Abstract
The remarkable variation in prostate cancer clinical behavior represents an opportunity to identify and understand molecular features that can be used to stratify patients into clinical subgroups for more precise outcome prediction and treatment selection. Significant progress has been made in recent years in establishing the composition of genomic and epigenetic alterations in localized and advanced prostate cancers using array-based technologies and next generation sequencing approaches. The results of these efforts shed new light on our understanding of this disease and point to subclasses of prostate cancer that exhibit distinct vulnerabilities to therapeutics. The goal of this review is to categorize the genomic data and, where available, corresponding expression, functional, or related therapeutic information, from recent large-scale and in-depth studies that demonstrate a new appreciation for the molecular complexity of this disease. We focus on how these results inform our growing understanding of the mechanisms that promote genetic instability, as well as routes by which specific genes and biological pathways may serve as biomarkers or potential targets for new therapies. We summarize data that indicate the presence of genetic subgroups of prostate cancers and demonstrate the high level of intra- and intertumoral heterogeneity, as well as updated information on disseminated and circulating tumor cells. The integrated analysis of all types of genetic alterations that culminate in altering critical biological pathways may serve as the impetus for developing new therapeutics, repurposing agents used currently for treating other malignancies, and stratifying early and advanced prostate cancers for appropriate interventions.
INTRODUCTION
Prostate cancer is the second most commonly diagnosed cancer in United States men with more than 240,000 cases reported annually. These carcinomas exhibit a remarkable diversity in behavior ranging from decades of indolence to rapid growth, dissemination and lethality. Though pathological grading provides a powerful indicator of disease behavior, clinical outcomes of tumors with the same histological patterns can vary substantially. While significant morbidity results from the overtreatment of indolent tumors, delayed diagnosis and under-treatment of aggressive malignancies contributes to an excess of 30,000 deaths per year from metastatic prostate cancers. A better understanding of the genetic and molecular characteristics defining indolent and lethal prostate cancers is key for improved patient stratification and selection of optimal therapies.
This review will focus on the field of prostate cancer genomics, highlighting chromosomal alterations that may drive cancer behavior and serve as biomarkers to guide future therapeutic directions. Genomic studies have recently strengthened our understanding of prostate cancer by clarifying: 1) the frequency, types, and mutation characteristics in prostate cancer relative to other cancers, 2) the progression of genomic alterations during disease evolution, and 3) tumor heterogeneity and clonality. Collectively, these studies indicate that integrated analyses of genetic aberrations, changes in gene expression and resulting contributions to biological functions are necessary to understand the key features underlying prostate cancer behavior.
The mutational landscape of prostate cancer
Prostate cancer is characterized by extraordinary genomic complexity1, 2, including somatic copy number alterations, point mutations, and structural rearrangements. Advanced prostate cancer may be aneuploid or have large regions of copy neutral loss-of-heterozygosity (cnLOH)3. Recent advances that collectively involve detailed analyses of hundreds of primary and metastatic prostate cancers now provide a clearer picture of genomic aberrations that accompany indolent and aggressive disease.
Somatic copy number alterations (SCNA)
SCNAs are genetic gains or losses that arise during cancer development. They are evident in nearly 90% of primary prostate tumors, with deletions typically outnumbering amplifications. These SCNAs tend to be focal (≤1–5 Mb), affecting only a small portion of the genome4, 5. Metastatic prostate tumors, however, display dozens to hundreds of aberrations, which can affect a large portion of the genome. This difference suggests increased genomic instability as the disease progresses. A recent detailed comparison of SCNAs among cancer types determined that prostate cancer displayed more SCNAs (averaging 46 per sample) than most of the other 26 cancer types4. Frequent deletions are seen on chromosomes 6q, 8p, 10q, and 13q and include genes such as NKX3-1, PTEN, BRCA2 and RB1. Castration-resistant metastatic tumors (CRPC) show frequent amplification of chromosomes X, 7, 8q, and 9q, which include the androgen receptor (AR) and MYC oncogenes. Table 1 summarizes the most frequent SCNAs in different stages of prostate cancer development.
Table 1.
