. [DOI] [PubMed] [Google Scholar]
- 25.Gnant M, Dubsky P, Hadji P. Bisphosphonates: prevention of bone metastases in breast cancer. Recent Results Cancer Res. 2007;192:65–91. doi: 10.1007/978-3-642-21892-7_3. [DOI] [PubMed] [Google Scholar]
- 26.El-Mabhouh AA NP, Abele JT, Riauka T, Postema E, McEwan AJ, Mercer JR. A conjugate of gemcitabine with bisphosphonate (Gem/BP) shows potential as a targeted bone-specific therapeutic agent in an animal model of human breast cancer bone metastases. Oncology Res. 2011;19:287–295. doi: 10.3727/096504011x13021877989874. [DOI] [PubMed] [Google Scholar]