Abstract
Objective
To test whether and to what extent inhibin mediates Cyp17 mRNA expression in theca cells (TCs) in response to FSH stimulation of granulosa cells (GCs).
Design
Ex-vivo and in vitro experimental study.
Setting
Department of Reproductive Medicine, University of California, San Diego, School of Medicine.
Animal(s)
Immature female Sprague Dawley rats.
Interventions(s)
Ovarian tissue explants and isolated theca-interstitial cell (TIC) preparations with or without GCs were treated with FSH, inhibin, inhibin antibody or β-glycan antibody.
Main Outcome Measure(s)
As a key enzyme in androgen production, Cyp17 mRNA levels were measured by real-time RT-PCR.
Result(s)
After 24 hours, Cyp17 mRNA expression was dose-dependently increased by FSH in ovarian tissue explants and TICs, suggesting that paracrine factor(s) secreted from GCs in response to FSH mediates Cyp17 mRNA expression in TCs. Antibodies against inhibin and inhibin co-receptor, β-glycan, blocked the stimulatory effect of FSH on Cyp17 mRNA expression. However, inhibin alone did not increase Cyp17 mRNA level to the same extent.
Conclusion(s)
These findings suggest a role for inhibin in the paracrine regulation of TC Cyp17 mRNA expression by GCs influenced by FSH; however, other paracrine factors produced by GCs by virtue of FSH seem to be required.
Keywords: Theca cell, granulosa cell, inhibin, Cyp17, polycystic ovary syndrome
Introduction
The production of ovarian androgen is driven by increased pituitary LH secretion, which activates CYP17. In vitro studies have shown that LH-induced increments in theca cell (TC) androgen production are dose-related (1). These findings correspond to the clinical observation that in women with polycystic ovary syndrome (PCOS), elevated serum LH levels positively correlate with circulating testosterone concentrations (2). In addition, in PCOS women treated with GnRH agonists, elevated LH levels are reduced or eliminated with a corresponding reduction in circulating androgen levels (3). Thus, increased secretion of LH may be pivotal in amplifying the production of excess androgen. However, another study looking at LH responsiveness of TCs found increased conversion of progesterone to androgen in PCOS women despite normal steroid responses to LH {Gilling-Smith, 1994 #2774}. Nevertheless, the action of LH on TC androgen production has as yet not been well quantified in PCOS or normal women.
We recently reported that in PCOS women, intravenous administration of FSH resulted in significant increases in serum 17-hydroxyprogesterone, androstenedione and dehydroepiandrosterone levels (4). By comparison, androgen levels in normal women remained unchanged after FSH stimulation. Interestingly, the serum androgen increase was accompanied by 5-fold elevated inhibin B levels in PCOS women, markedly greater than the 3-fold change observed in normal women. Moreover, in PCOS women, serum estrogen responses were also greater than those of the normal group. These findings suggested that granulosa cells (GCs) might be partially responsible for regulating TC androgen production in women with PCOS. Both in vitro and in vivo studies conducted in animals have indicated that FSH may amplify LH-induced ovarian androgen production (5–8). In cultured human TCs, androstenedione responses to LH in the presence of inhibin were clearly increased compared with those without inhibin (9, 10). In addition, inhibin was able to negate the inhibitory effect of activin on human TC androgen production. Accordingly, in our study the significant increases in ovarian androgens exhibited by PCOS women were accompanied by similar significant increments in FSH-stimulated inhibin B levels compared with those of normal women.