Most common somatic copy number aberrations (SCNAs) in human prostate cancer
| Cytoband | Event | Size (Mb) | Genes of interest | Reported frequency in prostate cancer | ||
|---|---|---|---|---|---|---|
| primary cancer | advanced cancer | DTC or CTC | ||||
| Xp11.22-q13.1 | Gain | 18–67.8 | AR | 50% CRPC 15, 24, 25, 68 | 45% AdvDTC6, 7, 69 | |
| 1p12-q43 | Gain | 117 | 45–65% CRPC24 | 50%69 | ||
| 1q32.1-q32.3 | Gain | 12.50 | ELK4, PTPRC, ELF3, PTPN7, MDM4, RAB7L1, RASSF5, IL24, IL10, CAMK1G | 5 | 24, 70 | 45% AdvDTC |
| 3q26.1 | Gain | 43.80 | GMPS, PIK3CA, MLF1, SKIL, CCNL1, ECT2 | 13–39%5, 71 | 24, 70 | 20%7 |
| 6q14.3-15 | Loss | 13.67 | CYB5R4, NT5E, SNX14, SYNCRIP, HTR1E, CGA, GJB7 | 40%5, 18, 64, 72, 73 | 55%64, 70, 72, 74 | 25%6, 7 |
| 7p22.3-q36.3 | Gain | 158.40 | 5, 18 | 25–55% CRPC68, 70, 72, 74 | 40%6, 7 | |
| 8p12-q24.3 | Gain | 97.64 | MYC, MAF, EYA1, MSC, TRPA1, KCNB2 | 20–30%5, 18, 64, 71–73, 75 | 64–82% CRPC15, 62, 68, 70, 72 | 50–65%6, 7, 69 |
| 8p23-p11 | Loss | 19.58 | NKX3-1 | 53–67%5, 18, 71–73, 75 | 67–74% of CRPC15, 68, 72 | 36–90% of AdvDTCs; 20-23% LocDTCs6, 7, 69 |
| 9q31.3 | Gain | 22.79 | PTPN3, AKAP2, DAPK1, SYK | 5 | 30%70, 72 | 25–45% AdvDTC6, 7, 69 |
| 10p13 | Loss | 1.12 | ITGA8, PTER, C1QL3, RSU1 | 18%64, 70, 73 | 25 | 6 |
| 10q11.21 | Loss | 0.58 | RET, RasGEF1A, HNRNPF, ZNF239, ZNF485, ZNF32 | 64, 73 | 55% AdvDTC6 | |
| 10q22-q24 | Loss | 24.91 | CFLP1, KILLIN, PTEN, RNLS, LIPJ, LIPF, LIPK, LIPN, LIPM, ANKRD22, STAMBPL1, ACTA2 | 12–25%5, 18, 62, 64, 72–75 | 36–80%15, 62, 68, 70, 74 | 36%69 |
| 11p13-p12 | Loss | 4.72 | 4, 6, 64, 70, 73 | 25 | 45% AdvDTC6 | |
| 12p13 | Loss | 1.46 | BCL2L14, LRP6, MANSC1, LOH12CR1, DUSP16, CREBL2, GPR19, CDKN1B, ETV6 | 30%5, 18 | 30–50%4, 64, 70, 72 | |
| 13q12.3- q14.2 | Loss | 2.63 | HSPH1, B3GALTL, RXFP2, EEF1DP3, FRY, ZAR1L, BRCA2, N4BP2L1, CG030, PDS5B, KL, STARD13, EXOSC8, FAM48A, CSNK1A1L, POSTN, TRPC4, UFM1, FREM2, KBTBD6, KBTBD7, MTRF1, NAA16, OR7E37P, C13ORF15, SPERT, SIAH2, RB1, FOXO1 | 11–40%5, 18, 62, 64, 71–73, 75 | 35–95% mets15, 62, 64, 68, 70, 72, 74 | 21–44% of LocDTC;36–55% AdvDTC6, 69 |
| 15q25.1-q26.3 | Loss | 21.30 | 64 | 40% CRPC | 20–25%7 | |
| 16q11.2-q24.3 | Loss | 33.56 | WWOX | 33–38%5, 18, 64, 71, 73, 75 | 57–82%72 | 33%7 |
| 17p13.1 | Loss | 4.28 | RPAIN, AIPL1, XAF, DLG4, PER1, TP53 | 20–30%5, 18, 64 | ||
| 17p13.3-p11.2 | Loss | 19.50 | 30%64, 73 | 51–61% CRPC | ||
| 17q21.31 | Loss | 0.15 | DHX8, ETV4 | 20%18 | 24 | |
| 17q24.2-q25.3 | Loss | 8.90 | 12–41% CRPC24, 70 | |||
| 18q22.3 | Loss | 0.29 | CBLN2, NETO1 | 20–25%5, 18, 71, 73 | 40%24, 72 | 50%7 |
| 21q22.3 | Loss | 0.25 | ERG, NCRNA00114, ETS2, PSMG1, BRWD1, HMGN1, WRB, LCA5L, SH3BGR, C21orf88, B3GALT5, IGSF5, PCP4, DSCAM, C21orf130, BACE2, PLAC4, FAM3B, MX2, MX1, TMPRSS2 | 33–50%5, 8, 18, 73, 75 | 33%74 | |
Note: SCNA regions are listed in chromosomal order. Well-characterized cancer genes are in bold. References are indicated for reported frequencies of SCNAs. In general, only SCNAs with a frequency >40% in at least one cancer category are listed.Size is based on reported results, and indicates the broader region of overlap across studies. Actual size reported in individual samples may vary, especially for studies using recently developed technologies such as high-density SNP CGH arrays and next-generation sequencing that permit a greater limit of resolution. In general SCNAs are smaller in primary tumors than those observed in metastases, and may only cover a portion of the region listed.
Clinically, detection of prostate SCNA from alternative tissue sources is of great current interest, as the success rate for prostate biopsy is only 60–70% even with CT guidance. Circulating and disseminated tumor cells (CTC and DTCs) in the blood and bone marrow present an opportunity for repeated testing. The difficulties lie in their rare numbers and complicated techniques for isolation. Nevertheless, new methods promise new results. Genomic profiling of DTCs from patients with advanced disease showed a large number of SCNAs, mostly concordant with corresponding metastases and previous tumors (Table 1),6, 7 although DTCs from men with localized disease generally have fewer SCNAs, which may not correspond well with the primary tumor SCNAs.
Structural rearrangements
Double-stranded breaks can occur when DNA unwinds during replication or transcription. Improper repair of these breaks can result in intra- and inter-chromosome rearrangement. Almost 50% of all primary prostate tumors have TMPRSS2:ERG rearrangement, which places the growth-promoting activity of the ERG oncogene under the control of the regulatory elements of androgen-responsive TMPRSS2 8. Rearrangements can also result in new fusion proteins that are constitutively active or have altered function or cellular localization, as in the example of ESRP1:CRAF rearrangement 3. Several other rearrangements have been described for prostate cancer, including other ETS family rearrangements 9, 10, and RAF kinase gene fusions 11 as reviewed previously 12.
Although ERG rearrangement does not affect the overall frequency of SCNAs, it is associated with deletions of 10q, 17p and 3p14 5. These tumors have a distinct expression signature8, 13. Tumors without ERG rearrangement are significantly enriched for 6q deletion, 7q gain, and 16q deletion5.
