Abstract
Cognitive rehabilitation has shown beneficial effects on cognition in patients with schizophrenia, which may also help to improve negative symptoms due to overlapping pathophysiology between these two domains. To better understand the possible relationship between these areas, we conducted an exploratory analysis of the effects of Cognitive Enhancement Therapy (CET) on negative symptoms. Early course schizophrenia outpatients (n = 58) were randomized to two years of CET or an Enriched Supportive Therapy (EST) control condition. Results revealed significant and medium-sized (d = .61) differential improvements favoring CET in overall negative symptoms, particularly social withdrawal, affective flattening, and motor retardation. Neurocognitive improvement was associated with reduced negative symptoms in CET, but not EST patients. No relationships were observed between improvements in emotion processing aspects of social cognition, as measured by the Mayer-Salovey-Caruso Emotional Intelligence Test, and negative symptoms. CET represents an effective cognitive rehabilitation intervention for schizophrenia that may also have benefits to negative symptoms. Future studies specifically designed to examine negative symptoms during the course of cognitive rehabilitation are needed.
Keywords: Cognitive rehabilitation, schizophrenia, negative symptoms, social cognition, neurocognition, psychosocial treatment
1. Introduction
Schizophrenia is a severe psychiatric disability that is characterized by significant impairments in social and non-social cognition at all phases of the illness (Penn et al., 1997; Heinrichs and Zakzanis, 1998; Mesholam-Gately et al., 2009), which significantly limit the ability of individuals to recover from the disorder (Green et al., 2000; Couture et al., 2006). While the benefits of pharmacologic treatment for these pervasive cognitive deficits are currently limited (Buchanan et al., 2011; Keefe et al., in press), cognitive rehabilitation approaches have emerged as effective intervention strategies for improving cognition in schizophrenia (McGurk et al., 2007; Wykes et al., 2011). The increasing evidence for the efficacy of cognitive rehabilitation in people with schizophrenia has raised important questions regarding the secondary benefits of enhancing cognition on other core aspects of the disorder. A growing body of research indicates significant effects on positive symptomatology and patient functioning (e.g., Eack et al., 2011; Subramaniam et al., 2012), highlighting the potential benefits of these rehabilitation strategies beyond the core cognitive domains that are the primary focus of treatment.
One critical area of impairment of schizophrenia that might also be positively affected by cognitive rehabilitation interventions is negative symptoms, which like cognitive impairments, have proven to be particularly resistant to current treatment approaches (Erhart et al., 2006). Previous studies have concluded that while negative symptoms and cognition are separable in the disorder, considerable overlap does exist (Harvey et al., 2006). Some of this overlap likely stems from conceptual blurring between these two constructs (e.g., when poor attention is included in operational definitions of negative symptoms), yet evidence from animal models also suggests that similar neural pathways may underlie the processes involved in components of both negative symptoms and cognitive impairments in schizophrenia (Karlsson et al., 2008; Labrie et al., 2008). Indeed, the potential shared pathophysiology of these areas of impairment in the disorder have led some to consider pharmacologic initiatives targeting both negative symptom and cognitive domains with the same agent (e.g., Buchanan et al., 2007; Marx et al., 2009).
To date, few studies have specifically examined the effects of cognitive rehabilitation interventions on negative symptoms in schizophrenia, as most studies have understandably been focused on isolating potential benefits to cognition. Bellucci and colleagues (2003) and Bark and colleagues (2003) both reported improvements in negative symptoms following short-term cognitive rehabilitation in patients with schizophrenia. Recently, we published the results of a 2-year randomized-controlled trial of Cognitive Enhancement Therapy (CET; Hogarty and Greenwald, 2006), a comprehensive cognitive rehabilitation approach targeting both social and non-social cognition, in early course patients with schizophrenia. We observed medium-to-large effect sizes in this trial on neurocognition (d = 0.46), social cognition (d = 1.55), and functional outcome (d = 1.53) (Eack et al., 2009). In addition to benefits on these important outcomes favoring CET, significant (d = 0.77) improvements were observed on a broad symptomatology composite (e.g., positive, negative, and affective symptoms). These differential benefits of CET on symptomatology were not expected; given that entry criteria for the trial included symptom stabilization and that the treatment is not focused on symptomatology. Further inspection suggested that composite symptom effects were not due to reductions in positive symptoms, but reflect, in large part, improvements in negative symptom domains.