A growing body of evidence supports the view that GC-derived factors are potent regulators of TC androgen secretion. For instance, members of the transforming growth factor-β (TGF-β) superfamily of proteins are well-established regulators of ovarian steroidogenesis and follicle development (11, 12). In regards to androgen production, bone morphogenetic protein (BMP)-6 potently suppressed basal and LH-induced secretion in ovine (13), bovine (14), porcine (15) and human TCs (16). TGF-β1, which is expressed by GCs and TCs (15), suppressed androgen secretion from rat (17) and human TCs (18). Activin decreased whereas inhibin increased androgen secretion from human (16, 19), rat (10), and bovine (20) TCs. Futhermore, insulin-like growth factor-1 (IGF-1) enhances the stimulatory effect of LH on CYP17 expression (21) and androgen synthesis (9, 19, 22). When combined with Stem Cell Factor/kit ligand (SCF), IGF-1 increased mRNAs of steroid acute regulatory protein (StAR), CYP11A1, CYP17, 3β-hydroxysteroid dehydrogenase and LH receptor (23). Although expressed by both GCs and TCs, hepatocyte growth factor reduced CYP17 expression and androgen secretion from rat TCs in the presence of LH (24). Overall it appears that there is a complex network of signals between GCs and TCs that act to fine-tune LH- and FSH-regulation of ovarian steroid production.
In the course of our long-term project identifying the GC-derived factor(s) that mediates FSH stimulation of androgen synthesis by TCs, we have focused here on the role of inhibin in the mechanism, based on our clinical data described above (4). With emphasis on Cyp17 as the pivotal enzyme in TC androgen synthesis, we studied FSH effects in isolated rat ovary explants and primary TICs from immature rats.
Materials and Methods
Reagents and Supplies
Female Sprague Dawley rats were purchased from Charles River Laboratories, Inc (Wilmington, MA). McCoy’s 5a and M199 medium were purchased from Gibco (Life Technologies, Carlsbad, CA). Diethylstilbestrol, collagenase type IA, hCG, penicillin/streptomycin, triton x-100, and DNase were purchased from Sigma Chemical Co. (St. Louis, MO). Silastic tubing was purchased from Dow Corning, Corp. (Midland, MI). Thin wall polyallomer tubes were obtained from Beckman-Coulter (Indianapolis, IN). Ovine FSH was obtained from Dr. A.F. Parlow at the National Hormone and Peptide Program of the NIDDK, Harbor-UCLA Medical Center (Torrance, CA). Inhibin antiserum was kindly provided by Dr. Nicholas Ling, which was derived from rabbits that were immunized with an amino terminal 30-amino acid chain of porcine inhibin a-subunit coupled to BSA (25). Goat anti-human β-glycan/TGFBRIII sc-6199 antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Recombinant inhibin A was gifted by Drs. Wolfgang H. Fischer and Wylie Vale. Nucleospin RNA II extraction kits were purchased from Clontech Laboratories, Inc. (Mountain View, CA). iQ SYBR Green Supermix qPCR reagent and iScript reverse transcriptase kits were purchased from BioRad (Hercules, CA). LabTek chamber slides were obtained from Thermo Fisher Scientific, Inc. (Chino, CA). Donkey anti-rabbit FITC-conjugated secondary antibodies were purchased from R&D Systems, Inc. (Minneapolis, MN). Prolong gold anti-fade DAPI reagent was obtained from Invitrogen (Life Technologies, Carlsbad, CA). ThinCert hanging tissue culture inserts and plates were purchased from Greiner Bio-One North America, Inc. (Monroe, NC).
Isolation and Culture of TICs
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego. Female Sprague Dawley rats (24 days old) were implanted subcutaneously with silastic implants containing 10 mg diethylstilbestrol (DES) to stimulate follicle growth, without luteinizing the follicles. Four days after DES implantation, ovarian follicles were punctured with needles to release GCs and oocytes. Oocytes were then removed from the GCs by passing the cell suspension through nylon mesh having a 40-μm pore size (26). The remaining ovary tissue was finely minced with a scalpel and digested in 100 μL/ovary of M199 medium with 0.35 mg/mL Collagenase Type IA, 10 ug/mL DNase, and 10 mg/mL BSA at 37°C. The tissue was digested for a total of 30 minutes and gently agitated with a Pasteur pipette every 5 minutes then centrifuged for 4 minutes at 1,000 rpm at RT. Media containing collagenase was aspirated and cells were resuspended in 5 mL fresh M199. Large debris and oocytes were subsequently removed using 100 and 40 μm cell strainers, respectively.
