Abstract
The traditional path of drug development passes from in vitro screening and response assessment to validation of drug efficacy in cell line xenografts. While xenografts have their merits, historically, more often than not, they have not served as an accurate predictor of drug efficacy in humans. The refinement and increased availability of genetically engineered mouse models (GEMMs) of cancer has made GEMMs an attractive avenue for the preclinical testing of therapeutic agents. The histopathologic and genetic resemblance of GEMMs to human cancer are an important measure to evaluate their suitability for pre-clinical studies and a number of studies using kinase inhibitors have now been performed in GEMMs. We have highlighted several of the salient advantages and challenges associated with GEMM studies. Well-characterized GEM models of human cancer should aide in the prioritization of both established and novel therapeutics.
Keywords: Genetically engineered mouse models, Drug development, Oncomouse, Attrition, Preclinical models, Kinase inhibitor
1 Introduction
Although a cancer researcher might become optimistic in these heady days of rationally targeted anti-cancer therapies, three trends remain a cause for concern (reviewed in Kola and Landis 2004; Peterson and Houghton 2004; Sharpless and Depinho 2006). First, the number of registered pharmaceutical new chemical entities (NCEs) is not growing rapidly, but instead is leveling off or even declining over the last decade. As registration of an NCE is the first real stage of drug development, this observation suggests that novel anti-cancer drugs are still very difficult and expensive to produce. This fact reflects the second concerning trend: the failure rate of oncologic drugs that enter human testing is still very high, perhaps greater than 95%. This observation in turn reflects the third worrisome trend: by a variety of metrics, most novel anti-cancer therapies fail in human testing because of the lack of efficacy. For example, the attrition rate of compounds at the Phase II stage may be over 70%, and the response rate of phase I trials remains low compared to the incidence of toxicity in such studies (Decoster et al. 1990; Roberts et al. 2004). In aggregate, these trends indicate that oncologic drugs remain very hard to develop, largely because the rare successes have to subsidize the testing of ineffective compounds in humans, and that these problems will be with us for much time. Clearly, one reason for the high attrition rate of anti-cancer compounds is the lack of predictive preclinical models for drug efficacy testing prior to human use. Several approaches have been suggested to prioritize would-be therapeutics, and in this chapter, we will focus on efforts to improve drug efficacy testing in mice using human cancer xenografts and genetically engineered murine models (GEMMs).
2 Classical Preclinical Testing in Xenograft Models
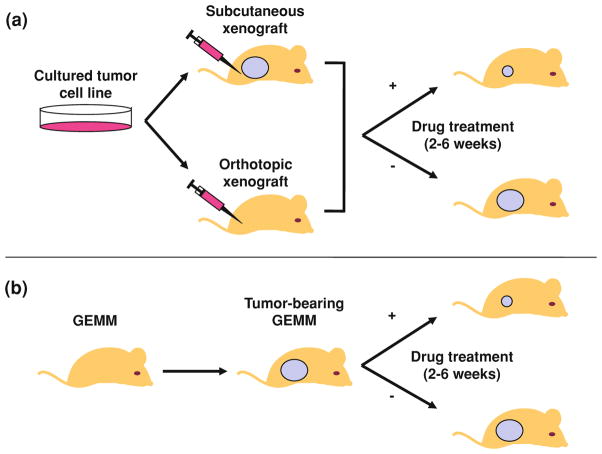
As described in the previous chapter, drug development usually follows a defined course. Potential cancer therapies are discovered through in vitro screens or more recently by targeted drug design. Such compounds are then tested for activity in vitro using a variety of cancer cell lines, and if sufficiently promising, then tested in vivo. In vivo testing typically consists of several parts including an analysis of pharmacology, pharmacodynamics and toxicology. For several decades, in vivo efficacy testing has largely been done in one way: through the use of xenograft models. In these models, cell lines are grown, generally subcutaneously, in immunodeficient (e.g. SCID) mice. Typically, 106 malignant cells are injected subcutaneously into the flank, a brief wait occurs, and then therapy with the potential anti-neoplastic is begun at the first sign of tumor growth, or even before a tumor appears. An agent is usually considered “active” in these assays if it slows tumor development (i.e. decreases progression) (Fig. 1a).
Fig. 1.
Generation of mice bearing xenograft or GEMM tumors. a Xenograft tumors are generated by bolus injections of human cell lines derived from cell cultures. The cells are amplified to sufficient numbers in vitro and then injected subcutaneously, (or far more rarely orthotopically) and allowed to develop tumors. Drug treatment can be initiated prior to tumor formation or in the setting of a measurable tumor. Subcutaneous xenograft tumor growth is typically assessed by bidimensional tumor measurements using calipers. b GEMM tumors occur spontaneously or after conditional oncogene activation or tumor suppressor gene inactivation. Depending on the GEM model and site of tumor development, mice are screened for tumor formation by palpation (e.g. melanoma or breast GEMMs) or radiographically (e.g. MRI or microCT for lung tumors). Drug treatment is usually initiated after tumor formation with the intent of shrinking a measurable tumor
Xenograft models offer a number of advantages, including their ease of use, public availability, and relatively modest infrastructural needs (e.g. long-term animal housing, imaging, etc). Assuredly, many important studies leading to changes in clinical care have been done using representative samples of human cancers grown as xenografts (see for example Peterson and Houghton 2004; Sausville and Burger 2006). Moreover, in some instances, e.g. certain rare cancers, no other small animal model for such cancers exists, and their use in these cases is undoubtedly preferable to the alternative of none in vivo efficacy testing.