Paired-end whole genome sequencing suggests that rearrangements are much more common and complex than previously appreciated, and implies the importance of surrounding chromatin structure12, 14. Sequencing of primary tumors from ‘high-risk’ prostate cancer patients showed a median of 90 rearrangements, often complex, per tumor genome. Moreover, breakpoints in TMPRSS2:ERG rearranged tumors were precise and located in accessible chromatin that was enriched in transcription factors associated with androgen-regulated transcription14. In contrast, in tumors without TMPRSS2:ERG rearrangement, breakpoints were located in transcriptionally-repressed chromatin.
Point mutations
Primary prostate cancer has a somatic mutation rate of 1~2×10−6, similar to breast, renal and ovarian cancers 15–17. Although several thousand mutations may exist in each prostate tumor genome, only ~20 per genome are likely to impact protein stability or function. However, mutation of the DNA mismatch repair enzyme MSH6 is associated with a hypermutator phenotype 5, 17–19, resulting in 25-fold more mutations than normally seen in prostate cancer. Mutations of common tumor suppressor genes, including TP53, PTEN, RB1 and PIK3CA, have also been defined in prostate cancer 15, 18, 20, 21, as have activating mutations in the oncogenes KRAS and BRAF. Additional recurrent mutations are detected in factors that mediate AR function, chromatin modification and transcription. These are detailed below.
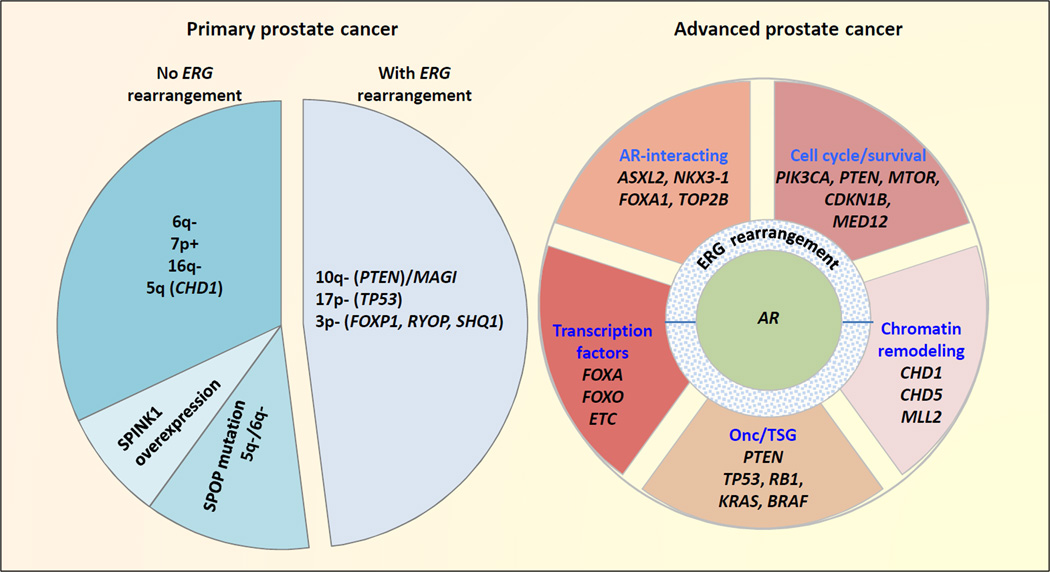
A new molecular subtype of prostate cancer has been suggested as defined by SPOP mutations 15, 18 (Figure 1). Point mutations at evolutionarily conserved residues of the substrate-binding cleft of this E3-ubiquitin ligase subunit were identified in up to 13% of primary tumors. SPOP mutations were enriched in tumors with somatic deletions of 5q21 and 6q21, which encode genes including the chromatin-modifying enzyme CHD1 and the tumor suppressor PRDM1 and FOXO3. But these tumors did not display ETS-rearrangement or mutations in TP53, PTEN, and PIK3CA. SPOP mutations have recently been shown to influence the stability of the SRC3/NCOA3 protein and alter AR signaling in prostate cancer cells22.
Figure 1. Relationships of common genomic alterations identified in localized and advanced in prostate cancer.
Approximately 50% of both primary and advanced prostate cancer harbor ERG gene fusion or other ETS-family gene rearrangement. Primary prostate cancers without ERG rearrangement can be subclassified based on SPINK1 overexpression10, SPOP mutations, and select somatic copy number aberrations (SCNAs) which are often mutually exclusive. The size of the pie chart pieces on the left represents approximate frequencies of each subgroup. Nearly all advanced stage prostate cancers have amplification or mutation of AR, or abnormalities of other AR pathway components. However, the genetic complexity associated with most advanced stage prostate cancers precludes their classification into distinct subgroups based on genetic profiles. Among this complexity exists a number of commonly observed genetic aberrations as shown. Metastatic tumors may display some or all of these aberrations. Therefore, the pie chart pieces on the right simply help define functional groups of various genes with mutations observed in advanced prostate cancer, without indication for frequencies or mutual exclusivity. See text for detail.
(Abbreviations: Onc/TSG refers to oncogenes and tumor suppressor genes)
Integrating genetic information to identify novel therapeutic targets
As the spectrum of genetic aberrations becomes increasingly more complex in prostate cancer, integrated analysis of genetic aberrations, epigenetics, transcriptional regulation and expression profiles is necessary to understand the molecular pathways that contribute to tumorigenesis. Results from such integrated approaches are now poised to define key targets for future prostate cancer therapeutics.