Discontinuous Percoll gradients were created based on the methods of Magoffin and Erickson (27). Briefly, gradients were formed in 5 mL thin wall Polyallomer tubes. First, 750 μL of a 50% Percoll solution was added to the bottom of the tube, then 1.5 mL of a 36% Percoll solution followed by 2.25 mL the cell suspension carefully layered on top. TICs were separated by centrifuging the gradients at 2,000 rpm for 25 minutes at 4°C in a swinging bucket rotor. TICs migrated to the interface between the 36% and 50% Percoll layers and were collected from the tube using a 20-gauge needle and syringe. TICs were then washed twice in M199 and resuspended in McCoy’s 5a medium with penicillin/streptomycin. Cells were plated in serum-coated 24-well plates at 0.25 × 106 cells per well. For antibody neutralization studies, TICs were plated and immediately pre-treated with 0–2 μg of inhibin or β-glycan antibodies for 1 hour at 37°C. FSH (10 ng/mL) was added, cells were cultured for 48 hours, and total RNA was analyzed by RT-PCR.
Isolation of Total RNA and RT-PCR
TIC preparations (0.25 × 106 cells) were cultured with increasing concentrations of FSH or hCG (1, 3, or 10 ng/mL) in serum-coated 24-well plates with 500 μL serum-free McCoy’s 5a medium for 48 hours. In separate experiments, cells were cultured with either inhibin alone or FSH (10 ng/mL) and increasing concentrations of β-glycan or inhibin antibodies (0.02, 0.2, or 2 μg/mL) as mentioned. After 48 hours, cell culture medium was aspirated and total RNA was isolated from cells using the Clontech Nucleospin RNA II kit according to the manufacturer’s instructions. Total RNA and purity was quantified by measuring sample absorbance at 260 and 280 nm. Samples were stored at −80°C until assayed. Primers were designed to span exon boundaries to exclude possible genomic DNA amplification based on NCBI nucleotide sequences as follows: rat Cyp17 (NM_012753.1) and rat L19 (BC058135.1). Primer sequences used were: Cyp17 (Forward: 5′-ACAAGGCTAACGTTGACTCC-3′) and (Reverse, 5′-TGGTCAATCTCCTTTTGGATCTTC-3′) and L19 (Forward, 5′-ATGTAAAGAGCAGCCGGAAC-3′) and (Reverse, 5′-ATTGGTCTGATCCACCACAC-3′).
Relative abundance of Cyp17 mRNA was calculated by the ΔΔ-CT method and normalized to L19 expression. Briefly, the delta-CT (Δ-CT) was calculated by subtracting the cycle threshold (CT) of the housekeeping gene from that of the target gene. The CT of the sample with the lowest Cyp17 gene expression was subtracted from every sample (ΔΔ-CT). Lastly, the relative expression level was calculated using the 2− ΔΔ-C T method.
Immunofluorescence
Polyclonal antibodies against human cytochrome P450c17 (28) and porcine follistatin (29, 30) were generously gifted by Drs. Alan Conley and Nicholas Ling, respectively. TIC preparations (0.25×105) were cultured in 8-well chamber slides in McCoy’s 5a medium without treatment overnight. The following morning, culture media was removed and cells were washed twice with PBS. Cells were fixed for 10 minutes at 4°C in 4% paraformaldehyde, washed twice with PBS, and permeabilized in PBS with 0.1% triton X-100 at room temperature for 30 minutes. Nonspecific binding was blocked with 2% BSA and 0.1% triton X-100 in PBS (blocking buffer) for 1 hour. Primary antibody was diluted to 1:250 in blocking buffer and applied to cells for 1 hour followed by 3 washes in PBS for 10 minutes each. Cells were incubated with donkey anti-rabbit FITC-conjugated antibodies (1:200) for 30 minutes at room temperature protected from light. Three PBS washes were performed and nuclei were stained with Prolong Anti-gold with DAPI reagent. Evaluation of TC-specific Cyp17 and GC-specific follistatin immunostaining confirmed that these TIC preparations were predominantly TCs with only 5% GC contamination.