Although almost every FDA-approved, anti-cancer agent developed in the modern era has shown activity in some xenograft system, these systems have a mixed record of predicting efficacy in humans (Johnson et al. 2001; Voskoglou-Nomikos et al. 2003). It is clear that many ineffective compounds are passing the xenograft filter and then later failing, at great expense, in human phase II trials. Of equal concern, however, is the possibility that potentially active agents are not making it to human testing because of a lack of efficacy in these systems. We contend that the predictive value of these assays would be sharply improved with three relatively straightforward modifications to standard xenograft testing:
Determining a compound’s activity in several cell lines of a given tumor type rather than one or two examples, usually chosen in a non-random way.
Considering tumor regression or prolonged stabilization, rather than mere non-progression (or tumor growth inhibition “TGI”), as success in these assays.
Assuring that drug treatments are given with pharmacologically realistic doses and schedules with regard to human use of the same agent.
Furthermore, the use of orthotopically transplanted xenograft tumors (e.g. Kerbel et al. 1991; Mitsiades et al. 2003; Rofstad 1994) or uncultured, patientspecific ‘tumorgrafts’ (Garber 2009) represent meaningful advances over traditional xenografting of established human cancer cell lines.
Although these changes seem straightforward, the current literature suggests that much academic xenograft testing fails to meet even these modest standards. When carefully done, drug efficacy testing in xenograft models has clear merit and will continue to play an important role for years to come. However, the limited predictive power of these models suggests that drug development decisions should not be based solely on drug performance in these models and instead be complemented by drug efficacy testing in other disease-relevant animal models. In the following section, we will suggest that GEMMs could fill this important void in the current drug development paradigm.
3 Harnessing Genetically Engineered Murine Models for Drug Efficacy Testing
The design and production of GEMMs have been described at length elsewhere (Frese and Tuveson 2007; Jonkers and Berns 2002). In brief, transgenic and knockout alleles are combined to produce strains of mice that are predisposed to autochthonous tumors. Generally, such models rely on the use of transgenic alleles directing the expression of oncogenic “driver” proteins in the tissue of interest. For example, expression of mutant K-Ras can be induced in the adult murine pancreas or lung to cause pancreatic adenocarcinomas (Bardeesy et al. 2006; Hingorani et al. 2005) or non-small cell lung cancer (Johnson et al. 2001; Meuwissen et al. 2001) respectively. To shorten tumor latency, such systems often also include deletion of relevant tumor suppressor genes (e.g. p16Ink4a, p53, Pten) in the same tissues (see for example Ji et al. 2007). Importantly, the use of modern CRE-Lox and doxycycline-inducible technologies allows for the somatic and tissue-specific induction of these genetic lesions, thereby providing an opportunity to direct almost any combination of oncogene and tumor suppressor mutations to the presumed cell of origin of the cancer type under investigation.
Compared to their widespread use in the study of human cancer biology, the use of GEMMs for the development of novel anti-cancer therapeutics, such as kinase inhibitors, is far less established. In this latter scenario, genetically predisposed animals are observed for tumor development, and therapy is usually begun after advanced tumors develop. An agent is considered ‘active’ if it causes significant tumor regression, or at the very least, prolonged stable disease (Fig. 1b). Tumors arising in GEMMs can also be used to derive a large number of genetically related cell lines which can help to better define the activity of agents that have already shown at least some activity in xenograft assays. For example, inhibitors of poly-ADP-Ribosylase (PARP) demonstrated considerable activity in combination with cytotoxics in a variety of xenograft assays (see e.g. Donawho et al. 2007), but GEMM studies from Jonkers and colleagues were instrumental in convincingly defining the potent single-agent activity of these compounds specifically in BRCA1/2-deficient, as opposed to proficient, cancers (Evers et al. 2008; Rottenberg et al. 2008). This specific efficacy of PARP inhibitors in BRCA1/2- deficient tumors, which has been convincingly confirmed in human patients (Fong et al. 2009), was not evident solely from the xenograft testing of these compounds.
GEMMs differ from xenografts in several important aspects that may be very relevant for drug efficacy testing (Table 1). First, GEMM tumors occur in animals with an intact immune system and unperturbed DNA repair mechanisms. Second, as GEMMs rely on the accumulation of stochastic genetic events for tumor progression, these models follow a stepwise progression and recapitulation of the tumor heterogeneity of human solid tumors. Third, as cancers occur autochthonously in these models, GEMMs afford a more faithful recapitulation of tumor–stroma interactions found in human cancer. Which of these differences between GEMMs and xenografts is most relevant for the evaluation of any particular new agent is currently unclear, but a number of recent observations clearly point toward an important role of the tumor microenvironment in the initiation, progression and maintenance of tumors. Chronic inflammation, for example, is a risk factor for certain cancers, and once tumors have formed, the interaction between neoplastic cells with various components of the surrounding microenvironment including inflammatory cells, vascular and lymphatic networks, and the extracellular matrix can either promote or inhibit tumor growth (reviewed in Mueller and Fusenig 2004). It is not hard to see why xenograft models might not accurately recapitulate this aspect of cancer biology: fully transformed cells—often selected for their propensity to grow as rapidly as possible after transplantation—are injected as a bolus into a stroma that is deranged and disorganized by the ectopic transplant of a large number of foreign cells. In contrast, the neoplastic cells of GEM tumors form in a stepwise manner similar to in situ human tumors in the setting of an appropriately responsive tumor microenvironment. Such differences between tumor development in the xenograft versus autochthonous setting have been suggested to explain the marked differences observed in the role of hypoxia and Id proteins in tumor angiogenesis in the two settings (Ruzinova et al. 2003; Sikder et al. 2003). Likewise, recent work from Pollard and colleagues has suggested differences in the gene expression of tumor-associated macrophages (TAMs) in xenograft versus GEM models (Ojalvo et al. 2009). We expect these differences to be of significance as TAMs have been suggested to augment tumor angiogenesis, invasion and matrix remodeling; modulate drug delivery, and modulate a host anti-tumor immune response. Given these multiple differences in the reaction of diverse stromal elements, the ability to more faithfully model the stroma–tumor interaction appears to be a particular strength of GEMMs compared to xenograft assays.
