Abstract
The abasic (AP) sites, the major mutagenic and cytotoxic genomic lesions, induced directly by oxidative stress and indirectly after excision of damaged bases by DNA glycosylases, are repaired by AP-endonucleases (APEs). Among two APEs in Saccharomyces cerevisiae, Apn1 provides the major APE activity, and Apn2, the ortholog of the mammalian APE, provides back-up activity. We have cloned apn1 and apn2 genes of Schizosaccharomyces pombe, and have shown that inactivation of Apn2 and not Apn1 sensitizes this fission yeast to alkylation and oxidative damage-inducing agents, which is further enhanced by Apn1 inactivation. We also show that Uve1, present in S.pombe but not in S.cerevisiae, provides the back-up APE activity together with Apn1. We confirmed the presence of APE activity in recombinant Apn2 and in crude cell extracts. Thus S.pombe is distinct from S.cerevisiae, and is similar to mammalian cells in having Apn2 as the major APE.
INTRODUCTION
Mitochondria continuously generate reactive oxygen species (ROS) as respiration by-products. ROS are genotoxic and induce a multitude of DNA damage, including oxidized bases, abasic (AP) sites due to direct glycosylic bond cleavage in DNA, and strand breaks caused by fragmentation and oxidation of deoxyribose in the DNA backbone with generation of 3′-phosphoglycolaldehyde and 3′-phosphoglycolate termini (1). AP sites are also generated as repair intermediates after excision of alkylated and oxidatively damaged bases by DNA glycosylases and are produced due to spontaneous depurination (2,3). Oxidized base-specific DNA glycosylases possess intrinsic AP-lyase activity, cleaving the DNA backbone at AP sites and producing 3′-phospho-α,β unsaturated aldehyde or 3′-phosphates. AP sites, arguably the most abundant endogenous lesions in DNA, have been shown to be cytotoxic and mutagenic (3–5). Repair of AP sites and single-strand breaks with 3′-blocked termini occurs primarily via the DNA base excision repair (BER) pathway, which is essential for mammalian survival. Inactivation of some BER proteins including the major AP-endonuclease (APE), DNA polymerase β (Polβ) or XRCC1 protein in the mouse causes embryonic lethality (6). Repair of AP sites is initiated with an APE which cleaves the DNA strand 5′ to the AP site and generates 3′-OH and 5′-phosphodeoxyribose termini. The resulting 5′-phosphodeoxyribose group is removed by the lyase activity of Polβ to generate a 1 nt gap. Subsequently, Polβ fills in the gap, and DNA ligase seals the single-strand nick to complete repair [reviewed in Mitra et al. (7)]. APEs also have intrinsic 3′-phosphodiesterase activity responsible for removing the 3′-blocking groups generated at strand breaks caused by either ROS directly or due to AP lyase activity of some DNA glycosylases (8). Two major classes of APEs are present in organisms ranging from the bacteria to mammals whose prototypes are Xth (exonuclease III), representing ∼90% of the total AP repair activity, and Nfo (endonuclease IV) in Escherichia coli. In Saccharomyces cerevisiae, Apn1, the first APE to be cloned, belongs to the E.coli Nfo family (9), while Apn2 shares homology with E.coli Xth and human APE1 and APE2 (10,11). Both Apn2 and Apn1 possess APE and DNA 3′-phosphodiesterase activities (12,13). However, the apn1 but not the apn2 mutant is sensitive to the methylating genotoxicant methyl methanesulfonate (MMS), while the apn1Δ apn2Δ double mutant is much more sensitive to MMS than the apn1Δ mutant (10,11,14). These results confirm the earlier conclusion that Apn1 is the major APE in S.cerevisiae, and that Apn2 has a back-up role in repair of AP sites in the absence of Apn1.
Fission and budding yeasts diverged early in evolution and often have distinct functional differences. Phylogenetically, the fission yeast Schizaosaccharomyces pombe is generally believed to be closer to mammalian cells than S.cerevisiae, particularly in functions of many regulatory pathways (15,16). However, the BER pathway has not been studied in detail. Although the candidate genes for Apn1 and Apn2 were identified in the S.pombe genome, and the Apn1 gene was cloned (17), the APE activity in the recombinant protein was not shown, presumably because of the presence of an intron in the genomic clone. Although Uve1, a UV photoproduct-specific endonuclease identified in S.pombe, but not S.cerevisiae, was shown to possess APE activity (18), attempts to demonstrate APE activity in cell-free extracts of S.pombe were unsuccessful (17). Although Apn2 was identified in the S.pombe genome database (10,11), its repair activity was not shown. Based on these observations, it was suggested that S.pombe does not have active APE (17). We show in this report that S.pombe does possess active Apn1 and Apn2 in addition to Uve1. Furthermore, we show a distinct difference in APE activity of the fission and budding types in that, in contrast to the situation in S.cerevisiae, Apn2 is the major APE in S.pombe. Moreover, the UV photoproduct-specific Uve1, present in S.pombe but not in S.cerevisiae, provides back-up activity along with Apn1 in repair of AP sites.
MATERIALS AND METHODS
Strains and media
The genotypes of strains used in this study are listed in Table 1. Standard genetic techniques were used for construction and growth of strains (19). Schizosaccharomyces pombe were routinely grown on yeast extract medium (YE) or EMM minimal medium containing appropriate supplements (19).
Table 1. Schizosaccharomyces pombe strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| PO4 | leu1-32 h– | TCP1 Invitrogen |
| PO10 | his4-239 ura4-D18 h– | M. Sipiczki |
| PO20 | lys4-95 ura4-D18 h+ | M. Sipiczki |
| PO29 | arg3-D 4ura4-D18 leu1-32 ade7-152 h– | V. Bashkirov |
| PO24 | apn2::ura4+lys4-95 leu1-32 h+ | This study |
| PO61 | apn1::ura4+his4-239 h– | This study |
| PO60 | apn1::ura4+apn2::ura4+leu1-32 his4-239 h+ | This study |
| PO89 | uve1::ura4+ ura4-D18 ade6-210 leu1-32 h– | P. Doetsch |
| PO74 | apn1::ura4+apn2::ura4+uve1::ura4+ his4-239 h+ | This study |
| PO90 | apn2::ura+4 uve1::ura4+h+ | This study |
Deletion of apn1 and apn2 genes
A microhomology-mediated PCR targeting method was used (20), following PCR-mediated generation of the entire ura4+ gene cassette flanked by 80 bp segments from the 5′ and 3′ regions of the gene to be disrupted. Oligonucleotides used to generate disruption cassettes for apn1+ are: ‘DEL1’, TAC TTG ATG AGT CAA GAA GAA GAA ATT AAA GAA AGT TCT CCT GAA AAG GAC CTT AAA CAA GAG GAT TCTGAG GAA AAG CTT AGC TAC AAA TCC CAC TGG C; and ‘DEL2’, AAC AGC GTT GCT CAT GGG AAA TGG TAA AAT GAT AAA ATT ATA AGT AAT GTA TCG TTA TAT GAT TAA AAA TTC CGT ATT CCA GTG AAG CTT GTG ATA TTG ACG AAA; and for apn2+ are ‘DELAPN2F’, TTT AGA AAG AAT CAT TAG ATT GTT TAC AGT TGT AAC TCA ATA TTA GTC AAT CTA GAA TTT TTT TGA AAA TCA ACA TTC AAA AGC TTA GCT ACA AAT CCC ACT GGC; and ‘DELAPN2B’, GTC CGG TGT GCA GAA GAA AAG TAC ATC CAA ACA AAG TTA TAT GTT TGG AAA TGA TGC TCG GAT CTC AAA AAA AGA AAT CTA AGC TTG TGA TAT TGA CGA AAC TTT. Following transformation of haploid strains his4 ura4-D18 h– (PO10) and lys4 ura4-D18h+ (PO20), ura+ progeny colonies were screened for the correct integration pattern by diagnostic PCR using primer pairs spanning the presumptive recombination sites, and used for further genetic crossing. The genotype of resulting progeny was verified by PCR analysis.