Ovary Organ Cultures
Ovaries from 4-day DES-treated Sprague-Dawley rats were harvested and dissected to remove fat and connective tissue. Each ovary was dissected into 1 mm3 fragments using a sterile scalpel. Ovary pieces were cultured on 3 μm PET ThinCert hanging well culture inserts for 12-well plates in 1 mL McCoy’s 5a medium for 24 hours with 0–10 ng/mL FSH or hCG. Total RNA was extracted and gene expression measured by qRT-PCR as mentioned above.
GCs and TICs Co-Cultures
GCs (1.5×106) and TICs (0.25×106) were co-cultured in the upper and lower compartments, respectively, of the above mentioned culture plates. Both the inserts and culture plates were prepared and serum coated according to the manufacturer’s instructions prior to adding cells, which were cultured in 1 mL McCoy’s 5a serum free medium with 0 or 10 ng/mL FSH.
Statistical Analysis
Data from two or three replicate experiments were pooled for statistical analyses. Comparisons among multiple groups were analyzed by ANOVA followed by Tukey’s posthoc analysis. Statistical analyses were carried out using StatPlus for Excel.
Results and Discussion
FSH stimulates Cyp17 mRNA expression in whole follicles
According to the two-cell/two-gonadotropin theory, ovarian steroid synthesis is governed by a specific pattern of enzyme expression whereby androgens are produced by Cyp17 in TCs and then subsequently aromatized in GCs. Based on findings that GCs can regulate TC androgenic pathways in a paracrine fashion in in vitro cell culture models (6, 7, 31), we sought to confirm that this phenomenon exists in our rat follicle model. The rationale of the use of this model is attributed to our previous in vivo finding that FSH stimulates androgen production in PCOS women (4). To closely mimic the in vivo environment of intact follicles, ovary organ cultures were used to elucidate the indirect regulation of Cyp17 by FSH. Fragments (1 mm3) of rat ovarian tissue were cultured for 24 hours with increasing concentrations of FSH (Fig. 1A) or hCG (Fig. 1B). Both FSH and hCG dose dependently increased Cyp17 mRNA levels in whole follicles. The effect of hCG, the canonical regulator of Cyp17 expression, was slightly stronger that that of FSH at inducing Cyp17 mRNA levels.
Fig. 1. FSH indirectly stimulates Cyp17 mRNA expression in whole follicles.
Fragments of ovarian tissue were treated with increasing concentrations (0, 1, 3, or 10 ng/mL) of FSH (A) or hCG (B) for 24 hours. Cyp17 mRNA levels were measured by quantitative real-time PCR and normalized to L19 mRNA levels. Results were evaluated by a one-way ANOVA followed by Tukey’s posthoc analysis. Different letters indicate a significant difference (p < 0.05) between groups within each treatment. Values shown are mean ± SEM. N = 6 per treatment group.
In this system, an active network between GCs and TICs with oocytes allows for extensive crosstalk and intercellular signaling. In this regard, a role for oocyte-secreted factors in regulating androgen production has been reported. For instance, in immortalized rat TICs, oocyte-derived GDF-9 directly upregulated basal and forskolin-induced Cyp17 mRNA expression in addition to androstenedione and progesterone secretion (32). Furthermore, in vivo GDF-9 treatment increased Cyp17 protein levels in rat ovaries (33). Because of the intact signaling networks present in whole follicles, paracrine feedback loops between the cell types may also enhance steroid production. It is likely that other oocyte-factors, which have not been identified, are involved in regulating follicular androgen production.
FSH stimulates Cyp17 and Cyp19 mRNA expression in GC and TIC Co-Cultures
To study the paracrine stimulation of Cyp17 mRNA expression, 1.5×106 GCs (insert) and 0.25×106 TICs (well) were co-cultured in hanging well insert plates with FSH for up to 72 hours. In the presence of FSH, CYP19 mRNA expression in the GC compartment was increased as expected, reaching 16-fold control levels by 48 hours (Fig. 2A). Interestingly, Cyp17 mRNA expression in the TIC compartment was also increased approximately 38-fold compared with untreated controls by 24 hours and more than 90-fold by 48 hours (Fig. 2B). This suggests that, when co-cultured at a 6:1 ratio of GCs:TICs in the presence of FSH, GC factors exert paracrine upregulation on Cyp17 mRNA expression in TICs.