The pattern of psychopathological improvement observed in this trial of CET in early course schizophrenia provided a unique opportunity to explore the effects of cognitive rehabilitation on negative symptom improvement in the disorder. This study investigates the differential effects of CET on individual negative symptom domains, as well as the relationship between cognitive improvement and changes in negative symptoms during the course of this trial, in an effort to elucidate the potential benefits of cognitive enhancement during cognitive rehabilitation on negative symptoms in schizophrenia.
2. Method
2.1. Participants
Participants included all 58 individuals in the early course of schizophrenia (n = 38) or schizoaffective disorder (n = 20) participating in a randomized trial of Cognitive Enhancement Therapy for early course schizophrenia; the study design, sample characteristics, and primary effects of which have been reported elsewhere (Eack et al., 2009). Individuals were included in this study if they had a diagnosis of schizophrenia, schizoaffective, or schizophreniform disorder verified by the Structured Clinical Interview for DSM-IV (First et al., 2002), had experienced their first psychotic symptom within the past 8 years, had an IQ ≥ 80, as estimated by the Ammon’s Quick Test (Ammons and Ammons, 1962), did not have a recent (within past 2 months) history of substance abuse, and demonstrated significant social and cognitive disability, using pre-defined cut-off scores on the Cognitive Styles and Social Cognition Eligibility Interview (Hogarty et al., 2004). Enrolled participants were young, with an average age of 25.92 (SD = 6.31) years, had been ill on average 3.19 (SD = 2.24) years since the emergence of their first psychotic symptom, and the majority were male (n = 40) and Caucasian (n = 40). Many of the participants had received some college education (n = 39), but most were not employed at study baseline (n = 43).
2.2. Treatments
Eligible participants were randomized to two years of either CET or an Enriched Supportive Therapy (EST) control. Both of these intervention approaches have been described in detail elsewhere (Hogarty et al., 2004; Eack et al., 2009). Briefly, CET is a comprehensive cognitive rehabilitation approach that integrates approximately 60 hours of computer-based training in attention, memory, and problem-solving with 45 social-cognitive small group sessions focused on enhancing the ability of patients to take the perspective of others, accurately appraise spontaneous social contexts, be foresightful, and understand the social “gist” from novel interpersonal encounters, in addition to teaching psychoeducation, illness management and coping skills for stress management. Together, these rehabilitation activities are designed to address the core social and non-social cognitive deficits that limit functional recovery from schizophrenia. The contrasting psychosocial treatment condition was EST, which is based on Personal Therapy (Hogarty, 2002), and is an individual therapy focusing on illness management and psychoeducation designed to help patients learn about their disorder, identify early cues of stress, and implement coping strategies to minimize the impact of stress on their lives and promote psychiatric stability. In EST, strategies for managing stress are tailored to the level of recovery of the patient. For example, for patients in the earlier phases of recovery, stress management approaches rely upon simple behavioral techniques, including passive distraction and avoiding stressful situations. As individuals proceed, more advanced stress management techniques are learned, such as diaphragmatic breathing and criticism management. Individual sessions lasting approximately 30–60 minutes are held weekly in the early phase of the treatment, and then progress to biweekly sessions in the later phases of the intervention. No attempt was made to artificially match the number of hours of treatment exposure between CET and EST, and those receiving CET by design received a greater number of hours of treatment, due to the increased frequency and lengthy of treatment sessions in that condition (Eack et al., 2009). All individuals were maintained on antipsychotic medications approved by the U.S. Food and Drug Administration for the treatment of schizophrenia or schizoaffective disorder by a study psychiatrist.
2.3. Measures
2.3.1. Negative symptoms
Negative symptoms were assessed using the Wing Negative Symptom Scale (Wing, 1961) and the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962). The Wing Negative Symptom Scale consists of 6 items rated on a 5-point Likert scale from 1 (“No Evidence”) to 5 (highly present, e.g. “Almost always disinterested and unmotivated, no spontaneous interest in work, play, reading, conversations, etc.”) that cover core negative symptom domains in schizophrenia including affective flattening, poverty of speech, slowness of movement, under-activity, social withdrawal, and amotivation. This scale has been used in previous psychiatric research (McEvoy et al., 1991; Hogarty et al., 1997), and demonstrated good internal consistency reliability in this sample (α = .80). The BPRS is a widely used measure of psychopathology that assesses positive, negative, and affective symptoms, as well as thought disturbance and hostility on a 1 (not observed) to 7 (very severe) scale. Previous factor-analytic studies have identified a clear negative symptom factor for the BPRS consisting of the items covering emotional withdrawal, motor retardation, and blunted affect (Shafer, 2005). For primary analyses, items from the Wing Negative Symptom Scale and BPRS were scaled to a common (z) metric based on baseline scores for the entire sample and averaged to form an overall negative symptom composite index, which demonstrated excellent internal consistency (α = 0.86).