MMS and phleomycin D1 treatment
Cells were grown overnight in YE or EMM medium for survival studies after treatment with MMS and phleomycin D1 (Zeocin; Invitrogen, Carlsbad, CA). The cells were washed with distilled water and resuspended (2 × 107/ml) in phosphate-buffered saline (PBS) before addition of the appropriate amount of MMS. Aliquots were removed at the indicated times and, after inactivating MMS with 10% Na-thiosulfate, the suspensions were serially diluted and plated on solid growth media. In separate studies, the yeast cells were treated with various concentrations of phleomycin D1 at 30°C for 1 h with shaking. Cell suspensions were then diluted 10-fold with cold water, and appropriate dilutions were plated on YEA for viability determination. In all cases, colonies were counted after 4–6 days of growth at 30°C. The data shown here are representative of three independent experiments.
Assay of MMS-induced canr mutagenesis
Cells grown overnight in YE medium were washed with PBS, treated with MMS for 30 min and then appropriate dilutions were spread on YEA plates for measuring viability, and also on EMM plates containing glutamate as the sole nitrogen source, and 75 µg/ml canavanine for canr colonies which were counted after 8 days of incubation at 30°C (21).
Growth inhibition assay
The agar diffusion assay for examining complementation (22) was used with the following modification. Transformed cells were grown overnight in EMM medium lacking leucine and containing thiamine, and 5 µl (106 cells) aliquots were streaked in parallel lines on a thiamine-containing EMM plate (square-form Petri dish). Then 10% MMS (v/v)-soaked filter paper strips were placed orthogonally on the streaks. After incubation for 3 days at 30°C, the growth inhibition zone was measured. The data represent results of an average of three or more experiments.
Plasmids
The wild-type apn1 cDNA was generated by PCR amplification of an S.pombe cDNA library (Machmaker Library, Clontech, Palo Alto, CA). apn2 cDNA was cloned by RT–PCR amplification using total RNA isolated (23) from wild-type S.pombe (Perkin Elmer GENE Amp RNA PCR Kit). The amplified insert was cloned into pNMT1-TOPO vector in-frame with a His6 tag (Invitrogen, Carlsbad, CA). The recombinant plasmids were checked by PCR with primer pairs flanking the intron region, in order to distinguish cDNA clones from those resulting from contaminating genomic DNA or non-spliced mRNA. After confirming the cDNA sequence, the expression plasmids pNBR117 for Apn1 and pNBR110 for Apn2 were generated. Two point mutations in the putative proliferating cell nuclear antigen (PCNA)-binding motif (F402A and F403A), and three point mutations in the putative TOP3-binding domain (P456A, L457A and C458A) were generated in the Apn2 cDNA in pNBR110 by PCR using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The following oligonucleotide primers were used. For PCNA: F402A, F403A (PCN-F-5′ TCG AAG CTT CTT TCA GCC GCC GCG AAG CAA AAG GAA 3′, PCN-B-5′ TTC CTT TTG CTT CGC GGC GGC TGA AAG AAG CTT 3′) and for TOP3: P456A, L457A, C458A (TOP-F-5′ AGT GGA CGA GCT CCT GCC GCC GCC GAG GGT CAC AAG GAA 3′, TOP-B-5′ TTC CTT GTG ACC CTC GGC GGC GGC AGG AGC TCG TTC ACT 3′).
The authenticity of the mutant plasmids, named pNBR114 (apn2A402A403) and pNBR115 (apn2A456A457A458), was verified by sequencing.
To over-express GST–Apn2 fusion protein, we subcloned the Apn2 cDNA from pNBR110 into pESP1 vector (Stratagene, La Jolla, CA) using PCR amplification, and generated plasmid pEBR116.
The human APE1 and APE2 cDNAs were gifts of Drs S. Seki and K. Akiyama, Okayama University, Japan. The APE1 cDNA was subcloned into pREP3XHIS vector (23) using BamHI and SalI restriction sites, to produce pRBR113. The cDNA of APE2 was subcloned into pNMT1-TOPO vector using PCR amplification to generate pNBR103.
Expression and purification of S.pombe Apn2
The GST–Apn2 fusion protein was over-expressed from the plasmid pEBR116 in the S.pombe apn1Δapn2Δ mutant (PO60). Cells were grown to stationary phase in EMM medium containing 5 µM thiamine and lacking leucine. The culture was washed three times with water and diluted in fresh EMM medium to an OD600 of 0.05. After additional incubation for 16 h at 30°C with shaking (180 r.p.m.), the cells were collected by centrifugation, and resuspended in cell breakage buffer containing 0.3 M sorbitol, 0.1 M NaCl, 5 mM MgCl2, 10 mM Tris–HCl pH 7.4, with protease inhibitor mixture (Mini-Complete, Roche, Indianapolis, IN), and disrupted in a French press. After removing cell debris by centrifugation (20 000 g, 30 min) and filtration, the extract was loaded on a gluthatione–Sepharose 4B column [Amersham Pharmacia (12)] which was then washed extensively with cell breakage buffer supplemented with 1 M NaCl. The GST–Apn2 protein was finally eluted from the column with the buffer containing 20 mM gluthatione. The peak fractions containing GST–Apn2 were pooled, and concentrated in a Centricon 30 concentrator.
Assay of AP-endonuclease
A 75mer synthetic nucleotide, 5′-AGC TAC CAT GCC TGC CTC AAG AGT TCG TAA XAT GCC TAC ACT GGA GTA CCG GAG CAT CGT CGT GAC TGG GAA AAC-3′, containing a single tetrahydrofuran (THF), an AP site analog, at position 31 (indicated by X), was purchased from IDT (12). The complementary 75mer contained C opposite THF. The THF-containing oligo was 32P labeled at the 5′ end, using T4 polynucleotide kinase and [γ-32P]ATP, and then annealed to the complementary oligo before using in an APE assay. In Figure 7, a 43mer THF-containing oligo was used as before (24). The assay mixture (10 µl) contained 20 mM Tris–HCl pH 7.4, 2 mM MgCl2, 0.5 mM EDTA, 30 mM KCl, 0.1 mg/ml bovine serum albumin (BSA) and 1 mM dithiotreithol (DTT). 32P-labeled duplex oligo (50 fmol) was incubated with 300 ng of GST–Apn2, at 30°C for various times. After stopping the reaction by addition of formamide/EDTA gel loading buffer, the cleaved oligo was separated by electrophoresis in a denaturing polyacrylamide gel (12%) containing 7 M urea, and the radioactivity was quantitied by PhosphorImager analysis using the ImageQuant Software (Molecular Dynamics, Inc., Sunnyvale, CA).