Table 1.
Comparison of xenograft models and genetically engineered mouse models (GEMMS)
| Xenograft | GEMM | |
|---|---|---|
| Functional immune system | No | Yes |
| Intact DNA repair mechanisms | No | Yes |
| Stochastic genetic events during tumor progression | No | Yes |
| Faithful tumor stromal interactions | No | Yes |
| Variability of tumor penetrance | No | Yes |
| Tumor latency | Short | Long |
| Ease of tumor response assessment | Easy | Difficult (imaging) |
| Infrastructure needed | Small | Large |
| Differences in human and mouse genomes | N/A | Yes |
4 Comparing the Track Record of Xenograft and GEM Models
While many arguments can be made for and against the utility of xenografts versus GEMMs for in vivo drug efficacy testing, the most relevant question ultimately is which model better predicts the clinical efficacy of anti-cancer therapeutics in humans. There currently is not a large body of drug development experience using both xenograft and GEMM approaches to allow a rigorous comparison between both approaches. However, a few examples do exist.
One of the oldest examples involves the use of the peroxisome proliferator-activated receptor γ (PPARγ) agonists for the treatment of colorectal cancer. Previous work had shown that PPARγ agonists could promote the differentiation and cell cycle arrest of primary cells derived from human liposarcomas (Demetri et al. 1999). Because PPARγ was noted to be highly expressed in colonic epithelium as well as the wealth of epidemiologic data connecting dietary fat intake with colorectal cancer risk, two separate groups evaluated the effect of PPARγ agonists on colorectal cancer, using different systems, publishing starkly contrasting results. PPARγ agonist treatment of subcutaneous xenografts derived from established colorectal cancer cell lines appeared to slow their growth rate and promote their differentiation (Sarraf et al. 1998). In contrast, PPARγ agonist treatment of mice genetically engineered to develop intestinal polyps secondary to germ line deletion of a single copy of the Apc gene (Min mice) resulted in tumorigenic acceleration, causing an increased number of colonic polyps (Saez et al. 1998). The xenograft studies led to a single institution, phase II clinical trial evaluating the effectiveness of an FDA-approved PPARγ agonists, troglitazone, in patients with metastatic colorectal cancer. Of the 25 patients enrolled, there were no objective responses noted and the median progression-free survival (PFS) was a mere 1.6 months (Kulke et al. 2002), which compares poorly even to historical PFS noted with best supportive care.
A second example of discrepant results of drug efficacy testing between xenografts and GEMMs comes from the experience of testing anti-angiogenic agents such as endostatin and angiostatin. These agents demonstrated impressive activity in xenograft studies (O’Reilly et al. 1997; O’Reilly et al. 1996; O’Reilly et al. 1994) compared to a much more limited activity in GEMMs (Bergers et al. 1999). These discrepancies may be explained by the described differences in angiogenesis between xenograft and autochthonous tumor models (reviewed in Alani et al. 2004); but in any event, the results in GEMMs were more consistent with the limited anti-tumor activity of these agents in clinical trials in humans.
A more recent example is the comparison of gemcitabine efficacy in autochthonous versus transplanted pancreatic tumors (Olive et al. 2009). Pancreatic cancer GEMMs, like human pancreatic cancers, are generally resistant to gemcitabine chemotherapy (objective response rates of 5–10%). This chemoresistance however, does not appear to be cell-autonomous as transplantation of cell lines derived from pancreatic cancer GEMMs into a syngeneic flank increases the sensitivity of these tumors to gemcitabine to that of cultured human cell line xenografts. Indeed, the relative chemoresistance of autochthonous tumors appears to be a reflection of suboptimal drug delivery into fibrotic and poorly vascularized pancreatic GEM tumors, a characteristic that accurately reflects its human counterpart and speaks to the importance of the interactions between tumor cells and stroma discussed in our previous section. Our group has measured intra tumoral drug levels in GEMM versus xenograft models, and seen large and unanticipated differences in drug exposure between the two types of systems (NES, WYK and William Zamboni, submitted). Ongoing efforts will attempt to further determine the physiologic bases for these differences between GEMMs and xenografts with regard to intra-tumor drug delivery.
To more systematically address the utility of GEMMs for drug development, Johnson and colleagues recently examined this issue in KRas mutant lung and pancreatic cancers (Singh et al. 2010), examining the effects of combining cytotoxic chemotherapy with agents inhibiting epidermal growth factor receptor (EGFR) and vascular endothalial growth factor receptor (VEGFR) in highly faithful GEMMs. Comparison of data from large phase III randomized trials in humans with the results seen in lung and pancreatic cancer GEMMs treated similarly suggests many similarities. For example, the combination of EGFR inhibition and chemotherapy worsens survival of both mice and humans with KRas mutant NSCLC. Importantly, some discrepancies were noted as well. For example, combined gemcitabine and VEGF inhibition in KRas mutant pancreatic cancer prolonged survival in GEM models of pancreatic cancer, while this approach has not been of benefit in human patients with pancreatic cancer. This particular discrepancy appeared to result from heterogeneity among the GEMM tumors, with a subset benefitting from angiogenesis inhibition, and another subset displaying behavior more similar to the human disease. This heterogeneity may provide an opportunity to better understand the efficacy of anti-VEGF agents in human tumors. Such discrepancies were modest, however, and the authors concluded that for these models, GEMMs performed well in predicting human Phase III results.