2.3.2. Cognition
Assessments of neurocognitive and social-cognitive domains were included in this study to examine the degree to which the previously-reported benefits of CET on these domains (Eack et al., 2009) were associated with any improvements in negative symptoms. Neurocognition was assessed using a standardized neuropsychological testing battery mostly reflective of the domains later outlined by the NIMH MATRICS committee (Green et al., 2004). This included assessments of verbal memory (immediate and delayed recall items from stories A and B of the Revised Wechsler Memory Scale [Wechsler, 1987]; List A total recall, short-term free recall, and long-term free recall from the California Verbal Learning Test [Delis et al., 1987]), working memory (digit span from the Revised Wechsler Adult Intelligence Scale [WAIS-R; Wechsler, 1981]), language (vocabulary items from the WAIS-R), executive functioning (Trails B time to completion [Reitan and Waltson, 1985]; perseverative and non-perseverative errors, categories completed, and percentage of conceptual responses from the Wisconsin Card Sorting Test [Heaton et al., 1993]; total move score and ratio of initiation to execution time from the Tower of London [Culbertson and Zillmer, 1996]; and picture arrangement score from the WAIS-R), and neurological soft signs (cognitive-perceptual and repetition-motor subscales from the Neurological Evaluation Scale [Buchanan and Heinrichs, 1989]). All items were scaled to a common (z) metric and averaged to form an overall neurocognitive composite, which demonstrated excellent internal consistency (α = 0.87). Full details on the construction of this composite index have been reported elsewhere (Eack et al., 2009).
Social cognition was assessed using the MATRICS-recommended Managing Emotions branch of the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer et al., 2003). The MSCEIT is a computerized 141-item performance-based measure of emotion processing that covers the four domains of emotional intelligence proposed by Salovey and Mayer (1990), which include emotion perception, facilitation, understanding, and management. Each domain is assessed using two performance-based tasks that come together to form a branch in one of the four domains of emotional intelligence. The measure has demonstrated excellent psychometric properties in previous studies (Mayer et al., 2003), and been shown to be a reliable and valid measure of social cognition in patients with schizophrenia (Nuechterlein et al., 2008; Eack et al., 2010a). For the purposes of this research, the fourth branch of the instrument (Managing Emotions) that was recommended by the NIMH MATRICS committee (Nuechterlein et al., 2008) for assessing social cognition in schizophrenia was used. Analyses were also conducted with total MSCEIT scores, which did not change the results, and thus are not presented here.
2.4. Procedures
Participants in the early course of schizophrenia were recruited for a randomized-controlled trial of Cognitive Enhancement Therapy from outpatient clinics at Western Psychiatric Institute and Clinic in Pittsburgh, PA and surrounding community clinics. Upon recruitment, individuals were assessed for eligibility in consensus conferences containing at least two master’s- or doctoral-level trained clinical team members to verify psychiatric diagnosis, and significant cognitive and social disability. Eligible individuals were then randomly assigned by the project statistician to either CET (n = 31) or EST (n = 27) and treated for two years. Clinical assessments of negative symptoms were completed prior to treatment and at 1- and 2-years, respectively, by trained master’s level clinicians who were not blind to treatment assignment. Interim 1-year treatment effects were not examined, and analyses focused only on 2-year effects on negative symptoms. Performance-based assessments of neurocognition and social cognition were completed by trained neuropsychological testers prior to treatment and then annually for the remaining course of the study. All patients, with the exception of 1 participant (at baseline) were maintained on second-generation antipsychotic medications throughout the course of the study. Medication changes were allowed, although no significant differences between treatment groups were observed in medication dosage at any timepoint during the study. In addition, no significant differences in pre-treatment demographic, cognitive, or symptom characteristics were observed between treatment groups (Eack et al., 2009). Of the 58 patients randomized, 49 patients completed 1 year of the study, and 46 completed the full two years of treatment. There were no significant differences between treatment groups in rates of attrition (Eack et al., 2009). To avoid the well-known biases associated with completer analyses (Schafer & Graham, 2002), all 58 patients who were randomized and received some exposure to their respective treatment condition were included in intent-to-treat analyses, with missing data handled using the expectation-maximization approach (Dempster et al., 1977). All participants provided written, informed consents prior to participation, and the study was reviewed and approved annually by the University of Pittsburgh Institutional Review Board.