Androgen signaling pathway
Because the growth of prostate cancer is largely dependent on androgens, therapies blocking the AR signaling pathway are effective for most patients. However, several mechanisms can restore AR signaling and promote the development of castration-resistant metastatic disease (CRPC). These mechanisms include AR amplification, gain-of-function AR mutations, splice variants, and overexpression of AR or its coactivators. AR amplification is observed in metastases from ~50% of patients, and can occur through focal amplification 23 or through gain of the entire X-chromosome, on which AR resides 5, 24. AR is also frequently mutated in advanced disease 5, 15, 25. The oncogenic H874Y AR mutation increases the binding affinity of AR for testosterone 26. Additional mutations in the ligand-binding domain (K580R, T877A, L701H and V715M) permit inappropriate AR activation by other steroid hormones such as estrogens, progestin, and glucocorticoids 27. A new AR mutation, F876L, confers resistance to the potent AR antagonist, MDV310028, attesting to the plasticity of the prostate cancer genome in responding to selective therapeutic pressures.
Beyond AR itself, other components of the AR signaling pathway are altered in up to half of primaries and nearly all metastases, indicating the critical nature of this pathway to prostate cancer at all developmental stages 5. The oncogenic transcriptional coactivator NCOA2, on 8q13.3, is amplified in 24% of metastases and 1.9% of primary tumors, and correlates with elevated NCOA2 transcripts. Overexpression of NCOA2 primes AR to respond to reduced androgen levels and boosts the total magnitude of AR transcriptional response. Mutations in the Ser/Thr-rich regulatory domain and the transcriptional activation domain of NCOA2 are also frequent. Clinically, NCOA2 may be an important AR-pathway biomarker in primary prostate cancer as noncastrate patients who had NCOA2 alterations showed significantly more recurrences 29.
Androgen signaling can also promote co-recruitment of AR with topoisomerase II beta (TOP2B) to DNA, resulting in TOP2B-mediated double-stranded breaks and rearrangements, including TMPRSS2-ERG 30. Furthermore, in response to genotoxic stress - as may be experienced by cells during radiation or other anti-cancer therapeutics - AR recruits the enzymes AID (activation-induced cytidine deaminase) and the LINE-1 encoded ORF2 endonuclease 31, which may also contribute to formation of rearrangements. Given that chromosomes form three-dimensional ‘transcriptional hubs’ that simultaneously coordinate the chromatin structure and transcriptional activity of multiple genes 32, ‘transcriptional hubs’ may facilitate rearrangements of genes that spatially co-localize when errors in DNA processing cause DNA breaks in response to androgen signaling 33. These new factors warrant further investigation.
Transcription factors and chromatin modifiers
Transcription factors and chromatin modifiers work cohesively to mediate sequence-specific chromatin modifications that regulate gene expression. They play important roles in embryonic stem cells, cellular differentiation, and are altered in many cancer types, including prostate cancer. Given the potentially broad effects of alterations in chromatin structure and transcriptional regulation, disruptions in these genes may have extensive effects. These global changes may contribute to the high degree of genetic instability that is characteristic of metastatic prostate cancer, perhaps through increasing accessibility of DNA to factors that induce DNA breaks or by altering three-dimensional chromatin structures and interactions. Most notable are recurrent aberrations in the chromodomain-helicase-DNA-binding (CHD) proteins and FOXA and FOXO transcription factor families. Nevertheless, their contribution to prostate cancer oncogenesis is not fully understood to date.
Alterations in CHD genes have been commonly detected in primary and metastatic prostate cancer, and may distinguish a new subgroup of prostate cancers with increased aggressiveness. CHD proteins function in ATP-dependent chromatin remodeling. CHD1 on 5q21 is disrupted by focal deletions or mutations in up to 17% of all prostate tumors14, 15, 34. Intragenic breakpoints in CHD1 yield truncated proteins14. Knockdown of CHD1 in prostate cancer cell lines has been associated with morphological changes and increased cell invasiveness34. CHD1-deficiency is strongly correlated with lack of ETS-family gene rearrangements, and may represent a novel subclass of aggressive prostate cancer 15, 18. Another CHD protein, CHD5, is a tumor suppressor whose expression is altered in several solid tumor types by focal deletions, mutations or DNA methylation. CHD5 mutations have been detected in multiple prostate tumors 25. Loss of CHD5 correlates with increased proliferation and decreased apoptosis via the p53 pathway35–37. Finally, the H3/K4-specific methyltransferase gene MLL2 is also frequently mutated in prostate cancer, as has previously been seen in other cancer types15, 38, 39.
The Fork-head box protein A (FOXA) and O (FOXO) families belong to the larger group of highly conserved forkhead proteins, which are deregulated in several tumor types 40. FOXO and FOXA members are transcription factors that bind to AR and regulate its association with androgen response elements 15, 41. However, their roles in prostate cancer appear to be diverse based on frequent amplification or activation of FOXA1 but loss of FOXO in tumors. FOXA1 is required for prostate epithelial cell differentiation and promotes proliferation42. Focal amplifications of FOXA1 and mutations in the transactivation and DNA-binding forkhead domains have been reported in ~10% of prostate cancer cases5, 15, 18, 25. Increased expression of FOXA1 correlates with PSA and Gleason score, and is associated with biochemical recurrence and poor prognosis 43. FOXA1 likely functions by repressing AR signaling, therefore leading to dedifferentiated tumors that are more aggressive and have a higher risk for metastatic relapse. In prostate cancer cell lines, increased FOXA1 activity promotes proliferation, tumorigenesis and xenograft growth15.