5 Refining Kinase Inhibitor Therapy in GEMMs
There are several examples demonstrating the utility of GEMMs for the developmentof kinase inhibitors and related molecules (e.g. rapamycin) for solidtumors. Even when these results only confirm established findings in cancerpatients (e.g. the demonstration of efficacy of anti-EGFR agents in EGFR-mutantlung cancer), the tractability of GEM systems compared to human testing is ofsignificant value as these systems can be used to rapidly test novel combinations oftherapeutics and model secondary resistance in ways not possible in human clinical trials. A few particularly instructive examples are:
1. EGFR mutant lung cancer in GEMMs
As discussed in detail in “EGFR Mutant Lung Cancer”, most patients with non-small cell lung cancer do not respond to therapeutic inhibition of the EGFR. However, a subset of patients experience rapid and dramatic responses (Shepherd et al. 2005). This sensitivity has subsequently been shown to correlate with somatic activating mutations in the EGFR kinase domain (Lynch et al. 2004; Paez et al. 2004; Pao et al. 2004). In mice, transgenic expression of tetracycline inducible alleles of either of the two most common EGFR activating mutations (the point mutant, L858R, in exon 21 or deletion of a four amino acid sequence LREA in exon 19) is sufficient for the development of lung adenocarcinoma with brochioloalveolar features (BAC) (Ji et al. 2006a; Politi et al. 2006). Furthermore, confirming the effects seen in humans, treatment of established EGFR-driven tumors with the small molecule EGFR inhibitors, erlotinib and HKI-272, resulted in dramatic radiographic responses. Notably however, foretelling the results to be shown in both human lung and colorectal cancer, mutant K-Ras driven lung tumors appeared to be relatively resistant to EGFR inhibitor therapy.
In addition to the activating mutations in the kinase domain of EGFR, in-frame deletions of the extracellular domain of EGFR, so-called EGFRvIII, have been found in other solid tumors (most notably in glioblastoma) as well as a small subset of lung cancers. In contrast to EGFR kinase domain mutants, however, which showed equivalent tumor response to both reversible (erlotinib) and irreversible (HKI-272) inhibitors of EGFR, EGFRvIII-driven lung tumors were considerably more sensitive to irreversible inhibitors of EGFR than reversible inhibitors such as erlotinib (Ji et al. 2006b). These data suggest that irreversible inhibitors of EGFR may be more effective in the treatment of tumors harboring EGFRvIII mutations such as glioblastomas (GBM).
Lung cancers treated with EGFR inhibitors eventually develop resistance to therapy. The majority of patients who develop drug resistance also acquire a secondary mutation in the kinase domain of EGFR, T790M, which is thought to sterically hinder the binding of reversible inhibitors (Balak et al. 2006; Kosaka et al. 2006; Pao et al. 2005). Generation of a doxycycline inducible GEMM that expressed both the L858R and the T790M alleles demonstrated, as predicted, that these bitransgenic mice were resistant to erlotinib but remained relatively sensitive to HKI-272 (Li et al. 2007). In a separate report, Pao and colleagues show that L858R/T790M tumors were resistant to erlotinib, but could be rendered sensitive by simultaneous treatment with the anti-EGFR monoclonal antibody, cetuximab (Regales et al. 2009). While yet to be tested clinically, these results suggest a possible therapeutic value of irreversible EGFR inhibitors and/or combined EGFR targeting.
2. Ras-mutant lung cancer in GEMMs
Ras and related small GTPases have been considered ‘undruggable’ based on biochemical characteristics as well as the discouraging number of failed anti-RAS efforts. Unlike the successful development of nM binding ATP-competitive inhibitors for blocking the low μM ATP binding activity of protein kinases, it has not been feasible to develop analogous inhibitors to block the 10 pM Kd of GTP-binding to RAS (Bos et al. 2007). Instead, more recent anti-RAS drug discovery efforts have focused on blocking key downstream signaling molecules such as AKT and RAF/MEK/ERK (Cox and Der 2002). As predicted from our knowledge of signaling redundancies, K-Ras driven murine lung adenocarcinomas do not respond substantially to pharmacologic inhibition of MEK, EGFR or the PI3K alone. When MEK inhibitors and PI3K inhibitors were combined, however, marked and unexpected synergy was observed (Engelman et al. 2008). This therapeutic combination appears to be effective in both HER2 and EGFR driven lung cancers as well (Faber et al. 2009). This synergy, first convincingly demonstrated in GEMMs, has led to related human clinical trials in RAS-mutant cancers. Moreover, while inactivation of LKB1 on a background of KRas activation imparts resistance to dual MEK and PI3K inhibition, sensitivity can be restored by the addition of the Src inhibitor, dasatinib (Carretero et al. 2010).
An additional attractive therapeutic target in K-Ras driven lung cancers is c-MET. K-Ras-driven murine lung cancers express high levels of activated c-MET as well as increased levels of its ligand hepatocyte growth factor (HGF) (Yang et al. 2008). Preclinical testing of the c-MET inhibitor, PHA-665752, in K-Ras driven lung cancer GEMM, results in a decreased number of tumors as well as an increase in apoptosis of both tumor and endothelial cells (Yang et al. 2008). While this result has yet to be confirmed in the clinic, trials are in progress using various strategies to inhibit the c-MET pathway.