Fig. 2. FSH stimulates Cyp19 and Cyp17 mRNA expression in GC and TIC Co-Cultures.

GCs (1.5×106) and TICs (0.25×106) were co-cultured in the upper and lower compartments, respectively, of serum-coated 12-well hanging well insert plates for up to 72 hours with 0 or 10 ng/mL FSH. Total RNA was extracted from each compartment separately then Cyp19 mRNA levels in GCs (A) and Cyp17 mRNA levels in TICs (B) were measured by quantitative real-time PCR and normalized to L19 mRNA levels. Results were evaluated by a one-way ANOVA followed by Tukey’s posthoc analysis. Different letters indicate a significant difference (p < 0.05) between groups within each treatment. Values shown are mean ± SEM. N = 6 per treatment group.
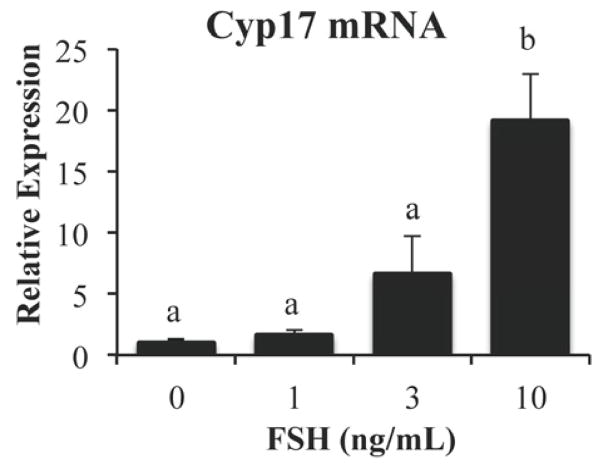
To further examine the paracrine stimulation by GCs, TIC preparations were cultured alone and intriguing results were obtained. Treatment with 0–10 ng/mL FSH for 24 hours showed a 19-fold increase of Cyp17 mRNA levels with the highest FSH dose (Fig. 3) despite the presence of only a very small number (5%) of contaminating GCs in the TIC preparations. This suggests that a potent stimulator of Cyp17 mRNA expression is released from GCs only upon FSH stimulation. Together, these data show that FSH regulates Cyp17 mRNA expression in both dispersed TICs preparations and intact follicles.
Fig. 3. FSH indirectly stimulates Cyp17 mRNA expression in TIC cultures.
TICs were treated with increasing concentrations (0, 1, 3, or 10 ng/mL) of FSH for 24 hours. Cyp17 mRNA levels were measured by quantitative real-time PCR and normalized to L19 mRNA levels. Results were evaluated by a one-way ANOVA followed by Tukey’s posthoc analysis. Different letters indicate a significant difference (p < 0.05) between groups within each treatment. Values shown are mean ± SEM. N = 6 per treatment group.
Inhibin Antibody Blocks FSH Stimulation of Cyp17 mRNA expression in TICs
Elevated circulating inhibin B and androgen levels were observed in PCOS women compared with controls when treated with FSH (4). Therefore, we sought to determine whether inhibin plays a role in the indirect stimulation of ovarian androgen production by FSH. Specifically, we examined the effects of an inhibin antibody on TICs in the presence of FSH. TIC preparations were pre-treated with 0, 0.02, 0.2, or 2 μg of antibody for 1 hour before stimulation with 10 ng/mL FSH. After 24 hours, FSH clearly stimulated Cyp17 mRNA expression (Fig. 4A). The presence of the inhibin antibody resulted in a dose dependent suppression of FSH stimulatory actions with an 80% reduction in the presence of 2 μg of antibody. The inhibin antibody alone had a very slight stimulatory effect on Cyp17 expression at the highest concentration. Normal rabbit IgG did not affect the stimulation of Cyp17 mRNA expression by FSH, indicating a specific effect of the inhibin antibody. These results directly indicate that inhibin is involved in the FSH upregulation of Cyp17 mRNA expression in TICs.