3. Results
3.1. Effects of Cognitive Enhancement Therapy on Negative Symptoms
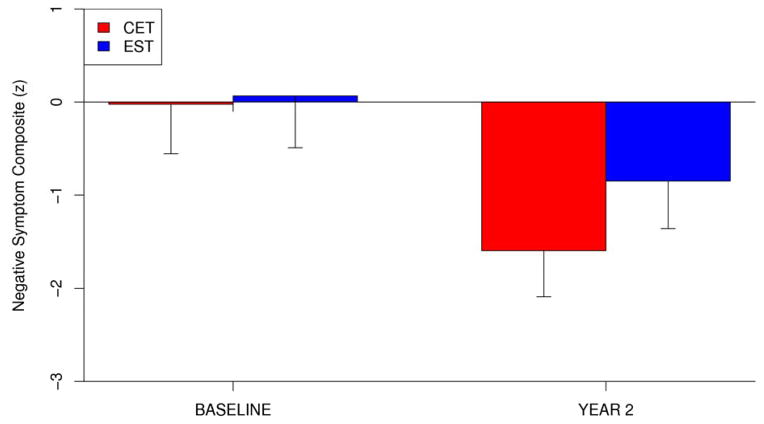
We began our analysis of the effects of CET on negative symptoms by conducting a series of intent-to-treat linear mixed-effects models examining the effects of group assignment on an overall negative symptom composite, which consisted of the Wing Negative Symptom Scale total score and the BPRS negative symptom factor score. Adjusting for potential a priori selected demographic (age, gender, IQ, and illness duration) and medication (dose) contributors to outcome, analytic models were designed to predict negative symptom outcomes over time from initial negative symptom status, baseline demographic characteristics, time-varying medication dose, time (baseline = 0, year 1 = 1, year 2 = 2), treatment assignment (1 = CET; 2 = EST), and a treatment by time interaction. Significant treatment by time interactions, indicating differential effects of one of the treatment conditions on negative symptoms, were the primary effects of interest and these analytic models are identical to those previously used for assessing the efficacy of CET on cognitive and functional domains in this early course trial (Eack et al., 2009). Results revealed a significant and medium-sized effect favoring CET for overall two-year improvement in negative symptoms, t(82) = 2.63, p = 0.01, d = 0.61 (see Figure 1). Within-group improvement in both the CET, t(46) = −8.69, p < 0.001, d = 1.44, and EST, t(43) = −4.75, p < 0.001, d = 0.84, was considerable, although patients treated with CET maintained an advantage in greater reductions in negative symptoms compared to those treated with EST.
Figure 1.
Two-Year Effects of Cognitive Enhancement Therapy and Enriched Supportive Therapy on Negative Symptoms.
Having found evidence for greater improvement in overall negative symptoms among patients treated with CET, we proceeded to examine the differential effects of CET versus EST on individual negative symptom domains. Analyses again made use of intent-to-treat linear-mixed effects models adjusting for demographic and medication covariates, and indicated that findings favoring CET were a result of specific differential improvements in social withdrawal, motor retardation, and affective flattening, with the largest effects observed in the domain of social withdrawal (see Table 1). No significant differential effects were observed between CET and EST on other areas of negative symptoms. Analyses of within-group effects revealed reductions in every negative symptom domain assessed among CET (all p < 0.006) and EST participants (all p < 0.05), with the exception of motor retardation in EST, which was trend-level (p = 0.056), after adjusting for multiple inference testing using Benjamini and Hochberg’s (1995) correction.
Table 1.
Two-Year Effects of Cognitive Enhancement Therapy on Negative Symptom Domains in Early Schizophrenia (N = 58).