Figure 7.
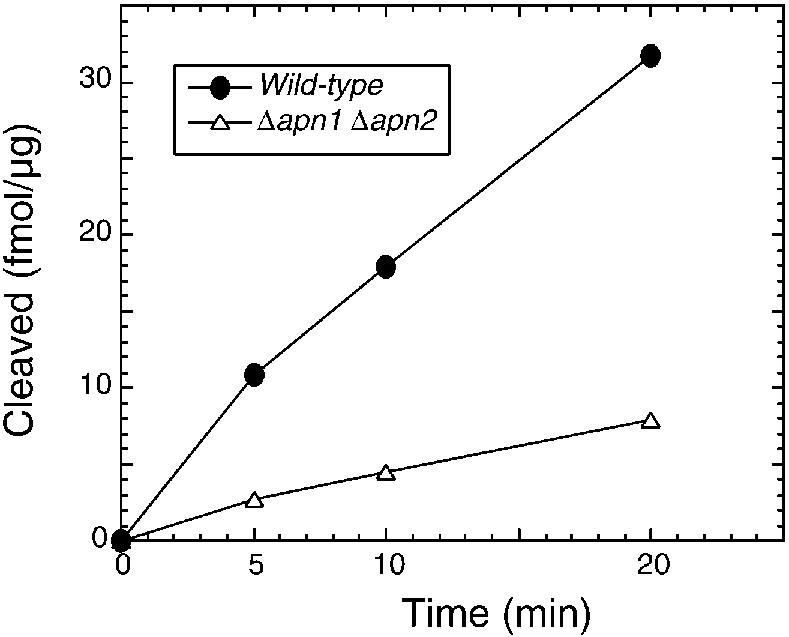
Analysis of APE activity in S.pombe extract. Wild-type and mutant cells were broken by French press and centrifuged. Crude cell extract (1 µg) was mixed with 50 fmol of the oligonucleotide containing the AP site in reaction mixture (20 µl) containing 20 mM Tris pH 8.0, 100 mM NaCl, 1 mM MgCl2, 1 mM DTT and 100 µg/ml BSA. After incubation at 37°C, the 5′-labeled 30mer product was identified with PhosphorImager (Molecular Dynamics) after electrophoretic separation on 20% polyacrylamide gels containing 7 M urea (26,45).
RESULTS
Existence of an intron in the apn1 gene of S.pombe, and cloning of Apn1 cDNA
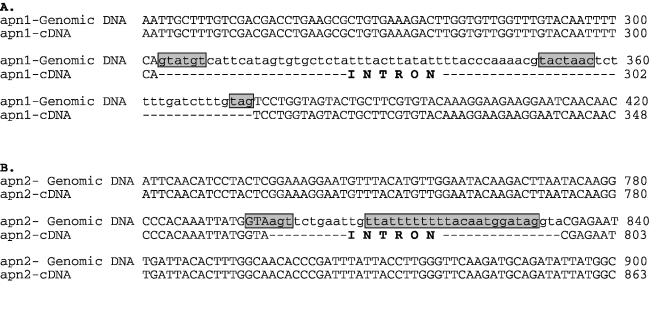
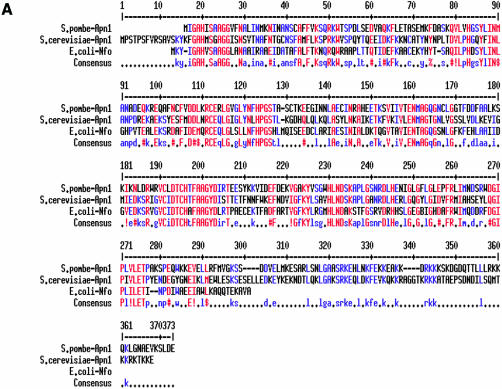
Ramotar et al. (17) predicted the presence of a 72 bp intron sequence in the apn1 gene of S.pombe, flanked by 5′- and 3′-splice sites, which is in-frame with the open reading frame (ORF) of the putative coding sequence. To confirm the coding sequence, we cloned Apn1 cDNA from a cDNA library as described in Materials and Methods. The cDNA contained an ORF with 1029 bp, and lacked the intron (Fig. 1). Apn1, with predicted Mr of 40 kDa, shares 46% identity with S.cerevisiae Apn1 and 43% with E.coli Nfo (Fig. 2A) (25).
Figure 1.
Intron-containing region of the apn1+ (A) and apn2+ (B) genes. The intron sequences are shown in lower case. The consensus sequences for the 5′ splice, branch and 3′ splice sites are boxed [(17) and http://www.sanger.ac.uk/Projects/S_pombe].
Figure 2.
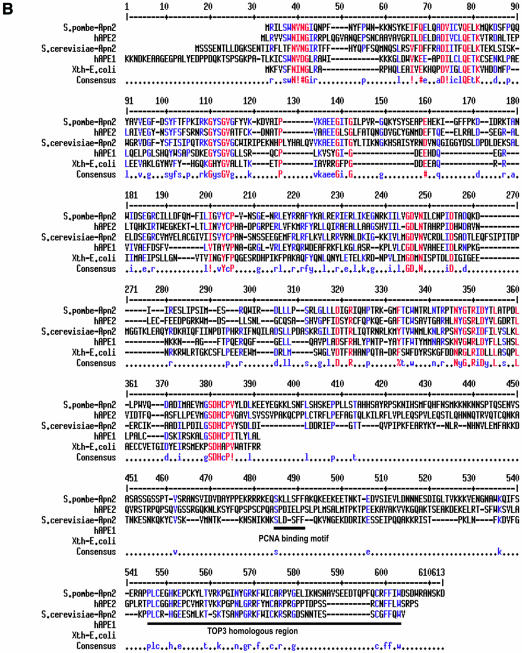
(A) Apn1 sequence alignment of S.pombe and S.cerevisiae Apn1with Nfo of E.coli. (B) Apn2 sequence alignment of S.pombe and S.cerevisiae, human APE2, human APE1 and E.coli Xth proteins. Identical and highly conserved residues are indicated in red. Spaces indicate gaps introduced to optimize alignment by the MultiAlin program (23): http://prodes.toulouse.inra.fr/multalin/multalin.html. GenBank accession numbers AY483157 for apn1+ and AY483158 for apn2+.
The X-ray crystallographic structure of E.coli Nfo showed it to be a single domain protein with eight parallel β-strands surrounded by eight peripheral α-helices. Its active site contains a trinuclear Zn center (26). These residues and the flanking sequences are highly conserved in S.pombe Apn1 and other members of the Nfo family.
