Abstract
Objective
To test the hypothesis that a left-dominant brain immune network (LD-BIN) might affect the occurrence of infection during inpatient rehabilitation of stroke and traumatic brain injury (TBI).
Design
A retrospective analysis was performed on electronic medical records between January 2009 and December 2010. All patients with left-or right-sided stroke or TBI were included into the study. The LD-BIN hypothesis was tested by comparing HAI rates depending on whether patients had left- or right-sided brain lesions.
Setting
A large inpatient rehabilitation hospital.
Participants
Among the patients (N=2236) with stroke or TBI who had either a left- or right-sided brain lesion, 163 patients were identified with HAIs.
Intervention
Not applicable.
Main Outcome Measure
Frequency of HAIs.
Results
In the 163 patients identified with HAIs with a diagnosis of stroke or TBI, chi-square analysis revealed a significantly higher proportion of HAIs among patients with left-sided (n=98; 60.1%) relative to right-sided (n=65; 39.9%) brain injuries (χ2=6.68, P<.01). These effects could not be attributed to either clinical or demographic factors.
Conclusions
Our findings are consistent with the hypothesis that an LD-BIN may mediate vulnerability to infection during rehabilitation of patients with stroke or TBI. Further translational research investigating novel means of managing patients based on brain lesion location, and modulating the LD-BIN via behavioral and physiologic interventions, may result in neuroscience-based methods to improve infection resistance in brain-injured patients.
Keywords: Brain, Infection, Injuries, Rehabilitation
Hospital-acquired infections (HAIs) are defined as infections first diagnosed 48 to 72 hours after admission to a hospital or health care facility.1 Minimizing HAIs is an important aim of brain injury care, because of the impact of HAIs on hospitalization outcomes and cost of care.2 HAIs are common after stroke and brain injury. One study3 observed that half of all patients with brain injuries had at least 1 infectious complication at a level 1 trauma center,3 and researchers also reported that after stroke, minor or moderately severe HAIs developed in >20% of patients with ischemic stroke.4,5 HAIs are not limited to the acute care hospital setting; during inpatient rehabilitation, 15% of patients, including mainly survivors of traumatic brain injury (TBI), acute stroke, and subarachnoid hemorrhages, were diagnosed with HAIs, including Clostridium difficile diarrhea and other infections.6
An approach to reducing HAIs often emphasizes generic infection control methods. Although any kind of brain injury may activate the hypothalamo-pituitary-adrenal axis and the sympathetic nervous system,7 increasing vulnerability to infection, it is also possible that focal brain regions form a network interacting with the immune system. In this study, we looked for evidence supporting this left-dominant brain immune network (LD-BIN). Previous studies8–13 in both animals and humans suggested that left-sided brain lesions might result in decreased T-cell immune function. Other recent reports suggest that in healthy subjects, greater activation in the left side as compared with the right side of the brain, especially prefrontal cortical regions, might predict vaccine response,14 and that mental activity stimulating these regions may also enhance immune indices.15 In a prospective study16 of asymptomatic human immunodeficiency virus—positive patients, researchers reported that greater functional activity in the left side, as compared with the right side of the brain, predicted positive immune competence 2 to 3 years later. Thus, the incidence of HAIs may be different depending on whether brain injury affects critical nodes of the LD-BIN.
Studies on brain-injured patients also suggest a strong link between LD-BIN and immune function. Differences in both white blood cell counts and C-reactive protein between patients after left-sided brain stroke and patients after right-sided brain stroke suggested that survivors of left-sided stroke had a deficit in immune control.17 Meador et al18 found that patients undergoing epilepsy resection surgery had reduced cellular (Th1) immunity (decreased T lymphocytes) and decreased allergic responses (histamine reaction) when areas of the left hemisphere relative to the right hemisphere were ablated. Although prefrontal cortical areas may be critical to supporting the LD-BIN, these studies suggest that damage elsewhere in the left hemisphere may indirectly affect functional prefrontal activity and thus reduce a left-greater-than-right functional balance needed to maintain optimal immune homeostasis. Although a recent review19 emphasized that left-sided brain stroke may be highly associated with immune suppression, it is not completely clear that this influences the occurrence of clinically relevant infections. Minnerup et al20 suggested that lesion size, and not location, predicted vulnerability to infection in a sample of stroke patients.