| Variable | CET
|
EST
|
Analysisa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year 2 | Baseline | Year 2 | ||||||||
|
| |||||||||||
| M | SD | M | SD | M | SD | M | SD | t | p | d | |
| WING Negative Symptom Scale | 18.27 | 4.14 | 11.02 | 3.44 | 18.27 | 3.63 | 14.00 | 3.79 | 2.43 | 0.017 | 0.72 |
| Affective Flattening | 3.25 | 0.96 | 2.06 | 1.02 | 3.00 | 1.00 | 2.45 | 0.86 | 2.03 | 0.046 | 0.68 |
| Poverty of Speech | 2.54 | 0.92 | 1.81 | 0.70 | 2.86 | 0.64 | 2.26 | 0.81 | .49 | 0.627 | 0.14 |
| Slowness of Movement | 2.76 | 1.12 | 1.31 | 0.64 | 2.95 | 0.99 | 1.90 | 0.92 | 1.31 | 0.193 | 0.36 |
| Underactivity | 3.07 | 0.93 | 1.93 | 0.90 | 3.17 | 0.95 | 2.51 | 1.01 | 1.58 | 0.119 | 0.52 |
| Social Withdrawal | 3.42 | 0.72 | 2.06 | 1.02 | 3.23 | 0.94 | 2.65 | 0.97 | 3.04 | 0.003 | 1.08 |
| Amotivation | 3.26 | 0.90 | 1.83 | 0.87 | 3.07 | 0.83 | 2.18 | 0.81 | 1.68 | 0.096 | 0.60 |
| BPRS Withdrawal/Motor Retardation | 2.75 | 1.20 | 1.63 | 0.60 | 2.94 | 1.06 | 2.29 | 0.78 | 1.98 | 0.051 | 0.40 |
| Emotional Withdrawal | 2.04 | 1.20 | 1.40 | 0.93 | 2.41 | 1.05 | 1.76 | 1.01 | −0.03 | 0.978 | −0.01 |
| Motor Retardation | 2.93 | 1.29 | 1.50 | 0.78 | 2.96 | 1.37 | 2.34 | 0.89 | 2.49 | 0.015 | 0.63 |
| Blunted Affect | 3.31 | 1.61 | 2.02 | 0.98 | 3.45 | 1.24 | 2.77 | 1.03 | 1.97 | 0.052 | 0.38 |
Note. CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy
Results from linear mixed-effects models adjusting for demographic (age, gender, IQ, illness duration) and medication (dose) covariates, comparing rates of improvement in negative symptom domains among patients receiving Cognitive Enhancement Therapy versus Enriched Supportive Therapy.
3.2. Associations Between Cognitive and Negative Symptom Improvement
After observing the additional benefit of cognitive rehabilitation on negative symptoms in this sample of early course schizophrenia patients, we then examined the associations between cognitive and negative symptom improvement among individuals treated with CET or EST using a series of intent-to-treat linear mixed-effects growth models adjusting for potential demographic and medication covariates. These models predicted negative symptoms over time from cognitive scores over time to assess the degree to which cognitive change was associated with negative symptom change in each of the treatment conditions. Cognitive change by treatment interactions were also explored to examine the degree to which associations between changes in cognition and negative symptoms were significantly different between patients treated with CET or EST. As can be seen in Figure 2, two-year improvements in neurocognition were significantly associated with greater reductions in negative symptoms among individuals treated with CET (R2 = 0.16, β = −0.34, p = 0.009), but not those treated with EST (R2 = 0.00, β = 0.11, p = 0.473), and moderator models indicated that the association between changes in neurocognition and negative symptoms was significantly greater in the CET compared to EST condition, F(1, 79) = 4.72, p = 0.033. Social-cognitive improvement, as measured by the Managing Emotions branch score of the MSCEIT, was not associated with negative symptom composite change in either treatment group (all p > 0.230).
Figure 2.
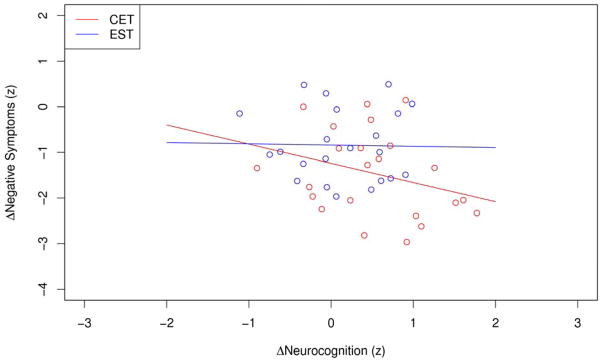
Association Between Two-Year Changes in Neurocognition and Negative Symptoms Among Early Course Patients Treated with Cognitive Enhancement Therapy or Enriched Supportive Therapy.a
aRegression lines and data points represent raw, unadjusted data from all treatment completers.
With regard to individual negative symptom domains, reductions in social withdrawal (R2 = 0.15, β = −0.28, p = 0.022) and BPRS-measured blunted affect (R2 = 0.10, β = −0.30, p = 0.024) were significantly associated with improved neurocognition in patients treated with CET, whereas these relationships were not observed in EST patients (all p > 0.144), and moderator analyses again revealed that these relationships were significantly stronger in patients treated with CET, all F(1, 79) > 5.60, all p < 0.021. Associations between motor retardation and Wing-measured affective flattening with neurocognitive improvement were in the same direction as other negative symptom domains among those treated with CET, but trend-level and non-significant (all p < 0.104). Unexpectedly, neurocognitive decline was significantly associated with improved affective flattening scores on the Wing in EST patients (R2 = 0.05, β = 0.32, p = 0.037), and no significant associations between motor retardation and neurocognitive change were observed in patients treated with EST (R2 = 0.00, β = −0.08, p = 0.652). No significant associations between changes in social cognition and negative symptoms were observed across any domain among those treated with CET or EST (all p > 0.191).