3. Prostate Cancer GEMs with PI(3)K Pathway Activation
Prostate adeno-carcinomas are characterized by a high rate of inactivation of the PTEN tumor suppressor gene with consequent upregulation of the PI3K/AKT/mTOR pathway. There are now several well-characterized GEMMs of prostate cancer based on PTEN inactivation or Akt activation in prostate epithelial cells, which result with variable penetrance and latency in prostate intraepithelial neoplasia (PIN) and prostate adenocarcinoma (Shappell et al. 2004). Of the numerous downstream effector pathways of activated Akt, early studies demonstrated that treatment of Akt-driven PIN with the inhibitor of the mammalian target of rapamycin (mTOR) inhibitor, RAD001 (everolimus), resulted in complete reversal of established PIN, suggesting that the mTOR pathway is a critical mediator of Akt-driven prostate adenocarcinomas (Majumder et al. 2004). Furthermore, in a separate prostate cancer GEM model built on the combined inactivation of the Nkx3.1 homeobox gene and Pten, while inhibition of either mTOR or the MEK/ERK pathway using rapamycin and PD0325901 appeared to restrain tumor growth, combined mTOR and MEK inhibition had marked synergistic effects (Kinkade et al. 2008). Based in part on this work, there are now ongoing clinical trials evaluating the effectiveness of mTOR inhibitors in prostate cancer (Figlin et al. 2008).
4. BRAF Mutant Melanoma in GEMMs
Malignant melanoma is characterized by a frequent incidence of concomitant B-Raf activation and PTEN loss (Daniotti et al. 2004; Tsao et al. 2004). Correspondingly, melanocyte-specific expression of mutant, activated BRafV600E with concomitant Pten deletion results in the rapid formation of pigmented and highly metastatic melanoma (Dankort et al. 2009). These genetic lesions result in the activation of both the PI3K and mTOR pathways. In keeping with the above results, therapeutic treatment of established Pten deficient, B-Raf mutant melanomas with either a MEK inhibitor (PD325901) or the mTORC1 inhibitor, rapamycin, modestly inhibited their growth, but did not result in tumor regression. As in the other aforementioned systems, concurrent inhibition of both pathways results in tumor regression that correlates with inhibition of MEK and mTOR signaling, suggesting that dual inhibition of the MEK/ERK and PI3K/AKT/mTOR pathways may be efficacious in B-Raf/PTEN mutant cancers.
6 “Credentialing” GEMMs as Genetically Faithful Models
Human and murine genomes differ in important ways, and these differences may affect the nature of cooperating tumorigenic events and suitability of these models for drug efficacy testing. While there have been relatively few comprehensive comparisons between the transcriptional profiles and genetic complexity of cancer GEMMs and their human counterparts, a few analyses in this regard have proven informative and are described below:
1. Cooperating Genetic Events in GEMMs
With regard to genetic lesions in GEM tumors, the published results have suggested that GEMMs may not possess the same degree of genetic complexity in terms of copy-number variants, aneuploidy and point mutations as human tumors (see for example Ellwood-Yen et al. 2003; Kim et al. 2006). A period of telomere dysfunction which may occur at an early stage of most human but not murine tumor progression has been suggested as one cause for this discrepancy, and tumor-bearing mice with ‘humanized’ telomere length exhibit increased cytogenetic complexity reminiscent of the human disease (Artandi et al. 2000; Maser et al. 2007). Despite these genome-wide differences, a number of secondary genetic events important in the evolution of human tumors appear to be conserved in the progression and metastasis of GEM tumors such as the amplification of KRas2 and Myc in pancreatic ductal adenocarinomas, Nedd9 in Ras induced melanomas, and loss of Fbxw7 and Pten in Atm; p53 deficient lymphomas (Bardeesy et al. 2006; Ellwood-Yen et al. 2003; Kim et al. 2006; Maser et al. 2007). In a similar light, murine hepatocellular carcinomas (HCCs) induced by transplanted, genetically defined, liver progenitor cells were found to have focal amplifications of Myc and Yap, both known oncogenic events in the development of HCC (Zender et al. 2006).
2. Genome-Wide Expression Profiling
A recent study by Perou et al. of GEMM transcriptional profiles is particularly informative. The authors performed a microarray- based, comprehensive assessment of gene expression in more than 20 breast cancer GEMMs, and compared these expression profiles with a large number of human tumors using a novel cross-platform method for transcriptome normalization (Herschkowitz et al. 2007). The authors found great heterogeneity among breast cancer GEMMs, and were able to identify a few models with significant transcriptional overlap to human breast cancer subtypes. For example, the C3-Large T Antigen model appeared transcriptionally similar to the basal-like subtype of breast cancer. In retrospect, this similarity likely reflects the fact that RB and p53 inactivation are signature events of most basal-like cancers, and Large T Antigen similarly inactivates these two archetypal tumor suppressor genes. Likewise, the MMTV-Her2/ Neu model was transcriptionally similar to human Her2 + breast cancer, again consistent with the molecular genetics of these tumors. Surprisingly, no good murine model was identified for human estrogen receptor expressing (ER + or luminal) breast cancer, at least in terms of transcriptional profiles.
Expression profiling and assessment of copy number alterations by array CGH of PTEN, TP53 and Rb deficient mouse gliomas showed remarkable similarity in syntenic regions of gene amplification and loss (Chow et al. 2011). In contrast to the expression profiling of breast cancer GEMMs, which suggested that each GEMM can be classified into a single molecular subtype of human breast cancer, subgroups of these mice resembled the proneural, proliferative and mesenchymal molecular subtypes of glioblastoma previously shown to have prognostic significance suggesting that secondary genetic events are driving the molecular classification of glioblastomas. Similar studies using a Myc gene signature derived from a Myc-driven prostate cancer GEMM indicates that this model faithfully recapitulates the molecular changes seen in a subset of human prostate cancers harboring Myc activation (Ellwood-Yen et al. 2003). These observations suggest that faithful GEM models of some, but probably not all, human cancers can be identified, and reiterate the importance of GEMM credentialing.