FOXO proteins have tumor suppressor activity, and control the transcription of genes involved in metabolism, stress response, cell cycle arrest, cell death and DNA damage repair. In prostate cancer, FOXO1 and FOXO3A are inhibited by AKT-mediated phosphorylation, resulting in their nuclear exclusion and ubiquitin-mediated degradation44. Deletion of FOXO1 on 13q14 has been observed in approximately one-third of prostate cancer cell lines, primary tumors and xenografts45. Loss of FOXO1 increases the basal activity of AR and sensitizes it to lower androgen levels or other nonandrogenic ligands46. FOXO1 also inhibits the transcriptional activity of Runx2, a transcription factor that contributes to prostate cancer cell migration, invasion and metastasis47. Restoration of FOXO3A activity in cancer cell lines sensitizes them to radiation48, suggesting that combination of radiation with therapies that increase FOXO3A activity might be beneficial.
Phosphatidylinositol 3-kinase (PI3K) and AKTsignaling pathways
PI3K pathway is a critical regulator of cell survival and proliferation. In prostate cancer, aberrant activation of the PI3K pathway is associated with higher Gleason grades, earlier recurrence, and a higher risk of cancer-specific mortality49. Up to 50% primary prostate tumors and 100% metastases have aberrant expression, SCNAs or mutations in PI3K pathway members.
The major route for PI3K pathway activation is via the loss of PTEN’s tumor suppressor function as a result of PTEN copy number loss, inversions or mutation5, 14, 25. Focal deletion of PTEN or aneuploidy of chromosome 10 is present in nearly half of primary prostate cancers and all metastatic tumors5, 50, while inactivating mutations of PTEN account for 5–10% of primary cancers18. In addition, loss-of-function mutations and rearrangements in a PTEN-associating protein, MAGI (membrane-associated guanylate kinase inverted) have been detected in prostate cancer 14. MAGI enhances PTEN’s ability to suppress AKT activation. Besides PTEN, several other phosphatases can regulate AKT activity, including PHLPP and INPP4B. Deletion or loss of their expression is correlated with greater Gleason score and shorter time to biological recurrence 51, 52.
Activation of the PI3K pathway can also occur following oncogenic activation or amplification of PIK3CA, AKT1 and MTOR25. PIK3CA on 3q26.32 encodes the catalytic subunit of PI3K. Amplification of PIK3CA has been detected in 13–39% of primary tumors and up to 50% of CRPCs53, 54. Activating mutations in PIK3CA and MTOR have also been detected in ~4% of primary prostate tumors18, 25, 54.
Clonal heterogeneity
The high level of genetic heterogeneity within and across prostate tumor foci likely contributes to a tumor’s ability to develop therapeutic resistance. Pathologically distinct tumor foci have few commonly shared mutations, supporting the largely independent clonal origin of each neoplastic region 55. Even among different sites within a tumor focus, unique mutation profiles are observed. These results are concordant with the high degree of intratumoral heterogeneity that has been characterized in breast, kidney and myeloproliferative cancers 56–59. Meanwhile, the shared pattern of aberrations in patients’ metastatic tumors supports a monoclonal origin of metastasis, and may indicate aberrations that are important to the progression of prostate cancer 23, 25. However, metastases also accumulate SCNAs that are unique, particularly in lymph node, brain and bone metastases 23. These may either be passenger events that do not influence disease progression, or may result from tissue-specific selection pressures.
Given the high levels of heterogeneity, integrated analysis of biological pathways that are altered in prostate cancer will be critical. Integrated analysis of genetic and transcription data has revealed new pathways in glioblastoma 60, and is promising to do so in prostate cancer. Recently, Taylor et al found that three well-known pathways, PI3K, RAS/RAF, and RB1 are altered in 34–43% primary tumors and 74–100% metastases 5. While RNA expression could not predict recurrence, they found that DNA copy-number profiling did significantly associate with outcomes. They also identified a separate group of patients whose tumors did not carry any major SCNA or aneuploidy, and who remained largely recurrence-free at five years. Thus, genomic analyses may also identify patients who are good candidates for active surveillance.
Conclusions and future studies
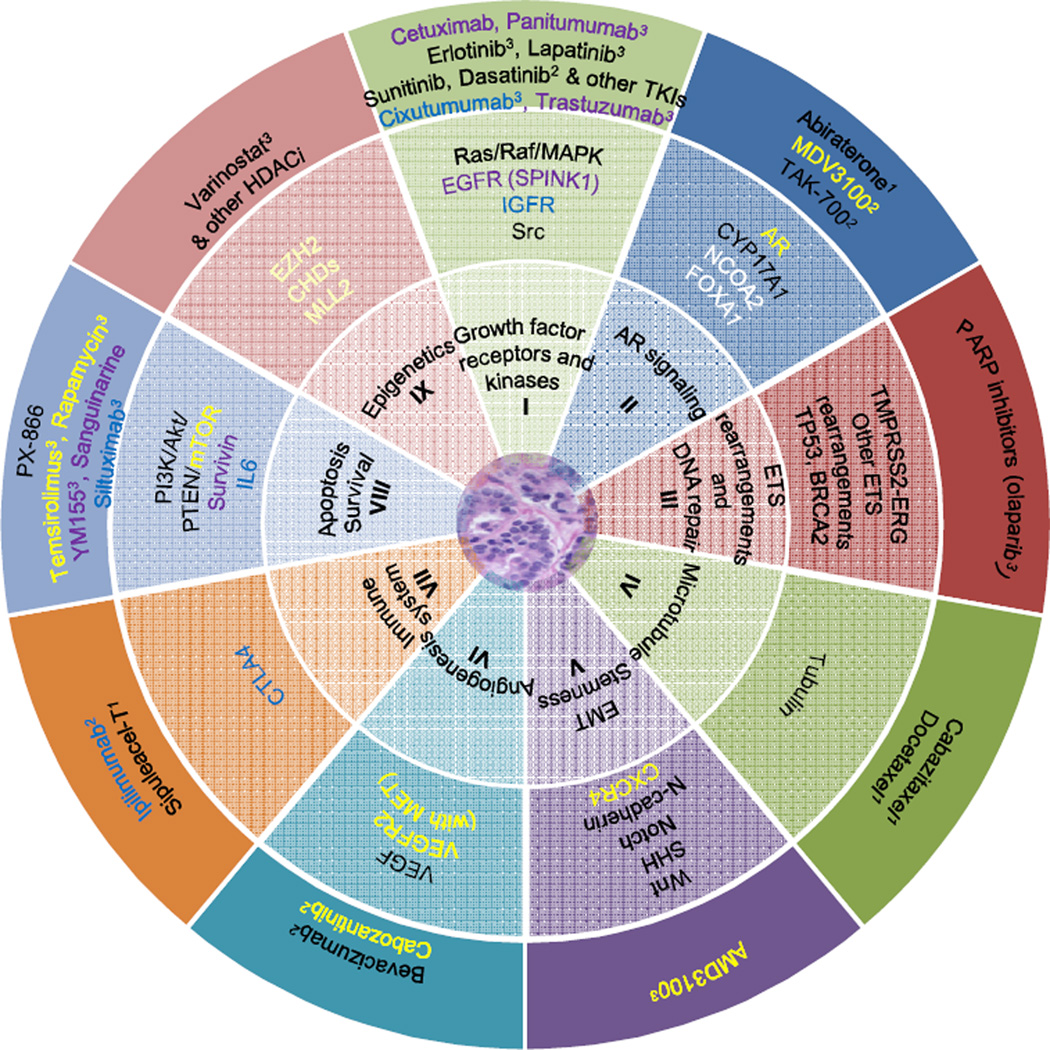