4. Discussion
Cognitive rehabilitation is emerging as an effective set of approaches that can produce meaningful and generalizable improvements in cognitive functioning in patients with schizophrenia (McGurk et al., 2007; Wykes et al., 2011). Intervention developers have hoped that such improvements in cognition that result from rehabilitation will have accompanying benefits on other important patient outcomes. Negative symptoms represent a critical area of impairment that may be positively affected by cognitive rehabilitation, given the consistent relationship between cognition and negative symptoms in schizophrenia (Harvey et al., 2006) and their potential shared pathophysiology (Karlsson et al., 2008; Labrie et al., 2008). This study explored the effects of CET on negative symptom improvement in patients in the early course of schizophrenia, and the association between cognitive enhancement and negative symptom change in this sample. Results revealed medium and significant differential improvements in overall negative symptoms favoring CET compared to EST, with specific effects observed in the areas of social withdrawal, affective flattening, and motor retardation. It should also be noted that the benefits of EST on negative symptoms were also considerable, likely due to the efficacy of Personal Therapy (Hogarty, 2002) in the treatment of schizophrenia, a therapy upon which EST was based. However, CET maintained a considerable advantage over EST in the treatment of negative symptoms and performance-based improvements in neurocognition were interestingly only related to reductions in negative symptoms among patients receiving CET, signifying a potential connection between cognitive and negative symptom improvement. When combined with several previous studies (Bark et al., 2003; Bellucci et al., 2003), these findings suggest that cognitive rehabilitation may offer an effective avenue for addressing some aspects of negative symptoms in patients with schizophrenia.
There are admittedly many caveats to these findings that limit conclusions regarding the benefits of cognitive rehabilitation on negative symptoms. This research represents an exploratory analysis of the effects of CET on negative symptoms that occurred in response to an unexpected benefit observed for the treatment on a broad symptomatology composite. As such, this trial was clearly not designed to rigorously examine negative symptom effects. Measurement of negative symptoms was limited and conducted by non-blind raters, which may have affected the results and did not now allow for the examination of whether effects represented improvements in primary negative symptoms or those that were secondary to other factors, such as medication side-effects. The lack of any differences in antipsychotic type or dosage between patients receiving CET and EST, as well as the modeling of potential antipsychotic dosage effects in treatment analyses, suggests that these findings may not be due to differences in medication response, but cannot rule out the possibility of changes in negative symptoms due to other secondary factors. Future cognitive rehabilitation studies will need to address this issue by selecting patients with prominent and persistent negative symptoms, and assessing for the variety of potential sources of secondary negative symptoms (e.g., medication side-effects, depressive symptomatology), as has been suggested for trials of pharmacologic cognitive enhancers (Kirkpatrick et al., 2006). In addition, subsequent studies will need to examine whether the effects of CET on negative symptoms can be maintained after the completion of treatment, as has been show with effects on cognition and functional outcome (Hogarty et al., 2006; Eack, Greenwald, Hogarty, & Keshavan, 2010). The treatment conditions were also not matched with regard to the number of hours of treatment provided, which could have also accounted for the greater benefits observed in CET. Further, the treatment mechanisms in CET that produced improvements in negative symptoms are also unclear. While neurocognitive improvement was uniquely associated with reductions in negative symptoms, suggesting the computer-based neurocognitive training may have been a strong contributor to these changes, CET is a comprehensive intervention that includes many other components that could have also resulted in negative symptom change. Particularly convincing of this possibility is that the largest area of negative symptom improvement was social withdrawal, which is specifically targeted in the social-cognitive group curriculum. Through the integration of neurocognitive training and social-cognitive group sessions, CET improves these important cognitive domains while also fostering the gradual improvement of mental and physical stamina (e.g., patients are taught strategies to improve motivation, develop a healthy daily schedule, and become more active thinkers).