Cloning of S.pombe Apn2 cDNA
The apn2/xth-like sequence (SPBC3D6.10) was identified in the S.pombe genome database by homology search using human APE1 (10,11,27). Using PCR, we isolated cDNA clones containing the predicted ORF from the Machmaker cDNA library. As in the case of Apn1, we identified a putative intron sequence, based on the genomic sequence, by using primer pairs flanking the intron region. Surprisingly, all (>40) clones were found to contain the intron sequence, which could have been derived from either genomic DNA contamination or unspliced transcripts. It is clear that the intron-containing cDNA does not encode active APE because of the presence of an in-frame stop codon in the intron. We then succeeded in cloning mature cDNA, by using RT–PCR of total RNA, although nearly half of the clones still contained the intron sequence. The Apn2 cDNA contains 1572 bp, corresponding to a polypeptide with 523 amino acid residues and predicted Mr of 58 kDa (Fig. 1).
Schizosaccharomyces pombe Apn2 is a member of the Xth family
Schizosaccharomyces pombe Apn2 shares several conserved and essential residues with E.coli Xth, human APE1 and S.cerevisiae Apn2 (Fig. 2B.) (10,11). The hAPE1 polypeptide with 318 amino acid residues contains two distinct domains, of which the N-terminal domain is dispensable for the endonuclease activity, but is required for its regulatory function (24). This region is absent in yeast Apn2 (Fig. 2B). The C-terminal sequence of APE1, essential for the endonuclease activity, is homologous to Apn2 (24,28).
The C-terminal regions of yeast Apn2/hAPE2-type proteins contain a PCNA-binding motif (29) and a sequence homologous to one present in the C-terminal region of the DNA topoisomerase III (TOP3) family (29,30). The C-terminal region of S.cerevisiae Apn2 is not required for its APE activity in vitro, but is required for in vivo repair of AP sites (12). Unk et al. subsequently showed physical and functional interaction of Apn2 with PCNA, which stimulates the 3′-phophodiesterase activities of Apn2 in vitro (31).
Apn1 and Apn2 are not essential proteins
The apn1 and apn2 mutants of S.pombe showed no obvious phenotype with regard to growth rate or viability, which indicates that both apn1 and apn2 are dispensable under normal conditions, as was also observed for S.cerevisiae (11).
MMS sensitivity of apn mutants
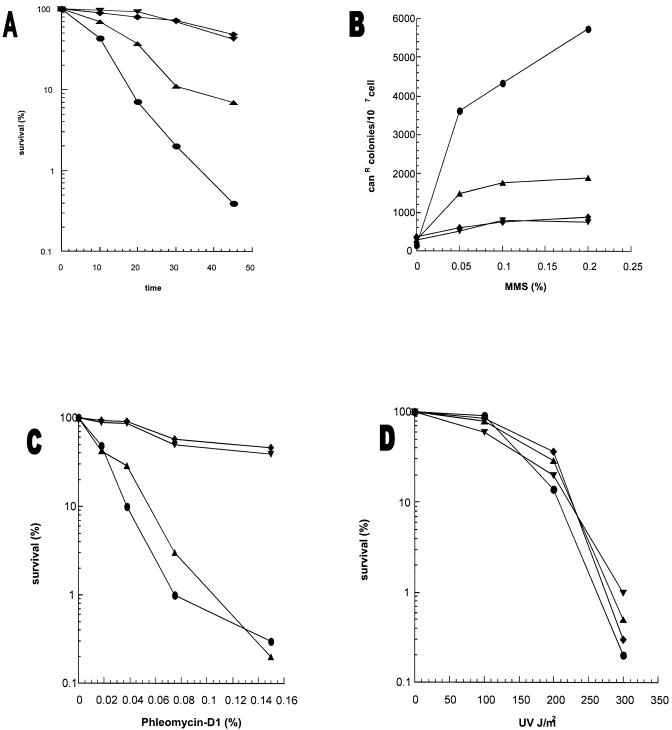
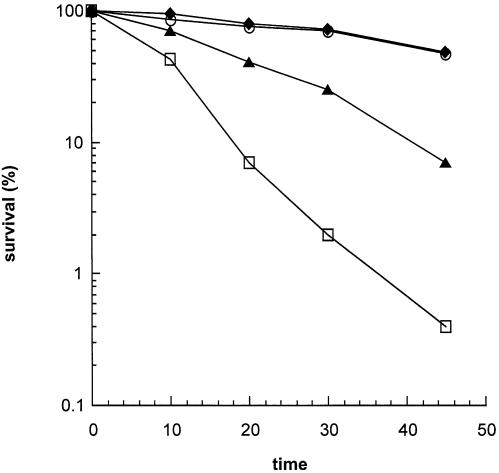
APEs play a central role in repair of methylated bases, e.g. 3-methyladenine and 7-methylguanine which are induced by MMS (32). Excision of the alkyl base adducts with mammalian N-methylpurine-DNA glycosylase, MPG (32), and mag1 in S.pombe (33), should generate AP sites whose repair requires APE. Thus MMS sensitivity of APE mutants is a measure of the in vivo repair activity of APEs. Figure 3A shows that the apn2Δ mutant was moderately more sensitive to MMS than the wild type, while apn1Δ mutation alone had no effect on MMS sensitivity. However, the apn1Δapn2Δ double mutant showed significantly higher sensitivity to MMS than the apn2Δ mutant.
Figure 3.
(A) MMS sensitivity of apn1Δ and apn2Δ strains. Cells grown overnight in YEL medium were treated with 0.2% MMS for various times. (B) MMS-induced mutagenesis at the can1 gene of S.pombe. The details are described in Materials and Methods. (C) Phleomycin D1 toxicity. Cells grown overnight in YEL medium were treated with phleomycin D1 for 1 h before spreading on YEA plates. (D) UV sensitivity of S.pombe apn1Δ and apn2Δ mutant strains. Sensitivity of the strains to UV was measured after exposure to various UV doses. PO29, wild type (diamonds); PO61, apn1Δ (inverted triangles); PO24, apn2Δ (upright triangles); PO60, apn1Δapn2Δ (circles).
These results strongly suggest that Apn2 and not Apn1 plays a rate-limiting role in protecting cells from alkylation damage. However, in the absence of Apn2, Apn1 provides protection and has a minor role in repair of AP sites. Thus there is an unexpected role reversal of Apn1 and Apn2 in S.pombe relative to S.cerevisiae in which Apn2 is completely dispensable in alkylation damage repair in the presence of Apn1 (11).
MMS-induced mutagenesis in the apn1Δ apn2Δ mutant
Because non-instructional AP sites are highly mutagenic, a deficiency in APE leads to enhanced mutation frequency after treatment with genotoxic agents such as MMS (34). We determined the frequency of MMS-induced forward mutation to canavanin resistance (canr1) in apn mutants. Figure 3B shows that the apn1Δ mutant and wild-type strain had a similar mutagenic response, while significant enhancement in frequency of canr1 mutation was observed in the apn2Δ mutant; the frequency was even higher in the apn1Δapn2Δ double mutant. These mutagenesis data, which mirror the survival data, clearly show that the APE activity of Apn2, and to a lesser extent Apn1, is needed to repair AP sites and prevent mutations.