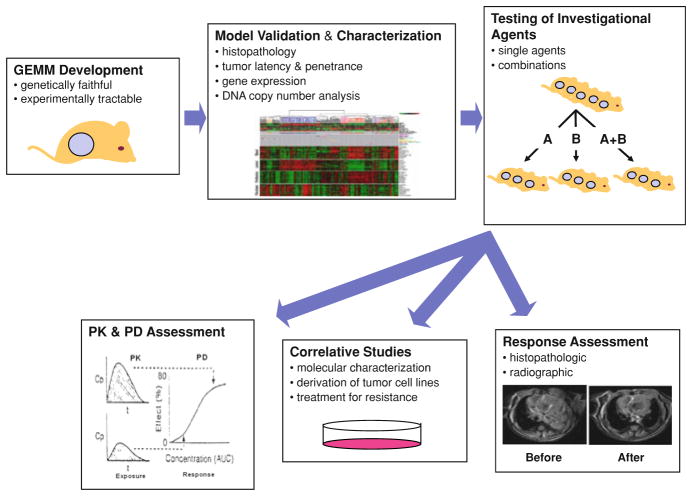
In summary, the available genomic data suggests that some aspects of human tumor progression are recapitulated in GEMMs, but that relevant inter-species differences also exist. Therefore, characterization and validation (e.g. ‘credentialing’) of a GEMM is requisite prior to widespread use for pre-clinical testing (Fig. 2). It is worth noting in this context that established human cancer cell lines—the alternative source of cancer cells for in vivo drug efficacy testing—are far from perfect with regard to genetic faithfulness to human cancer. Many tumor lines have been in culture for years or decades, and therefore subject to ex vivo genetic events associated with in vitro progression and drift. Moreover, the ability to be grown in vitro may differ markedly depending on a tumor’s attendant genetic lesions. For example, p16INK4a expression is an important barrier to in vitro growth for many cell types, and therefore not unexpectedly, the frequency of p16INK4a inactivation in cell lines often greatly exceeds the in vivo mutation frequency of p16INK4a in the same tumor type. Therefore, in vitro drift and the need for culture strongly influence in vitro and xenograft approaches.
Fig. 2.
Workflow and infrastructural needs for GEMM testing. GEM models are first designed and developed based on genetic events faithful to human cancers. The model is then characterized and validated histopathologically and molecularly prior to therapeutic testing. Once a model is characterized, investigational agents are tested as single agents or in combination. Treated and untreated mice are assessed for tumor response (typically radiographically or other imaging modalities using RECIST-like criteria) with pharmacokinetic (PK) and pharmacodymanic (PD) monitoring. Other correlative studies such as the molecular characterization of treated tumors and culture and in vitro evaluation of GEMM tumor cell lines can be performed as indicated
7 Logistic Considerations in Developing a “Mouse Clinic”
An important consideration when one considers the use of GEMMs for drug efficacy testing is the amount of time required to breed a sufficiently large cohort of animals for informative preclinical drug testing. While GEMMs possess one or more index genetic lesions, the neoplastic cells in these models require the acquisition of subsequent genetic alterations to progress into cancer. These secondary genetic events occur stochastically resulting in genetic heterogeneity as well as differences in penetrance, tumor latency and therapeutic responsiveness. Our experience has been that many efficacious therapeutics will cause tumor regression in only a subset of animals within a given GEM model. For example, some classical cytotoxics can induce profound tumor regression in a small fraction of breast GEM models, but such agents are not active in most tumors from animals of this genotype (unpublished data NES, WYK and Charles Perou). As these mice are syngeneic, this heterogeneity of response likely reflects the differences in cooperating genetic events during tumor progression in this model. While this presence of responders and non-responders within a given model is clearly similar to the experience of Phase II/III testing in humans with a given tumor type, such heterogeneity also necessitates the study of larger numbers of mice. For these reasons, we typically analyze 12–16 mice per initial therapeutic cohort, as opposed to cohort sizes of 4–8 mice that we use for xenograft testing.
Additionally, many cancer GEMMs are multiallelic, typically requiring a conditional knock-out, activatable knock-in, or transgenic allele as well as a Cre or tetracycline-inducible allele to control spatial expression of the genes of interest. The need for multiple alleles can decrease breeding efficiency if the employed alleles cannot be homozygosed, and also produces a need for rapid and accurate animal genotyping. Inefficient breeding and mutli-allele genotyping in turn can increase the needed cage space for GEMM testing and also significantly augment the experimental cost. One approach to these problems is to identify GEMMs that are relatively simple to use. For example, for the testing of anti-RAS therapeutics, we have relied extensively on the Tyr-RA-SInk4a/Arf−/− model from Chin and colleagues (Chin et al. 1997). This model is very faithful to the genetics of human melanoma featuring a melanocyte-specific, activated RAS transgene integrated on the Y-chromosome combined with germ line p16INK4a and Arf inactivation (Ink4a/ Arf−/−), an allele which can be homozygosed. Therefore, by crossing Tyr-RASInk4a/ Arf−/− males with Ink4a/Arf−/− females, a cohort is produced where no genotyping is required (all the males get cancer) and all the progeny mice are useful (the males develop tumors and the females are used for future breeding). While this degree of efficiency is not possible for all models, often more efficient breeding schemes can be developed to allow for low-cost and rapid cohort development.