The use of genomic profiling to identify robust subtypes of prostate cancer lags far behind efforts in other cancer types, where the molecular subclassification has improved our clinical ability to predict patients’ overall risk and response to treatments. Examples include: HER2 in breast cancer, BRAF in melanoma, and KRAS and EGFR in lung cancer. Thus, it is imperative that future studies correlate clinical data with molecular and genetic classification of cancer samples. With the high prevalence of aberrations in the AR and PI3K/AKT signaling pathways, additional treatments targeting these signaling programs are in order. Factors that regulate chromatin, epigenetics and transcription have also emerged as highly significant and deserve further investigation61. Ideally, targeted therapy based on key perturbed pathways, as illustrated in Figure 2, can be tested in prospective clinical trials.
Figure 2. Considerations for targeted therapy based on key pathways perturbed in prostate cancer.
Current standard-of-care involves active surveillance for low-risk localized prostate cancers; hormonal therapy, radical prostatectomy, or radiation therapy for higher-risk localized disease; and androgen pathway suppression for metastatic disease with chemotherapy and immunotherapy at the time of disease progression. This figure shows the potential for targeted therapy in molecularly-defined subtypes of prostate cancer. Genomic alterations are classified based on the class of molecular pathways affected (inner circle). Therapeutic agents (outer circle) targeting respective pathways are grouped with the genes (middle circle) commonly altered in these pathways, coordinated by color wherever possible. Selected agents in various phases of clinical trials are superscripted: 1FDA approved, 2Phase III clinical trials, 3Phase I/II clinical trials; preclinical development not marked. While anti-androgen therapy abiraterone, microtubule inhibitor cabazitaxel, and immunotherapy sipuleacel-T are already in clinical use, aberrations of NCOA2 and FOXA1 genes (white) are recent findings, the functional significance and therapeutic implications of which await further investigation.
An underlying question is how SCNAs, particularly heterozygous deletions and low-copy-number amplifications, affect expression of the genes located within the affected region and cause indirect effects on other genes. Kim et al show a modest correlation of copy number with gene expression; ~38% of amplified genes had concordant increases in expression 62. The area of copy-neutral LOH also warrants further attention, which can only be detected through next-generation sequencing approaches or by genomic arrays incorporating SNP markers63. Large cnLOH is typically associated with homozygous mutations of gene(s) residing in the respective sequence.
Additional meta-analysis of existing genetic information may help identify aberrations that work synergistically to promote tumorigenesis. In a limited example involving five metastatic tumors, all 19q13.32 losses occur in the presence of 1p22.1 loss, whereas 17q21.31 loss concurs with 18q22.3 loss, and 21q22.3 loss with 16q23.1 loss 64. Results such as these point to common regulation, such as through colocalization in three-dimensional space.
An important question that must be addressed centers on the molecular heterogeneity within and between primary prostate cancer foci and discrete metastasis. Developing approaches to assess distinct clones will have important implications for anticipating response and resistance to targeted therapeutics. Further, sampling multiple metastatic sites for genomic analyses poses technical and safety challenges. Enumeration of CTCs and DTCs has been shown to predict risk of relapse and quantifies patients’ treatment responses 65, 66. Building on these assessments of CTC numbers, technological advances now allow for the direct molecular profiling of these populations on a single-cell basis. Results such as these could provide a view of the heterogeneity of a patient’s tumor burden, and has the advantage of resampling over the course of disease. Direct sequencing of circulating cell-free DNA offers another avenue for identifying and monitoring genomic alterations that could influence therapy selection67.
In closing, rapidly expanding technologies and declining costs for genomic analysis are providing insights into the genetic underpinnings of prostate cancer at a rate faster than ever before. As additional studies are undertaken and new gene candidates emerge, putative driver events will be evaluated as therapeutic targets. With more novel therapies tested and approved, determining the best approach to handle genetic heterogeneity among patients will be a top research priority.