Sensitivity of the apn2Δ mutant to phleomycin D1
We assessed the relative 3′-phosphodiesterase activity of Apn1 and Apn2 in vivo by examining the sensitivity of various S.pombe mutants to phleomycin D1, a glycopeptide antibiotic structurally similar to bleomycin. These antibiotics react with deoxyribose to cleave phosphodiester bonds, and generate 3′-phosphoglycolate, and oxidized AP sites (1). Figure 3C shows that mutation in apn1 had no effect on phleomycin sensitivity, while the apn2Δ mutant or the apn1Δapn2Δ double mutant was ∼3-fold more sensitive to this drug than the wild-type strain. In contrast, apn2Δ mutation did not sensitize S.cerevisiae at all to the antibiotic (10). These results strongly suggest that Apn2 is the major contributor of not only APE but also DNA 3′-phosphodiesterase activity in S.pombe.
Inactivation of an alternative excision repair pathway enhances sensitivity of the apn2Δ mutant to alkylation damage
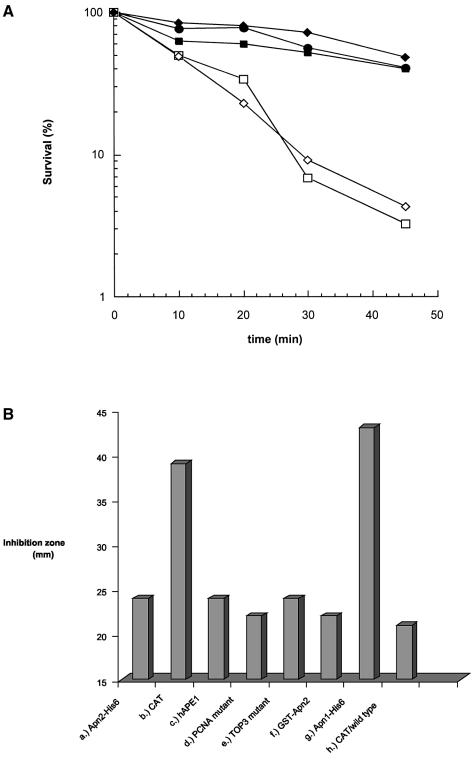
Uve1, expressed in S.pombe but absent in S.cerevisiae, was shown to repair UV photoproducts on the basis of its ability to phenotypically complement UV sensitivity of the E.coli uvrA recA phr mutant (35,36). In addition to its function as a mismatch-, and UV photoproduct-specific endonuclease (18,21,37), Uve1 possesses APE activity (18). We tested the contribution of Uve1 in repair of AP sites by measuring MMS and UV sensitivity of apn and uve1 mutants. The observed lack of UV sensitivity in apn1Δ and apn2Δ mutants was expected because UV damage repair does not involve BER in which Apns play a key role (Fig. 3D). The uve1Δ mutant was shown to be moderately sensitive to UV, which is consistent with its role in UV damage repair (38). On the other hand, mutation in uve1 did not sensitize S.pombe cells to MMS (Fig. 4D). However, this mutation acted like apn1 mutation in enhancing MMS sensitivity of the apn2Δ mutant. This indicates that both Uve1 and Apn1 provide back-up functions in repairing AP sites in the genome.
Figure 4.
Effect of uve1Δ mutation on MMS sensitivity of the apn2Δ mutant. Other details are as in Figure 3. PO29, wild type (diamonds); PO24, apn2Δ (triangles); PO89, uve1Δ (open circles); PO90, apn2Δuve1Δ (open squares).
Phenotypic complementation of the apn1Δapn2Δ mutant with S.pombe Apn2 and human APE1
We tested phenotypic complementation of the apn1Δapn2Δ mutant with S.pombe Apn2 or human APE1, using their expression plasmids in pNMT1 and pESP1 vectors with the thiamine-repressible nmt1 promoter, as described in Materials and Methods. Leaky expression of these proteins was observed in the presence of thiamine (39). In any case, as shown in Figure 5A, both Apn2 and human APE1 restored MMS resistance of the apn1Δapn2Δ mutant to the wild-type level. In contrast, human APE2 or S.pombe Apn1 could not complement apn1 apn2 deficiency (Fig. 5A and B). We conclude from these results that S.pombe Apn2 and human APE1 have a similar range of activity in repairing AP sites, and hence human APE1 could fully complement Apn2 deficiency.
Figure 5.
(A) Complementation of MMS sensitivity of the apn1Δapn2Δ (PO60) strain with wild-type S.pombe Apn2-His6 (pNBR110) (filled squares); human APE1 (pRBR113) (filled circles); and human APE2 (pNBR103) (open squares); wild-type control, leu1-32 (PO4), with control CAT expression vector (pNMT1-CAT; Invitrogen) (filled diamonds); sensitivity of apn1Δapn2Δ: (PO60) transformed with pNMT1-CAT (open diamonds). Other details are described in Materials and Methods. (B) Agar diffusion assay of complementation of apn1Δapn2Δ (PO60) and wild-type (PO4) strains as described in Materials and Methods. Data represent results from an average of three or more experiments. (a) Wild-type Apn2-His6 on plasmid pNBR110 in PO60; (b) control pNMT1-CAT plasmid in PO60; (c) human APE1 on plasmid pRBR113 in PO60; (d) Apn2-A402A403 mutation (PCNA) on plasmid pNBR114 in PO60; (e) Apn2-A456A457A458 mutation on plasmid pNBR115 in PO60; (f) wild-type GST–Apn2 on plasmid pEBR116 in PO60; (g) wild-type Apn1-His6 on plasmid pNBR117 in PO60; (h) pNMT1-CAT plasmid in PO4
PCNA-binding and TOP3-homologous region in Apn2 are dispensable for in vivo repair of alkylation damage
The C-terminal region in S.cerevisiae Apn2 contains a PCNA-binding motif which was shown to be required for its binding to PCNA. Such binding activates the 3′-phosphodiesterase activity of Apn2 (31). Because S.pombe and S.cerevisiae Apn2 have extensive sequence homology, including the PCNA-binding and TOP3-like motifs, we tested the role of these motifs in MMS resistance of S.pombe. We generated alanine substitution mutants at positions 402 and 403 in the PCNA-binding motif, and at positions 456–458 included in the TOP3-like motif in the Apn2 expression plasmid. These mutants were as effective in phenotypic complementation of the apn1Δapn2Δ mutant as wild-type Apn2 (Fig. 5B). Thus, even if the binding of PCNA to Apn2 occurs in vivo, this interaction, unlike in the case of S.cerevisiae, does not modulate Apn2 activity in S.pombe.