Drug efficacy testing in GEMMs requires substantial expertise, infrastructure to set up mouse colonies for efficient drug efficacy testing in GEMs (Fig. 2), and a commitment to test multiple agents, including ‘boring’ older cytotoxic agents in addition to more novel targeted therapies. Without assessment of multiple agents, we believe it is hard to assess the activity of any single compound. Likewise, several challenges must be solved including access to adequate cage space, the development of well-defined processes for animal husbandry and genotyping, meticulous record keeping, and for some models, infrastructure to permit serial animal imaging. Moreover, access to expertise in cancer histopathology is a critical and, in our view, still under appreciated need in GEMM experiments. Lastly, we believe in the modern era, where GEMM experiments require a modest amount of companion pharmacologic (PK) and pharmacodynamic (PD) monitoring to be fully informative. For example, the demonstration that a compound has activity in a given model is only helpful if complementary PK analyses indicate that drug levels associated with efficacy are levels that could be reasonably achieved in humans. Perhaps even more difficult is understanding why compounds that should work based on our understanding of tumor signaling still fail in certain models. In these instances, PD monitoring can be particularly informative; for example sometimes showing that failure of an agent correlates with a lack of in vivo inhibition of the relevant drug target. For these reasons, we have come to believe that GEMM analyses must necessarily be complemented by PK/PD monitoring.
A historical impediment regarding GEMM testing involves a series of legal issues related to the involved technologies. In particular, the topic of ‘Oncomouse’ patents has been covered extensively elsewhere (Hanahan et al. 2007; Marshall 2002; Sharpless and Depinho 2006). In our view, however, several recent developments have conspired to mitigate this problem including the expiration of some of these patents, the licensing of these patents by many large Pharma entities and decisions in the US and Canadian Supreme Courts that have limited the scope of these patents and bolstered the legal notion of ‘safe harbor’ which may shelter some GEMM testing. For whichever of these reasons, in the last few years, GEMM testing of novel therapeutics has now become commonplace in many institutions and has begun to be more widely embraced by large Pharma. While these patents may still pose problems for those industry workers in entities that have not taken license for these patents, this issue appears to no longer be a major experimental impediment in most settings.
8 Future Perspectives
Despite the relatively recent introduction of GEMMs for pre-clinical drug development, we believe that there have already been some remarkable successes that will speed up the development of active human therapeutics. In particular, there has been significant work with kinase inhibitors and related molecules in several GEM models of lung adenocarcinoma, prostate carcinoma and melanoma; and we expect publication of a raft of additional GEMM therapeutic stories in many other tumor types in the next few years. We believe the published experience suggests that GEMM testing adds immediate value to xenograft testing for pre-clinical efficacy studies.
Some cancer researchers believe that while GEMM testing is clearly of benefit, it is better left to the industry, which has the in-house wherewithal to meet the challenges of GEMM testing. In fact, these researchers are partially correct, as the forward-thinking industry has begun to move into GEMM testing with great enthusiasm, a move we wholeheartedly endorse. Unfortunately, however, we believe that such a desire to compartmentalize academic versus industry science may have contributed to a hesitation among large funding agencies to support laudable and highly translational GEMM efforts As a reflection of this, the most ambitious academic GEMM efforts have relied extensively on private, state and industry funding. Nonetheless, we believe that this line of research is too important to cede solely to the industry, and see an increasing need for such investigation in federally financed academic labs that have no commercial interest in the development of the agents that they are testing.
We have highlighted several of the challenges associated with GEMM studies, most notably the need for requisite infrastructure to support this work. Despite these challenges, we feel GEMM testing can be done cost-effectively with sufficient rapidity to directly shape human clinical trials of novel agents. Lastly, as mentioned, while we are cheered by significant recent industry investments in GEMM testing, it is crucial that widespread and vibrant GEMM testing also proceed in academia conducted by dispassionate scientists interested in improving clinical cancer care.
Acknowledgments
We would like to thank N. Bardeesy for useful discussions and K. Wong for MRI images.
Contributor Information
William Y. Kim, Email: wykim@med.unc.edu, The Lineberger Comprehensive Cancer Center, The University of North Carolina, CB# 7295, Chapel Hill, NC 27599-7295, USA
Norman E. Sharpless, Email: nes@med.unc.edu, The Lineberger Comprehensive Cancer Center, The University of North Carolina, CB# 7295, Chapel Hill, NC 27599-7295, USA
References
- Alani RM, Silverthorn CF, Orosz K. Tumor angiogenesis in mice and men. Cancer Biol Ther. 2004;3:498–500. doi: 10.4161/cbt.3.6.930. [DOI] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. 12/21/6494[pii] [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, Hanahan D, Redston MS, Chin L, Depinho RA. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. 0601273103[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. S0092-8674(07)00655-1[pii] [DOI] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, McNamara KL, Brandstetter KA, Walton ZE, Gu TL, Silva JC, Crosby K, Shapiro GI, Maira SM, Ji H, Castrillon DH, Kim CF, Garcia-Echeverria C, Bardeesy N, Sharpless NE, Hayes ND, Kim WY, Engelman JA, Wong KK. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, II, DePinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, Broniscer A, Ellison DW, Baker SJ. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19:305–316. doi: 10.1016/j.ccr.2011.01.039. S1535-6108(11)00051-1[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Der CJ. Ras family signaling: therapeutic targeting. Cancer Biol Ther. 2002;1:599–606. doi: 10.4161/cbt.306. 306[pii] [DOI] [PubMed] [Google Scholar]