It is interesting and unexpected that changes in social cognition were not related to reductions in any negative symptom domains, although the restriction of social-cognitive assessments to the MSCEIT in this analysis may have contributed to this finding, since it focuses only on the emotion processing aspect of social cognition. Perhaps improvements in other components of social cognition, such as the ability to shift to another person’s perspective, are more closely related to negative symptom change. The beneficial effects of CET on both cognition and negative symptoms may have also been related to the enhancement of reward system functioning, which has been shown to be abnormal in schizophrenia (for a review, see Ziauddeen and Murray, 2010). The role of the brain’s reward circuitry in the cognitive, emotional and behavioral deficits of schizophrenia has garnered increasing attention in recent years, particularly given the critical role of reward in learning and social interactions (Schultz, 2000; Wise, 2002; Berridge and Robinson, 2003) and the relationship between reward processing abnormalities and negative symptoms in schizophrenia (e.g., Barch and Dowd, 2010; Strauss et al., 2011; Dowd and Barch, 2012; Gold et al., 2012). Further exploration of the association between reward, cognition and negative symptoms, and as separable effects of CET, would aid in understanding the mechanisms by which CET may contribute to better cognitive and social outcomes in schizophrenia.
Finally, because this research examined associations between concurrent change in cognition and negative symptoms, causal inferences regarding the direction of these effects are limited. While we hypothesize that the cognitive improvements that result from CET are likely to have meaningful and specific impact on negative symptoms, it is also possible that improvements in negative symptoms could impact cognitive performance through a variety of avenues (e.g., increased motivation, improved test-taking behavior). Results of reverse analytic models indicated a considerably weaker effect of two-year improvements in negative symptoms on improved neurocognition, β = −0.21, p = 0.049, than did our models predicting changes in negative symptoms from changes in cognition. Further, the effect of negative symptom improvement on cognitive improvement did not differ significantly between those treated with CET or EST, F(1, 79) = 1.77, p = 0.188. Although future studies specifically designed to evaluate the contribution of improved cognition to negative symptoms are needed, these results suggest that the improvements in cognition observed during cognitive rehabilitation are stronger and produce far more specific effects on negative symptoms than negative symptom improvement does on cognition.
Despite these limitations, the results of this research raise important questions about the potential effects of cognitive rehabilitation on negative symptoms in schizophrenia. Previous studies of cognitive enhancing medications in the disorder have been designed to assess effects on both cognition and negative symptom targets given the potential overlap in some of their underlying neurobiologic substrates (Buchanan et al., 2007; Marx et al., 2009). Evidence is emerging that CET and other cognitive rehabilitation interventions can also positively affect some of these same neural substrates (Eack et al., 2010b; Subramaniam et al., 2012). The beneficial effects of CET on negative symptoms and the association between neurocognitive improvement and negative symptom change within the context of this longitudinal treatment trial suggest an important link between the enhancement of cognition and negative symptom improvement in patients with schizophrenia that should be more closely examined in future trials of cognitive rehabilitation.
Acknowledgments
This work was supported by NIH grants MH 60902 (MSK), MH 92440 (MSK and SME), MH 95783 (SME), DA 30763 (SME), and RR 24154 (SME). We thank the late Gerard E. Hogarty, M.S.W. for his leadership and direction as Co-Principal Investigator of this study, and Susan Cooley M.N.Ed., Anne Louise DiBarry, M.S.N., Konasale Prasad, M.D., Haranath Parepally, M.D., Debra Montrose, Ph.D., Diana Dworakowski, M.S., Mary Carter, Ph.D., and Sara Fleet, M.S. for their help in various aspects of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammons RB, Ammons CH. The Quick Test (QT): provisional manual. Psychological Reports. 1962;11 (1):111–161. [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal--striatal interactions. Schizophrenia Bulletin. 2010;36 (5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark N, Revheim N, Huq F, Khalderov V, Ganz ZW, Medalia A. The impact of cognitive remediation on psychiatric symptoms of schizophrenia. Schizophrenia Research. 2003;63 (3):229–235. doi: 10.1016/s0920-9964(02)00374-2. [DOI] [PubMed] [Google Scholar]
- Bellucci DM, Glaberman K, Haslam N. Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophrenia Research. 2003;59 (2–3):225–232. doi: 10.1016/s0920-9964(01)00402-9. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57 (1):289–300. [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26 (9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research. 1989;27 (3):335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The Efficacy of Glutamatergic Agents for Negative Symptoms and Cognitive Impairments. American Journal of Psychiatry. 2007;164 (10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RSE, Lieberman JA, Barch DM, Csernansky JG, Goff DC, Gold JM, Green MF, Jarskog LF, Javitt DC, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biological Psychiatry. 2011;69 (5):442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The Functional Significance of Social Cognition in Schizophrenia: A Review. Schizophrenia Bulletin. 2006;32 (Suppl1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. Unpublished manuscript. 1996. Tower of London-DX manual. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data using the EM algorithm. Journal of the Royal Statistical Society Series B (Methodological) 1977;39 (1):1–38. [Google Scholar]