Acknowledgments
The work is supported by P01 CA 085859 SUB (to M. Fang) and PNW Prostate Cancer SPORE CA097186 (to P. Nelson) from the National Cancer Institute.
Footnotes
Disclosure: P. Nelson served as a consultant to Johnson and Johnson and Astellas. The remaining authors have no conflict of interest to disclose.
References
- 1.Barbieri CE, Demichelis F, Rubin MA. Molecular genetics of prostate cancer: emerging appreciation of genetic complexity. Histopathology. 2012;60:187–198. doi: 10.1111/j.1365-2559.2011.04041.x. [DOI] [PubMed] [Google Scholar]
- 2.Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, Macdonald TY, et al. Targeted Next-generation Sequencing of Advanced Prostate Cancer Identifies Potential Therapeutic Targets and Disease Heterogeneity. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalgo ML, Isaacs WB. Molecular pathways to prostate cancer. The Journal of urology. 2003;170:2444–2452. doi: 10.1097/01.ju.0000085381.20139.b6. [DOI] [PubMed] [Google Scholar]
- 4.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb IN, Grove DI, Kinnunen M, Friedman CL, Gallaher IS, Morgan TM, et al. Genomic alterations indicate tumor origin and varied metastatic potential of disseminated cells from prostate cancer patients. Cancer research. 2008;68:5599–5608. doi: 10.1158/0008-5472.CAN-08-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H, et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–1556. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 9.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 10.Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13:519–528. doi: 10.1016/j.ccr.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF kinasepathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, et al. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. Journal of the National Cancer Institute. 2008;100:815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011;108:17087–17092. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Shendure J, Nelson PS. Genome interrupted: sequencing of prostate cancer reveals the importance of chromosomal rearrangements. Genome medicine. 2011;3:23. doi: 10.1186/gm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastham JA, Stapleton AM, Gousse AE, Timme TL, Yang G, Slawin KM, et al. Association of p53 mutations with metastatic prostate cancer. Clin Cancer Res. 1995;1:1111–1118. [PubMed] [Google Scholar]
- 21.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 22.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci U S A. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holcomb IN, Young JM, Coleman IM, Salari K, Grove DI, Hsu L, et al. Comparative analyses of chromosome alterations in soft-tissue metastases within and across patients with castration-resistant prostate cancer. Cancer research. 2009;69:7793–7802. doi: 10.1158/0008-5472.CAN-08-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins CM, Tembe WA, Baker A, Sinari S, Moses TY, Beckstrom-Sternberg S, et al. Copy number and targeted mutational analysis reveals novel somatic events in metastatic prostate tumors. Genome Res. 21:47–55. doi: 10.1101/gr.107961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askew EB, Gampe RT, Jr, Stanley TB, Faggart JL, Wilson EM. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. The Journal of biological chemistry. 2007;282:25801–25816. doi: 10.1074/jbc.M703268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah S, Hess-Wilson JK, Webb S, Daly H, Godoy-Tundidor S, Kim J, et al. 2,2-bis(4-chlorophenyl)-1,1-dichloroethylene stimulates androgen independence in prostate cancer cells through combinatorial activation of mutant androgen receptor and mitogen-activated protein kinase pathways. Molecular cancer research : MCR. 2008;6:1507–1520. doi: 10.1158/1541-7786.MCR-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, 3rd, Erdem H, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 30.Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nature genetics. 42:668–675. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–1083. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rickman DS, Soong TD, Moss B, Mosquera JM, Dlabal J, Terry S, et al. Oncogene-mediated alterations in chromatin conformation. Proceedings of the NationalAcademy of Sciences of the United States of America. 2012;109:9083–9088. doi: 10.1073/pnas.1112570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani RS, Tomlins SA, Callahan K, Ghosh A, Nyati MK, Varambally S, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009;326:1230. doi: 10.1126/science.1178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang S, Gulzar ZG, Salari K, Lapointe J, Brooks JD, Pollack JR. Recurrent deletion of CHD1 in prostate cancer with relevance to cell invasiveness. Oncogene. 2012;31:4164–4170. doi: 10.1038/onc.2011.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R, Yan Q, Lv J, Huang H, Zheng W, Zhang B, et al. CHD5, a tumor suppressor that is epigenetically silenced in lung cancer. Lung cancer. 2012;76:324–331. doi: 10.1016/j.lungcan.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Zhu Z, Li W, Fu X, Su D, Fu L, et al. Chromodomain helicase DNA binding protein 5 plays a tumor suppressor role in human breast cancer. Breast cancer research : BCR. 2012;14:R73. doi: 10.1186/bcr3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagchi A, Papazoglu C, Wu Y, Capurso D, Brodt M, Francis D, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128:459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 38.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JY, Hung MC. Deciphering the role offorkhead transcription factors in cancer therapy. Current drug targets. 2011;12:1284–1290. doi: 10.2174/138945011796150299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahu B, Laakso M, Ovaska K, Mirtti T, Lundin J, Rannikko A, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. The EMBO journal. 2011;30:3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 43.Imamura Y, Sakamoto S, Endo T, Utsumi T, Fuse M, Suyama T, et al. FOXA1 promotes tumor progression in prostate cancer via the insulin-like growth factor binding protein 3 pathway. PloS one. 2012;7:e42456. doi: 10.1371/journal.pone.0042456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer letters. 