In this study, we wished to learn whether the LD-BIN influences the relative risk of HAIs after left-sided versus right-sided brain stroke, as assessed via health care records during the acute rehabilitation hospital stay. If left-sided brain injury predicts a higher incidence of HAIs, this is important to our understanding of brain immune mechanisms, as well as to derive innovative methods to prevent these complications and reduce health care costs, improve functional ability, and perhaps reduce mortality.
Methods
Sample
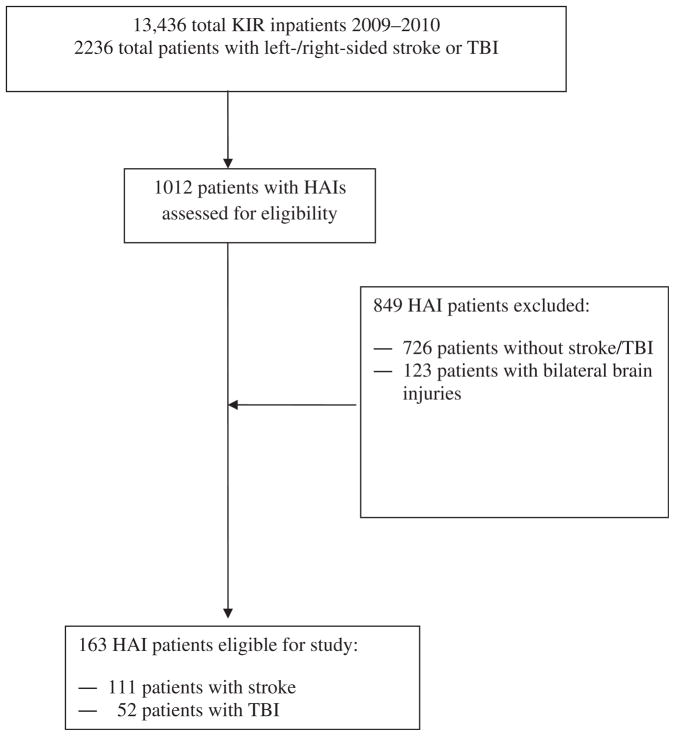
A retrospective chart review was completed from electronic medical records of HAIs among brain-injured patients in a large inpatient rehabilitation hospital system (Kessler Institute of Rehabilitation) between January 2009 and December 2010. This study was approved by the Kessler Foundation Research Center Institutional Review Board. As part of the routine quality-of-care assessment in this rehabilitation system, an HAI, defined as an infection that was identified >72 hours after admission, is tracked by an infection control specialist. An HAI requires that the infection control specialist identifies that no evidence of infection was present on admission; all HAIs are identified using the criteria of the Centers for Disease Control and Prevention.1 A dataset that included laboratory results for HAIs and patient demographic characteristics was provided by the infection control specialist. One of the authors (P.G.F.) then independently reviewed all episodes of HAIs and excluded all patients with diagnoses other than stroke or TBI according to the International Classification of Diseases, 9th Revision, Clinical Modification.21 We excluded patients with other forms of brain injury because these patients may have other body region injuries and comorbid conditions that increase the risk of HAIs. Based on documented acute care brain imaging, we classified brain injury location as right versus left hemisphere, and excluded patients with bihemispheric brain injury locations (fig 1).
Fig 1.
Patient inclusion in the current study. Abbreviation: KIR, Kessler Institute of Rehabilitation.
Materials and procedures
Data collected included demographic (ie, age, sex, ethnicity) and clinical characteristics (ie, rehabilitation diagnosis, dysphagia status, parenteral feeding, urinary/intravascular catheters, severity of disability at the time of admission by means of the FIM,22 length of hospital stay, and concurrent illnesses such as cardiac disease, hypertension, diabetes, renal and liver disease, autoimmune disease, thyroid disease, history of cancer, mood/psychological disorder, and prior stroke/head injury). Concurrent illnesses were examined because of their possible influence on infections and LD-BIN.19 We also noted any surgical or invasive procedures during acute care (eg, operations, urinary or intravascular catheters, tracheostomy, percutaneous endoscopic gastrostomy [PEG] tube placement), antibiotic treatment during acute care and inpatient rehabilitation, and steroid/gastric-suppressing drugs (ie, protein pump inhibitors) during inpatient rehabilitation. Lastly, data were collected on the frequency and type of HAIs, which included C difficile, vancomycin-resistant enterococcus (VRE), methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum β-lactamase (ESBL)-producing bacteria such as Escherichia coli or Klebsiella, and infections associated with urinary catheters.