APE activity of recombinant Apn2
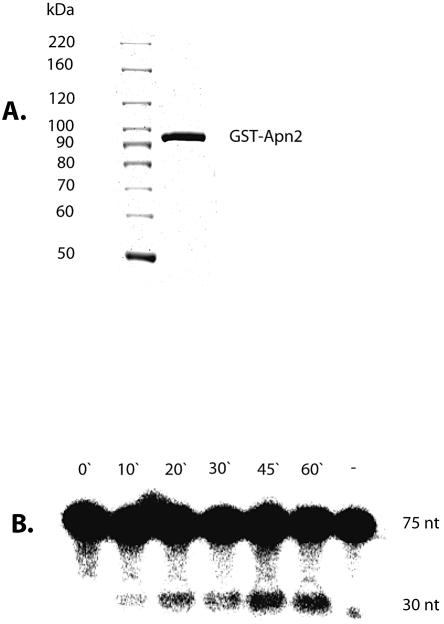
The in vivo studies described so far strongly suggest that Apn2 is active in vivo as an APE and a 3′-phosphodiesterase. We then directly tested recombinant Apn2 for APE activity. We attempted to over-express GST–Apn2 and His6-Apn2 fusion polypeptides in the S.pombe apn1Δ apn2Δ mutant. The recombinant Apn2-His6 was found to be mostly insoluble (data not shown). Furthermore, overexpression of the GST–Apn2 fusion protein appeared to be toxic because the transformed S.pombe stopped growing and became elongated (data not shown). Nevertheless, we were able to purify the GST–Apn2 fusion polypeptide in soluble form by affinity chromatography from extracts of these cells early after transformation. GST–Apn2 showed significant APE activity with THF-containing DNA oligo as described in Materials and Methods. The formation of the predicted 30 nt product indicated that Apn2 catalyzed hydrolysis of DNA 5′ to the abasic site linearly for up to 1 h (Fig. 6B). There are several possible reasons for very low specific activity of the Apn2 fusion protein. One obvious possibility is that even though present in a soluble form, a large fraction of the protein is not correctly folded, and the folding problem was exacerbated by the presence of the GST.
Figure 6.
(A) Purification of GST–Apn2. Eluted GST–Apn2 fusion protein was analyzed on a 7.5% denaturing polyacrylamide gel and stained with Coomassie blue. Lane 1, molecular weight standards; lane 2, 3 µg of eluted GST–Apn2 protein. (B) Kinetics of APE activity of GST–Apn2; lane (–) is the no-protein control. The details are described in Materials and Methods.
AP-endonuclease activity in S.pombe extracts
An earlier report raised the unlikely possibility that APE is absent in S.pombe in spite of the presence of apn genes (17). We examined cell-free extracts of wild-type and mutant S.pombe for APE activity. The APE activity was easily detectable in the wild-type cell extract which was stable at 37°C, and maintained linearity up to 20 min to generate the expected cleavage product (Fig. 7). As expected, the total APE activity was significantly decreased in the apn1Δapn2Δ mutant, and the residual activity could be due to Uve1 or other unidentified APEs.
DISCUSSION
Although both S.cerevisiae and S.pombe have been extensively used as model eukaryotes for elucidating various cellular processes, S.pombe, in spite of its closer similarity to human cells, has not been extensively investigated for DNA repair processes. Among various excision repair pathways, BER is involved in repair of endogenous oxidation and alkylation damage in which APE plays a central role. While two APEs in S.cerevisiae, Apn1 and Apn2, have been extensively characterized, much less is known about the Apns in S.pombe. One major problem in examining S.pombe apn genes is the presence of small introns and low efficiency of the cells to splice out these introns, especially in the case of apn2.
Ramotar et al. (17) predicted, but did not show experimentally, that the S.pombe apn1 gene contains a 72 bp intron. We confirmed the absence of this intron in cloned Apn1 cDNA. The Apn1 of S.pombe shares 46% identical residues with S.cerevisiae Apn1 which accounts for >90% of the APE/3′-diesterase activity in this yeast (11). This is consistent with the observed increase of spontaneous mutations as well as MMS sensitivity when the APN1 gene was inactivated in the budding yeast. In contrast, deletion of the apn1 gene in S.pombe did not affect its MMS sensitivity. Thus it appears that Apn1 is not the major contributor of APE activity. In our attempts to clone the S.pombe Apn2 cDNA, we discovered that due to inefficient splicing, the cDNA library contained mostly copies of Apn2 primary transcripts. This suggests that splicing plays a regulatory role in maintaining the Apn2 protein level. Similar regulation at the splicing level was observed for the mes1 gene in S.pombe whose product is developmentally regulated (40).
Deletion of apn1 and apn2 genes in S.pombe did not affect its growth and viability. Deletion of the apn2 gene in S.pombe caused MMS sensitivity, in contrast to a lack of phenotype of APN2 mutant in S.cerevisiae. Although apn1Δ mutation alone did not confer sensitivity to MMS in S.pombe, it enhanced MMS sensitivity of the apn2Δ mutant. These results strongly argue for the primary role of Apn2 in the removal of AP sites, and indicate that Apn1 provides the back-up activity in the absence of Apn2 in the fission yeast. An analogous situation was observed in E.coli in which nfo mutants do not show increased sensitivity to MMS, unlike the xth mutant. Xth is the major APE in E.coli and Nfo appears to provide the back-up activity. Accordingly, the nfo/xth double mutant is more sensitive to alkylating and oxidizing agents than the xth mutant (41). As expected, inactivation of apn1 and apn2 did not sensitize the cells to UV (254 nm, 300 J/m2) because the BER pathway is not involved in repair of UV-induced pyrimidine photoproducts.
Structure modeling shows that Uve1 and E.coli Nfo have similar secondary structure (42). Even though in vitro studies have indicated that Uve1 generates DNA strand breaks at AP sites (18,43), its function in BER was unknown. The present studies provide the first evidence for the ability of Uve1 to repair AP sites in vivo. As in the case Apn1, inactivation of Uve1 alone did not confer sensitivity to MMS. However, deletion of the uve1 gene in the apn2Δ mutant significantly increased MMS sensitivity, suggesting that Uve1 functions independently of Apn2 in removal of AP sites to enhance cell survival. A low level of APE activity was detected in the extract of a apn1Δapn2Δuve1Δ triple mutant, suggesting the presence of other unidentified APEs in S.pombe (data not shown). Interestingly, the apn1Δapn2Δuve1Δ triple mutant was no more sensitive to MMS than the apn2Δuve1Δ double mutant (unpublished observation). This suggests a common downstream pathway in repair of AP sites initiated by Uve1 and Apn1, which is distinct from the Apn2-initiated process. In any case, Apn2 appears to be the predominant APE in S.pombe while both Uve1 and Apn1 provide the back-up functions in BER. However, it should be noted that it was recently shown that the apn1Δapn2Δ double mutant of S.cerevisiae is viable but not in the absence of Rad1/Rad10. This suggests alternative repair of AP sites involving this nucleotide excision repair-specific endonuclease (44). An analogous situation may be present in S.pombe.