2012 doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Dong XY, Chen C, Sun X, Guo P, Vessella RL, Wang RX, et al. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 46.Liu P, Li S, Gan L, Kao TP, Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–10299. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- 47.Renault VM, Thekkat PU, Hoang KL, White JL, Brady CA, Kenzelmann Broz D, et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30:3207–3221. doi: 10.1038/onc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen MF, Fang FM, Lu CH, Lu MS, Chen WC, Lee KD, et al. Significance of nuclear accumulation of Foxo3a in esophageal squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2008;71:1220–1229. doi: 10.1016/j.ijrobp.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 49.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 50.Pourmand G, Ziaee AA, Abedi AR, Mehrsai A, Alavi HA, Ahmadi A, et al. Role of PTEN gene in progression of prostate cancer. Urology journal. 2007;4:95–100. [PubMed] [Google Scholar]
- 51.Hodgson MC, Shao LJ, Frolov A, Li R, Peterson LE, Ayala G, et al. Decreased expression and androgen regulation of the tumor suppressor gene INPP4B in prostate cancer. Cancer Res. 2011;71:572–582. doi: 10.1158/0008-5472.CAN-10-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS, O'Neill A, et al. Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell. 2011;20:173–186. doi: 10.1016/j.ccr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:5271–5281. [PubMed] [Google Scholar]
- 54.Sun X, Huang J, Homma T, Kita D, Klocker H, Schafer G, et al. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer research. 2009;29:1739–1743. [PubMed] [Google Scholar]
- 55.Lindberg J, Klevebring D, Liu W, Neiman M, Xu J, Wiklund P, et al. Exome Sequencing of Prostate Cancer Supports the Hypothesis of Independent Tumour Origins. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 56.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou Y, Song L, Zhu P, Zhang B, Tao Y, Xu X, et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell. 2012;148:873–885. doi: 10.1016/j.cell.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 58.Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JH, Dhanasekaran SM, Prensner JR, Cao X, Robinson D, Kalyana-Sundaram S, et al. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome research. 2011;21:1028–1041. doi: 10.1101/gr.119347.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JH, Dhanasekaran SM, Mehra R, Tomlins SA, Gu W, Yu J, et al. Integrative analysis of genomic aberrations associated with prostate cancer progression. Cancer Res. 2007;67:8229–8239. doi: 10.1158/0008-5472.CAN-07-1297. [DOI] [PubMed] [Google Scholar]
- 63.Fang M, Toher J, Morgan M, Davison J, Tannenbaum S, Claffey K. Genomic differences between estrogen receptor (ER)-positive and ER-negative human breast carcinoma identified by single nucleotide polymorphism array comparative genome hybridization analysis. Cancer. 2011;117:2024–2034. doi: 10.1002/cncr.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demichelis F, Setlur SR, Beroukhim R, Perner S, Korbel JO, Lafargue CJ, et al. Distinct genomic aberrations associated with ERG rearranged prostate cancer. Genes, chromosomes & cancer. 2009;48:366–380. doi: 10.1002/gcc.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doyen J, Alix-Panabieres C, Hofman P, Parks SK, Chamorey E, Naman H, et al. Circulating tumor cells in prostate cancer: A potential surrogate marker of survival. Crit Rev Oncol Hematol. doi: 10.1016/j.critrevonc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Panteleakou Z, Lembessis P, Sourla A, Pissimissis N, Polyzos A, Deliveliotis C, et al. Detection of circulating tumor cells in prostate cancer patients: methodological pitfalls and clinical relevance. Molecular medicine. 2009;15:101–14. doi: 10.2119/molmed.2008.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. The New England journal of medicine. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 68.Friedlander TW, Roy R, Tomlins SA, Ngo VT, Kobayashi Y, Azameera A, et al. Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer Res. 2012;72:616–625. doi: 10.1158/0008-5472.CAN-11-2079. [DOI] [PubMed] [Google Scholar]
- 69.Magbanua MJ, Sosa EV, Scott JH, Simko J, Collins C, Pinkel D, et al. Isolation and genomic analysis of circulating tumor cells from castration resistant metastatic prostate cancer. BMC Cancer. 2012;12:78. doi: 10.1186/1471-2407-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun J, Liu W, Adams TS, Sun J, Li X, Turner AR, et al. DNA copy number alterations in prostate cancers: a combined analysis of published CGH studies. The Prostate. 2007;67:692–700. doi: 10.1002/pros.20543. [DOI] [PubMed] [Google Scholar]
- 71.Cheng I, Levin AM, Tai YC, Plummer S, Chen GK, Neslund-Dudas C, et al. Copy number alterations in prostate tumors and disease aggressiveness. Genes, chromosomes & cancer. 2012;51:66–76. doi: 10.1002/gcc.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lapointe J, Li C, Giacomini CP, Salari K, Huang S, Wang P, et al. Genomic profiling reveals alternative genetic pathways of prostate tumorigenesis. Cancer research. 2007;67:8504–8510. doi: 10.1158/0008-5472.CAN-07-0673. [DOI] [PubMed] [Google Scholar]
- 73.Ishkanian AS, Mallof CA, Ho J, Meng A, Albert M, Syed A, et al. High-resolution array CGH identifies novel regions of genomic alteration in intermediate-risk prostate cancer. The Prostate. 2009;69:1091–1100. doi: 10.1002/pros.20959. [DOI] [PubMed] [Google Scholar]
- 74.Robbins CM, Tembe WA, Baker A, Sinari S, Moses TY, Beckstrom-Sternberg S, et al. Copy number and targeted mutational analysis reveals novel somatic events in metastatic prostate tumors. Genome research. 2011;21:47–55. doi: 10.1101/gr.107961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boyd LK, Mao X, Xue L, Lin D, Chaplin T, Kudahetti SC, et al. High-resolution genome-wide copy-number analysis suggests a monoclonal origin of multifocal prostate cancer. Genes, chromosomes & cancer. 2012;51:579–589. doi: 10.1002/gcc.21944. [DOI] [PubMed] [Google Scholar]