- Daniotti M, Oggionni M, Ranzani T, Vallacchi V, Campi V, Di Stasi D, Torre GD, Perrone F, Luoni C, Suardi S, Frattini M, Pilotti S, Anichini A, Tragni G, Parmiani G, Pierotti MA, Rodolfo M. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene. 2004;23:5968–5977. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoster G, Stein G, Holdener EE. Responses and toxic deaths in phase I clinical trials. Ann Oncol. 1990;1:175–181. doi: 10.1093/oxfordjournals.annonc.a057716. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA. 1999;96:3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristov B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. S1535610803001971[pii] [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. nm.1890[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo HB, Smith GC, Martin NM, Lau A, O’Connor MJ, Jonkers J. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14:3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, Lifshits E, Chen Z, Maira SM, Garcia-Echeverria C, Wong KK, Engelman JA. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci USA. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. 0905056106[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlin RA, Brown E, Armstrong AJ, Akerley W, Benson AB, III, Bursein HJ, Ettinger DS, Febbo PG, Fury MG, Hudes GR, Kies MS, Kwak EL, Morgan RJ, Jr, Mortimer J, Reckamp K, Venook AP, Worden F, Yen Y. NCCN task force report: mTOR inhibition in solid tumors. J Natl Compr Canc Netw. 2008;6(Suppl 5):S1–S20. quiz S21–S22. [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Garber K. From human to mouse and back: ‘tumorgraft’ models surge in popularity. J Natl Cancer Inst. 2009;101:6–8. doi: 10.1093/jnci/djn481. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Wagner EF, Palmiter RD. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 2007;21:2258–2270. doi: 10.1101/gad.1583307. [DOI] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, Mahmood U, Mitchell A, Sun Y, Al-Hashem R, Chirieac LR, Padera R, Bronson RT, Kim W, Janne PA, Shapiro GI, Tenen D, Johnson BE, Weissleder R, Sharpless NE, Wong KK. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006a;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Ji H, Zhao X, Yuza Y, Shimamura T, Li D, Protopopov A, Jung BL, McNamara K, Xia H, Glatt KA, Thomas RK, Sasaki H, Horner JW, Eck M, Mitchell A, Sun Y, Al-Hashem R, Bronson RT, Rabindran SK, Discafani CM, Maher E, Shapiro GI, Meyerson M, Wong KK. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci USA. 2006b;103:7817–7822. doi: 10.1073/pnas.0510284103. 0510284103[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer. 2002;2:251–265. doi: 10.1038/nrc777. [DOI] [PubMed] [Google Scholar]
- Kerbel RS, Cornil I, Theodorescu D. Importance of orthotopic transplantation procedures in assessing the effects of transfected genes on human tumor growth and metastasis. Cancer Metastasis Rev. 1991;10:201–215. doi: 10.1007/BF00050792. [DOI] [PubMed] [Google Scholar]
- Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, Flotte TJ, Duncan LM, Granter SR, Chin L. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, Tsuboi M, Tada H, Kuwano H, Mitsudomi T. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12:5764–5769. doi: 10.1158/1078-0432.CCR-06-0714. 12/19/5764[pii] [DOI] [PubMed] [Google Scholar]
- Kulke MH, Demetri GD, Sharpless NE, Ryan DP, Shivdasani R, Clark JS, Spiegelman BM, Kim H, Mayer RJ, Fuchs CS. A phase II study of troglitazone, an activator of the PPARgamma receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J. 2002;8:395–399. doi: 10.1097/00130404-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, Liang MC, Perera SA, Zaghlul S, Borgman CL, Kubo S, Takahashi M, Sun Y, Chirieac LR, Padera RF, Lindeman NI, Janne PA, Thomas RK, Meyerson ML, Eck MJ, Engelman JA, Shapiro GI, Wong KK. Bronchial and peripheral murine lung carcinomas induced by T790M–L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Marshall E. Intellectual property. DuPont ups ante on use of Harvard’s OncoMouse. Science. 2002;296:1212. doi: 10.1126/science.296.5571.1212. [DOI] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O’Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. nature05886[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen R, Linn SC, van der Valk M, Mooi WJ, Berns A. Mouse model for lung tumorigenesis through cre/lox controlled sporadic activation of the K-ras oncogene. Oncogene. 2001;20:6551–6558. doi: 10.1038/sj.onc.1204837. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, Bronson RT, Chauhan D, Munshi N, Treon SP, Maxwell CA, Pilarski L, Hideshima T, Hoffman RM, Anderson KC. Fluorescence imaging of multiple myeloma cells in a clinically relevant SCID/NOD in vivo model: biologic and clinical implications. Cancer Res. 2003;63:6689–6696. [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes: bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. nrc1477[pii] [DOI] [PubMed] [Google Scholar]
- Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40:837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, Koutcher JA, Spassova M, Ouerfelli O, Mellinghoff IK, Zakowski MF, Politi KA, Pao W. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. 38746[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TG, Jr, Goulart BH, Squitieri L, Stallings SC, Halpern EF, Chabner BA, Gazelle GS, Finkelstein SN, Clark JW. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. Jama. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- Rofstad EK. Orthotopic human melanoma xenograft model systems for studies of tumour angiogenesis, pathophysiology, treatment sensitivity and metastatic pattern. Br J Cancer. 1994;70:804–812. doi: 10.1038/bjc.1994.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Connor MJ, Martin NM, Borst P, Jonkers J. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S, Manova K, Mittal V, Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4:1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. doi: 10.1158/0008-5472.CAN-05-3627. discussion 3354. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the mouse models of human cancer consortium prostate pathology committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, Bou Reslan H, Ho CC, Cao TC, Lee CV, Nannini MA, Fuh G, Carano RA, Koeppen H, Yu RX, Forrest WF, Plowman GD, Johnson L. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–593. doi: 10.1038/nbt.1640. nbt.1640[pii] [DOI] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- Yang Y, Wislez M, Fujimoto N, Prudkin L, Izzo JG, Uno F, Ji L, Hanna AE, Langley RR, Liu D, Johnson FM, Wistuba I, Kurie JM. A selective small molecule inhibitor of c-Met, PHA-665752, reverses lung premalignancy induced by mutant K-ras. Mol Cancer Ther. 2008;7:952–960. doi: 10.1158/1535-7163.MCT-07-2045. 7/4/952[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. S0092-8674(06)00720-3[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]