An earlier attempt to demonstrate APE activity in S.pombe extracts was unsuccessful (17). Because the present studies predict the presence of APE activity in Apn polypeptides, we have shown directly that the recombinant Apn2 is an authentic APE. We were also able to measure APE activity in S.pombe extracts, in contrast to an earlier report (17). Although the reason for the discrepancy between the previous study and our experiments is not clear, it could be due to a difference in extract preparation. While we detected the APE activity in extract produced in a French press, the extract prepared with beating beads was inactive (data not shown). It is likely that Apns are easily inactivated during preparation. More importantly, a significant amount of APE activity in the apn1Δ apn2Δ double mutant could be due to Uve1, and other unknown APEs. Thus our results provide the first evidence for APE-dependent BER in the fission yeast, in line with other organisms.
The E.coli Xth family of APEs, including yeast Apn2 and human APE1, share critical active site motifs. A newly discovered human APE, hAPE2, shares significant sequence homology with yeast Apn2 (30). Although the recombinant hAPE2 was reported to have very weak APE activity (30), we were unable to detect such activity in the recombinant protein purified from insect cells (unpublished experiment). We examined the in vivo activity of hAPE1 and hAPE2 in a phenotypic complementation assay using the S.pombe apn1Δapn2Δ mutant. Expression of hAPE1 in the mutant was indicated by enhanced APE activity in cell-free extract (data not shown), and increased MMS resistance of the mutant. On the other hand, no complementation was observed when hAPE2 was expressed in this mutant strain (Fig. 5A). Thus, while hAPE2 may have some unknown enzymatic activity, it does not appear to function as an APE in vivo. The reason for the lack of complementation of the apn1Δ apn2Δ mutant with S.pombe Apn1 is not obvious. However, it is possible that Apn1 does not possess the same level of distinct activities of Apn2 or hAPE1 needed for repair of MMS-induced damage. Furthermore, the low sensitivity of the growth inhibition assay may mask the small extent of complementation with Apn1.
The C-terminal regions in proteins of the APE2 family contain a consensus sequence for a PCNA-binding motif that was shown to be functional in human APE2 (29). Unk et al. (12) reported that the C-terminal region of Apn2 of S.cerevisiae is dispensable for its APE activity in vitro, but not for in vivo suppression of MMS sensitivity of the apn1Δapn2Δ mutant. In contrast, we found in S.pombe that mutation in the putative PCNA-binding and TOP3-type motifs of Apn2 did not affect MMS resistance. These results suggest that the PCNA-binding and TOP3-type motifs of Apn2 in S.pombe are not directly involved in the repair of abasic sites in vivo.
Phleomycin D1 produces oxidized AP sites and strand breaks with 3′-phosphoglycolate termini which prevent DNA replication or repair by DNA polymerases (1). In S.cerevisiae, the apn1Δapn2Δ double mutant, but not the single mutants, displays enhanced sensitivity to phleomycin D1, indicating that these enzymes functionally overlap in repair of oxidized AP sites and in the repair of strand breaks with 3′-terminal lesions (10). We have shown here that Apn2 contributes significantly to repair of phleomycin D1-induced DNA damage in S.pombe, based on enhanced sensitivity of the apn2 mutant to the drug (Fig. 4). The sensitivity did not increase further when cells were also deficient in apn1. These results and identification of 3′-phospodiesterase and 3′–5′ exonuclease activities in S.cerevisiae Apn2 strongly suggest that S.pombe Apn2 possesses both of these activities.
In summary, the present study provides the first evidence for the presence of active APEs in S.pombe, and for distinct functions of these APEs in S.pombe and S.cerevisiae. In the fission yeast, as in mammals but unlike in S.cerevisiae, Apn2, an APE1 ortholog, is the major APE/3′-phosphodiesterase for repairing DNA damage caused by oxidation and alkylation. Furthermore, at least three APE genes, apn1, apn2 and uve1, are functional in vivo. Whether expression of these genes is regulated by ROS, and how the proteins interact with other BER components needs to be addressed in order to elucidate the BER process in the fission yeast.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr D. Konkel for careful editing of the manuscript, Drs V. Bashkirov, P. Doetsch, W. Heyer and M. Sipiczki for S.pombe strains, and Drs. S. Seki and K. Akiyama for the human APE1 and APE2 cDNA plasmids. This work was supported by USPHS grants R01 CA53791, ES08457 and P50 ES06676. B.R. is grateful to the Soros Foundation for a Fellowship award and to the Hungarian Science and Technology Foundation, for a travel grant.
REFERENCES
- 1.Breen A.P. and Murphy,J.A. (1995) Reactions of oxyl radicals with DNA. Free Radical Biol. Med., 18, 1033–1077. [DOI] [PubMed] [Google Scholar]
- 2.Mitra S., Hazra,T.K., Roy,R., Ikeda,S., Biswas,T., Lock,J., Boldogh,I. and Izumi,T. (1997) Complexities of DNA base excision repair in mammalian cells. Mol. Cell, 7, 305–312. [PubMed] [Google Scholar]
- 3.Haracska L., Unk,I., Johnson,R.E., Johansson,E., Burgers,P.M.J., Prakash,S. and Prakash,L. (2001) Roles of yeast DNA polymerases δ and ζ and of Rev1 in the bypass of abasic sites. Genes Dev., 15, 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature, 362, 709–715. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura J. and Swenberg,J.A. (1999) Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res., 59, 2522–2536. [PubMed] [Google Scholar]
- 6.Lindahl T., Barnes,D.E., Klungland,A., Mackenney,V.J. and Schar,P. (1997) Repair and processing events at DNA ends. Ciba Found. Symp. 211, 198–205. [DOI] [PubMed] [Google Scholar]
- 7.Mitra S., Izumi,T., Boldogh,I., Bhakat,K.K., Hill,J.W. and Hazra,T.K. (2002) Choreography of oxidative damage repair in mammalian genomes. Free Radical Biol. Med., 33, 15–28. [DOI] [PubMed] [Google Scholar]
- 8.Demple B. and Harrison,L. (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem., 63, 915–948. [DOI] [PubMed] [Google Scholar]
- 9.Popoff S.C., Spira,A.I., Johnson,A.W. and Demple,B. (1990) Yeast structural genes (APN1) for the major apurinic endonuclease: homology to Escherichia coli endonuclease IV. Proc. Natl Acad. Sci. USA, 87, 4193–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett R.A. (1999) The Saccharomyces cerevisiae ETH1 gene, an inducible homolog of exonuclease III that provides resistance to DNA-damaging agents and limits spontaneous mutagenesis. Mol. Cell. Biol., 19, 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson R.E., Torres-Ramos,C.A., Izumi,T., Mitra,S., Prakash,S. and Prakash,L. (1998) Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1 and its role in the repair of abasic sites. Genes Dev., 12, 3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unk I., Haracska,L., Johnson,R.E., Prakash,S. and Prakash,L. (2000) Apurinic endonuclease activity of yeast Apn2 protein. J. Biol. Chem., 275, 22427–22434. [DOI] [PubMed] [Google Scholar]