Fig. 4. Involvement of inhibin in the stimulatory effect of FSH on Cyp17 mRNA expression in TIC cultures.
TICs were pre-treated with increasing concentrations (0, 0.02, 0.2, or 2 μg/mL) of inhibin antibody (Inh) (A) or β-glycan antibody (BG) (B) then stimulated with FSH (0 or 10 ng/mL) for 24 hours. TICs were also treated with increasing concentrations of inhibin A (0, 30, 100, 300 ng/mL) for 24 hours. Cyp17 mRNA levels were measured by quantitative real-time PCR and normalized to L19 mRNA levels (C). Results were evaluated by a one-way ANOVA followed by Tukey’s posthoc analysis. Different letters indicate a significant difference (p < 0.05) between groups within each treatment. Values shown are mean ± SEM. N = 6 per treatment group.
Inhibin Co-Receptor, β-glycan, Blocks FSH Stimulation of Cyp17 mRNA expression in TICs
Inhibin binds to membrane bound co-receptor protein, β-glycan, to form a complex that has high affinity for activin type II receptors (34). Expression of β-glycan has been localized to the GCs, TCs, and oocytes of rat ovaries (34, 35). Therefore, it is hypothesized that GC-derived inhibin functions as an antagonist of activin signaling in TCs by sequestering activin type II receptors, resulting in eventual inhibition of Cyp17 expression within the TC. We investigated the role of β-glycan in the FSH upregulation of Cyp17 mRNA expression in rat TICs. TICs were pre-treated with 0, 0.02, 0.2, or 2 μg of β-glycan antibody for 1 hour before stimulation with 10 ng/mL FSH. After 24 hours, we observed a dose dependent effect of FSH on Cyp17 mRNA expression with maximal immunoneutralization in the presence of 2 μg of β-glycan antibody (Fig. 4B). The same amount β-glycan antibody on its own did not affect Cyp17 mRNA expression. Furthermore, normal goat IgG did not affect the stimulation of Cyp17 mRNA expression by FSH, indicating a specific effect of the β-glycan antibody. These results suggest that inhibin acting via its co-receptor, β-glycan, may be involved in the FSH upregulation of Cyp17 mRNA expression.
Inhibin Alone Does Not Stimulate Cyp17 mRNA Expression in TICs
We further tested whether addition of inhibin, instead of FSH, to the TIC culture is sufficient to increase Cyp17 mRNA expression. As inhibin B was not available for this study, TICs were treated with increasing concentrations of inhibin A (0, 3, 100, 300 ng/mL) for 24 hours (Fig. 4C). Cyp17 mRNA expression was stimulated by 4-fold only at a super-high concentration (300 ng/ml) of inhibin compared with untreated controls. This finding was surprising to us since we thought the direct effect of inhibin was much more potent than FSH that exerts an indirect effect on TC Cyp17 mRNA expression. There is little information available about the activity of inhibin B vs inhibin A, with one study showing that inhibin A was 2-fold less potent than inhibin B in suppressing FSH production in pituitary cells {Ling, 1985 #1532}. However, the authors stated that the difference was attributed to the purity of inhibin A being slightly less than inhibin B. Therefore, it is highly unlikely that inhibin B would exhibit much greater activity than inhibin A in our culture system. Collectively, we conclude that inhibin is essential but insufficient to stimulate Cyp17 mRNA expression in TCs, thus there must be other GC factors synergistically acting with inhibin to stimulate TC Cyp17 mRNA expression.
In summary, in line with our recent clinical findings that intravenous FSH treatment increased serum androgen levels in women with PCOS but not normal women, we show that FSH treatment of rat ovarian explants and TICs increased Cyp17 mRNA levels similar to hCG treatment. Further, as increases in ovarian androgens paralleled increases in inhibin B levels in FSH-treated PCOS women (4), we also evaluated the contribution of GC-derived inhibin on the FSH-induced stimulation of Cyp17 mRNA. While inhibin and β-glycan antibodies dose dependently blocked the stimulatory effect of FSH, inhibin alone only stimulated a partial increase in Cyp17 mRNA. Overall our study shows that FSH-stimulated GC-derived factors, including but not limited to inhibin, act in a paracrine manner to stimulate theca cell Cyp17 mRNA expression.