- Dowd EC, Barch DM. Pavlovian Reward Prediction and Receipt in Schizophrenia: Relationship to Anhedonia. PloS One. 2012;7 (5):e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophrenia Bulletin. 2010a;36 (2):370–380. doi: 10.1093/schbul/sbn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of Cognitive Enhancement Therapy on functional outcome in early schizophrenia. Schizophrenia Research. 2010b;120 (1):210–216. doi: 10.1016/j.schres.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS. Cognitive Enhancement Therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60 (11):1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of Cognitive Enhancement Therapy against gray matter loss in early schizophrenia: Results from a two-year randomized controlled trial. Archives of General Psychiatry. 2010c;67 (7):674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, Keshavan MS. Effects of Cognitive Enhancement Therapy on employment outcomes in early schizophrenia: Results from a two-year randomized trial. Research on Social Work Practice. 2011;21 (3):32–42. doi: 10.1177/1049731509355812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophrenia Bulletin. 2006;32 (2):234–237. doi: 10.1093/schbul/sbj055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Collins AGE, Frank MJ. Negative Symptoms and the Failure to Represent the Expected Reward Value of Actions: Behavioral and Computational Modeling Evidence. Archives of General Psychiatry. 2012;69 (2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the right stuff? Schizophrenia Bulletin. 2000;26 (1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56 (5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship. Schizophrenia Bulletin. 2006;32 (2):250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12 (3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hogarty GE. Personal Therapy for schizophrenia and related disorders: A guide to individualized treatment. New York: Guilford; 2002. [Google Scholar]
- Hogarty GE, Greenwald DP. Cognitive Enhancement Therapy: The Training Manual. University of Pittsburgh Medical Center: Authors; 2006. Available through www.CognitiveEnhancementTherapy.com. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Keshavan M, Cooley S, DiBarry AL, Garrett A, Parepally H, Zoretich R. Cognitive enhancement therapy for schizophrenia. Effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61 (9):866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of Cognitive Enhancement Therapy. Psychiatric Services. 2006;57 (12):1751–1757. doi: 10.1176/ps.2006.57.12.1751. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald D, Ulrich RF, Kornblith SJ, Dibarry AL, Cooley S, Carter M, Flesher S. Three-year trials of personal therapy among schizophrenic patients living with or independent of family: II. Effects on adjustment of patients. American Journal of Psychiatry. 1997b;154 (11):1514–1524. doi: 10.1176/ajp.154.11.1514. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2008;34 (6):1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Buchanan RW, Marder SR, Schooler NR, Dugar A, Zivkov M, Stewart M. Clinical Trials of Potential Cognitive-Enhancing Drugs in Schizophrenia: What Have We Learned So Far. Schizophrenia Bulletin. doi: 10.1093/schbul/sbr153. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS Consensus Statement on Negative Symptoms. Schizophrenia Bulletin. 2006;32(2):214. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology. 2008;200 (2):217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RSE, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, et al. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34 (8):1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3 (1):97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Archives of General Psychiatry. 1991;48 (8):739–745. doi: 10.1001/archpsyc.1991.01810320063009. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A Meta-Analysis of Cognitive Remediation in Schizophrenia. American Journal of Psychiatry. 2007;164 (12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23 (3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, III, Gold JM, et al. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. American Journal of Psychiatry. 2008;165 (2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10 :799–812. [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Racenstein J, Newman L. Social cognition in schizophrenia. Psychological Bulletin. 1997;121 (1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Waltson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Salovey P, Mayer JD. Emotional Intelligence. Imagination, Cognition, and Personality. 1990;9 (3):185–221. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7 (2):147–177. [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1 (3):199–208. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Shafer A. Meta-analysis of the Brief Psychiatric Rating Scale factor structure. Psychological Assessment. 2005;17 (3):324–335. doi: 10.1037/1040-3590.17.3.324. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biological Psychiatry. 2011;69 (5):424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized Cognitive Training Restores Neural Activity within the Reality Monitoring Network in Schizophrenia. Neuron. 2012;73 (4):842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- Wing JK. A simple and reliable subclassification of chronic schizophrenia. Journal Mental Science. 1961;107 :862–875. doi: 10.1192/bjp.107.450.862. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36 (2):229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry. 2011;168 (5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Murray GK. The relevance of reward pathways for schizophrenia. Current opinion in psychiatry. 2010;23 (2):91–96. doi: 10.1097/YCO.0b013e328336661b. [DOI] [PubMed] [Google Scholar]