- 13.Unk I., Haracska,L., Prakash,S. and Prakash,L. (2001) 3′-Phosphodiesterase and 3′→5′ exonulcease activities of yeast Apn2 protein and requirement of these activities for repair of oxidative DNA damage. Mol. Cell. Biol., 21, 1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramotar D., Popoff,S.C., Gralla,E.B. and Demple,B. (1991) Cellular role of yeast Apn1 apurinic endonuclease/3′-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol. Cell. Biol., 11, 4537–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsburg S.L. (1999) The best yeast? Trends Genet., 15, 340–344. [DOI] [PubMed] [Google Scholar]
- 16.Sipiczki M. (2000) Where does fission yeast sit on the tree of life? Genome Biol., 1, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramotar D., Vadnais,J., Masson,J.Y. and Tremblay,S. (1998) Schizosaccharomyces pombe apn1 encodes a homologue of the Escherichia coli endonuclease IV family of DNA repair proteins. Biochim. Biophys. Acta, 1396, 15–20. [DOI] [PubMed] [Google Scholar]
- 18.Kanno S., Iwai,S., Takao,M. and Yasui,A. (1999) Repair of apurinic/apyrimidinic sites by UV damage endonuclease; a repair protein for UV and oxidative damage. Nucleic Acids Res., 27, 3096–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfa C., Fantes,P., Hyams,J., Mcleod,M. and Warbrick,E. (1993) Experiments with Fission Yeast. A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 20.Manivasakam P., Weber,S.C., McElver,J. and Schiestl,R.H. (1995) Micro-homology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res., 23, 2799–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur B., Fraser,J.L., Freyer,G.A., Davey,S. and Doetsch,P.W. (1999) A Uve1p-mediated mismatch repair pathway in Schizosaccharomyces pombe. Mol. Cell. Biol., 19, 4703–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maris A.F., Kern,A.L., Picada,J.N., Boccardi,F., Brendel,M. and Henriques,J.A. (2000) Glutathione, but not transcription factor Yap1, is required for carbon source-dependent resistance to oxidative stress in Saccharomyces cerevisiae. Curr. Genet., 37, 175–182. [DOI] [PubMed] [Google Scholar]
- 23.Ribar B., Grallert,A., Olah,E. and Szallasi,Z. (1999) Deletion of the sep1(+) forkhead transcription factor homologue is not lethal but causes hyphal growth in Schizosaccharomyces pombe.Biochem. Biophys. Res. Commun., 263, 465–474. [DOI] [PubMed] [Google Scholar]
- 24.Izumi T. and Mitra,S. (1998) Deletion analysis of human AP-endonuclease: minimum sequence required for the endonuclease activity. Carcinogenesis, 19, 525–527. [DOI] [PubMed] [Google Scholar]
- 25.Corpet F. (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res., 16, 10881–10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosfield D.J., Guan,Y., Haas,B.J., Cunningham,R.P. and Tainer,J.A. (1999) Structure of the DNA repair enzyme endonuclease IV and its DNA complex: double-nucleotide flipping at abasic sites and three-metal-ion catalysis. Cell, 98, 397–408. [DOI] [PubMed] [Google Scholar]
- 27.Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. [DOI] [PubMed] [Google Scholar]
- 28.Xanthoudakis S., Smeyne,R.J., Wallace,.D. and Curran,T. (1996) The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc. Natl Acad. Sci. USA, 93, 8919–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchimoto D., Sakai,Y., Sakumi,K., Nishioka,K., Sasaki,M., Fujiwara,T. and Nakabeppu,Y. (2001) Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen. Nucleic Acids Res., 29, 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadi M.Z. and Wilson,D.,III (2000) Second human protein with homology to the Escherichia coli abasic endonuclease exonuclease III. Environ. Mol. Mutagen., 36, 312–324. [PubMed] [Google Scholar]
- 31.Unk I., Haracska,L., Gomes,X., Burgers,P.M.J., Prakash,S. and Prakash,L. (2002). Stimulation of 3′–5′ exonuclease and 3′-phosphodiesterase activities of yeast Apn2 by proliferating nuclear antigen. Mol. Cell. Biol., 22, 6480–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy R., Brooks,C. and Mitra,S. (1994) Purification and biochemical characterization of recombinant N-methylpurine-DNA glycosylase of the mouse. Biochemistry, 33, 15131–15140. [DOI] [PubMed] [Google Scholar]
- 33.Memisoglu A. and Samson,L. (1996) Cloning and characterization of a cDNA encoding a 3-methyladenine DNA glycosylase from the fission yeast Schizosaccharomyces pombe. Gene, 177, 229–235. [DOI] [PubMed] [Google Scholar]
- 34.Loeb L.A. and Preston,B.D. (1986) Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet., 20, 201–230. [DOI] [PubMed] [Google Scholar]
- 35.McCready S.J., Osman,F. and Yasui,A. (2000) Repair of UV damage in the fission yeast Schizosaccharomyces pombe.Mutat. Res., 451, 197–210. [DOI] [PubMed] [Google Scholar]
- 36.Takao M., Yonemasu,R., Yamamoto,K. and Yasui,A. (1996) Characterization of a UV endonuclease gene from the fission yeast Schizosaccharomyces pombe and its bacterial homolog. Nucleic Acids Res., 24, 1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowman K.K., Sidik,K., Smith,C.A., Taylor,J.S., Doetsch,P.W. and Freyer,G.A. (1994) A new ATP-independent DNA endonuclease from Schizosaccharomyces pombe that recognizes cyclobutane pyrimidine dimers and 6–4 photoproducts. Nucleic Acids Res., 22, 3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonemasu R., McCready,S.J., Murray,J.M., Osman,F., Takao,M., Yamamoto,K., Lehmann,A.R. and Yasui,A. (1997) Characterization of the alternative excision repair pathway of UV-damaged DNA in Schizosaccharomyces pombe. Nucleic Acids Res., 25, 1553–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsburg S.L. (1993) Comparison of Schizosaccharomyces pombe expression systems Nucleic Acids Res., 21, 2955–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishida M., Nagai,T., Nakaseko,Y. and Shimoda,C. (1994) Meiosis-dependent mRNA splicing of the fission yeast Schizosaccharomyces pombe mes1+ gene. Curr. Genet., 25, 497–503. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham R.P., Saporito,S.M., Spitzer,S.G. and Weiss,B. (1986) Endonuclease IV (nfo) mutant of Escherichia coli. J. Bacteriol., 168, 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aravind L., Walker,D.R. and Koonin,E.V. (1999) Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res., 27, 1223–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avery A.M., Kaur,B., Taylor,J.S., Mello,J.A., Essigmann,J.M. and Doetsch,P.W. (1999) Substrate specificity of ultraviolet DNA endonuclease (UVDE/Uve1p) from Schizosaccharomyces pombe. Nucleic Acids Res., 27, 2256–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillet M. and Boiteux,S. (2002) Endogenous DNA abasic sites cause cell death in the absence of Apn1, Apn2 and Rad1/Rad10 in Saccharomyces cerevisiae. EMBO J., 21, 2833–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izumi T., Malecki,J., Chaudhry,M.A., Weinfeld,M., Hill,J.H., Lee,J.C. and Mitra,S. (1999) Intragenic suppression of an active site mutation in the human apurinic/apyrimidinic endonuclease. J. Mol. Biol., 287, 47–57. [DOI] [PubMed] [Google Scholar]