Acknowledgments
We thank Dr. Alan Conley and Drs. Wolfgang Fischer and Wylie Vale for their gifts of the Cyp17 antibody and inhibin A. We also thank Dr. Nicholas Ling for gifts of inhibin and follistatin antibodies. This work was supported in part by the National Institutes of Health (NIH) Grants R01 HD41494 and by the National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Financial disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smyth CD, Miro F, Howles CM, Hillier SG. Effect of luteinizing hormone on follicle stimulating hormone-activated paracrine signalling in rat ovary. Hum Reprod. 1995;10:33–9. doi: 10.1093/humrep/10.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Lobo RA, Kletzky OA, Campeau JD, diZerega GS. Elevated bioactive luteinizing hormone in women with the polycystic ovary syndrome. Fertil Steril. 1983;39:674–8. [PubMed] [Google Scholar]
- 3.Chang RJ, Laufer LR, Meldrum DR, DeFazio J, Lu JK, Vale WW, et al. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1983;56:897–903. doi: 10.1210/jcem-56-5-897. [DOI] [PubMed] [Google Scholar]
- 4.Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ. Increased FSH-stimulated Androgen Release in Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2008;93:1827–33. doi: 10.1210/jc.2007-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortune JE, Armstrong DT. Androgen production by theca and granulosa isolated from proestrous rat follicles. Endocrinology. 1977;100:1341–7. doi: 10.1210/endo-100-5-1341. [DOI] [PubMed] [Google Scholar]
- 6.Moor RM. Sites of steroid production in ovine Graafian follicles in culture. J Endocrinol. 1977;73:143–50. doi: 10.1677/joe.0.0730143. [DOI] [PubMed] [Google Scholar]
- 7.Lischinsky A, Armstrong DT. Granulosa cell stimulation of thecal androgen synthesis. Can J Physiol Pharmacol. 1983;61:472–7. doi: 10.1139/y83-072. [DOI] [PubMed] [Google Scholar]
- 8.Smyth CD, Miró F, Whitelaw PF, Howles CM, Hillier SG. Ovarian thecal/interstitial androgen synthesis is enhanced by a follicle-stimulating hormone-stimulated paracrine mechanism. Endocrinology. 1993;133:1532–8. doi: 10.1210/endo.133.4.8404591. [DOI] [PubMed] [Google Scholar]
- 9.Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant inhibin on androgen synthesis in cultured human thecal cells. Mol Cell Endocrinol. 1991;75:R1–R6. doi: 10.1016/0303-7207(91)90234-j. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh AJW, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, et al. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci. 1987;84:5082–6. doi: 10.1073/pnas.84.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 12.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BK, Souza CJ, Skinner AJ, Webb R, Baird DT. Enhanced response of granulosa and theca cells from sheep carriers of the FecB mutation in vitro to gonadotropins and bone morphogenic protein-2, -4, and -6. Endocrinology. 2006;147:1608–20. doi: 10.1210/en.2005-0604. [DOI] [PubMed] [Google Scholar]
- 14.Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–92. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- 15.Brankin V, Quinn RL, Webb R, Hunter MG. BMP-2 and -6 modulate porcine theca cell function alone and co-cultured with granulosa cells. Domest Anim Endocrinol. 2005;29:593–604. doi: 10.1016/j.domaniend.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Dooley CA, Attia GR, Rainey WE, Moore DR, Carr BR. Bone morphogenetic protein inhibits ovarian androgen production. J Clin Endocrinol Metab. 2000;85:3331–7. doi: 10.1210/jcem.85.9.6835. [DOI] [PubMed] [Google Scholar]
- 17.Fournet N, Weitsman SR, Zachow RJ, Magoffin DA. Transforming growth factor-beta inhibits ovarian 17 alpha-hydroxylase activity by a direct noncompetitive mechanism. Endocrinology. 1996;137:166–74. doi: 10.1210/endo.137.1.8536609. [DOI] [PubMed] [Google Scholar]
- 18.Attia GR, Dooley CA, Rainey WE, Carr BR. Transforming growth factor beta inhibits steroidogenic acute regulatory (StAR) protein expression in human ovarian thecal cells. Mol Cell Endocrinol. 2000;170:123–9. doi: 10.1016/s0303-7207(00)00335-x. [DOI] [PubMed] [Google Scholar]
- 19.Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J Clin Endocrinol Metab. 1991;72:1206–11. doi: 10.1210/jcem-72-6-1206. [DOI] [PubMed] [Google Scholar]
- 20.Wrathall JH, Knight PG. Effects of inhibin-related peptides and oestradiol on androstenedione and progesterone secretion by bovine theca cells in vitro. J Endocrinol. 1995;145:491–500. doi: 10.1677/joe.0.1450491. [DOI] [PubMed] [Google Scholar]
- 21.Magoffin DA, Weitsman SR. Differentiation of ovarian theca-interstitial cells in vitro: regulation of 17 alpha-hydroxylase messenger ribonucleic acid expression by luteinizing hormone and insulin-like growth factor-I. Endocrinology. 1993;132:1945–51. doi: 10.1210/endo.132.5.8477646. [DOI] [PubMed] [Google Scholar]
- 22.Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–9. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 23.Huang CT, Weitsman SR, Dykes BN, Magoffin DA. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod. 2001;64:451–6. doi: 10.1095/biolreprod64.2.451. [DOI] [PubMed] [Google Scholar]
- 24.Zachow RJ, Weitsman SR, Magoffin DA. Hepatocyte growth factor regulates ovarian theca-interstitial cell differentiation and androgen production. Endocrinology. 1997;138:691–7. doi: 10.1210/endo.138.2.4950. [DOI] [PubMed] [Google Scholar]
- 25.Cuevas P, S-Y, Ling Y, Ueno N, Esch F, Guillemin R. Immunohistochemical detection of inhibin in the gonad. Biochem Biophys Res Commun. 1987;142:23–30. doi: 10.1016/0006-291x(87)90446-3. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA. 2002;99:8060–5. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122:2345–7. doi: 10.1210/endo-122-5-2345. [DOI] [PubMed] [Google Scholar]
- 28.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CRJ. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71:83–8. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara M, DePaolo L, Nakatani A, DiMarzo SJ, Ling N. Radioimmunoassay of follistatin: Application for in vitro fertilization procedures. J Clin Endocrinol Metab. 1990;71:1672–4. doi: 10.1210/jcem-71-6-1672. [DOI] [PubMed] [Google Scholar]
- 30.Nakatani A, Shimasaki S, DePaolo LV, Erickson GF, Ling N. Cyclic changes in follistatin messenger ribonucleic acid and its protein in the rat ovary during the estrous cycle. Endocrinology. 1991;129:603–11. doi: 10.1210/endo-129-2-603. [DOI] [PubMed] [Google Scholar]
- 31.Smyth CD, Miro F, Whitelaw PF, Howles CM, Hillier SG. Ovarian thecal/interstitial androgen synthesis is enhanced by a follicle-stimulating hormone-stimulated paracrine mechanism. Endocrinology. 1993;133:1532–8. doi: 10.1210/endo.133.4.8404591. [DOI] [PubMed] [Google Scholar]
- 32.Solovyeva EV, Hayashi M, Margi K, Barkats C, Klein C, Amsterdam A, et al. Growth differentiation factor-9 stimulates rat theca-interstitial cell androgen biosynthesis. Biol Reprod. 2000;63:1214–8. doi: 10.1095/biolreprod63.4.1214. [DOI] [PubMed] [Google Scholar]
- 33.Vitt UA, McGee EA, Hayashi M, Hsueh AJW. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology. 2000;141:3814–20. doi: 10.1210/endo.141.10.7732. [DOI] [PubMed] [Google Scholar]
- 34.Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–4. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 35.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]