Statistical analysis
The frequency of HAIs between patients with left-sided brain injury and those with right-sided brain injury was analyzed with the Pearson chi-square statistic for proportions, and results were considered statistically significant when P was less than .05. We also compared other clinical and demographic variables using the Pearson chi-square statistic for proportions and the independent samples t test.
Results
On the basis of the study criteria we adopted, 163 patients with left-sided or right-sided brain injuries who had HAIs were included in the main chi-square analysis (table 1). Among these patients, we observed a significantly higher proportion of HAIs among those with left-sided (n=98; 60.1%) relative to right-sided (n=65; 39.9%) brain injuries (χ2=6.68, P<.01). When we examined the proportion of HAIs relative to the entire group of brain-injured patients, the higher proportion of left-sided brain-injured patients was still present. The proportion of left-sided brain-injured patients with HAIs compared with the entire group of patients with left-sided brain injury (98 of 1115 total patients with left brain injury; 8.8%) exceeded the proportion of right-sided brain-injured patients with HAIs compared with the entire group of patients with right-sided brain injury (65 of 1121 patients; 5.8%) (χ2=7.39, P<.01). The proportion of HAIs was also significantly higher among all left-sided (68 of 1003 patients; 6.8%) relative to all right-sided stroke patients (43 of 983 patients; 4.4%) (χ2=5.44, P=.02). Finally, the proportion of HAIs was also significantly greater among patients with left-sided (30 of 112; 26.8%) versus right-sided TBI (22 of 138; 15.9%) (χ2=4.41, P<.05). All subsequent analyses are based on the 163 HAI cases, to examine whether demographic and clinical variables contributed to the differences in brain lateralization and infection within the final sample of brain-injured patients.
Table 1.
Characteristics of the brain-injured sample with HAIs
| Characteristics | Values |
|---|---|
| Stroke | 111 (68.10) |
| TBI | 52 (31.90) |
| Sex | |
| Men | 81 (49.69) |
| Women | 82 (50.31) |
| Ethnicity | |
| White | 103 (63.19) |
| Black | 26 (15.95) |
| Hispanic | 10 (6.13) |
| Other ethnicity | 24 (14.72) |
| Age (y) | 69.11316.61 |
| Duration of stay in rehab hospital (d) | 32.81326.58 |
| Admission FIM | 35.36316.19 |
NOTE. Values are n (%) or mean ± SD.
Abbreviation: rehab, rehabilitation.
We were unable to identify clinical or demographic differences accounting for the higher proportion of HAIs among patients with left-sided brain injuries. There were no statistically significant differences observed between the 2 groups as a function of age (t159 =.89, P=.37, not significant [NS]), sex (χ2=.75, P=.39, NS), occurrence of stroke (χ2=.19, P=.66, NS) versus other brain injury (χ2=.25, P=.62, NS), proportion of patients with dysphagia (χ2=2.14, P=.14, NS), parenteral feeding (χ2=.24, P=.62, NS), or urinary/intravascular catheters (χ2=.15, P=.70, NS; table 2). Independent samples t test also showed no significant differences between patients with left- and right-sided brain injuries on admission FIM (t160=−1.07, P=.28, NS; see table 2). Additional analyses performed to evaluate for differences in history of smoking/substance abuse, or other intercurrent illnesses potentially related to HAIs, failed to identify any significant left-sided versus right-sided brain group differences (all P values >0.3, NS). Table 2 summarizes all the clinical and demographic variables we collected in the survivor groups with left- and right-sided brain injuries that are potentially relevant to HAIs.
Table 2.
Demographic and clinical characteristics for HAI patients with left- or right-sided brain injury
| Clinical/Demographic Variables | Left-Sided Brain Injury | Right-Sided Brain Injury |
|---|---|---|
| Age (y) | 70.07±16.76 | 67.69±16.41 |
| Sex (men) | 46 (46.94) | 35 (53.85) |
| Stroke | 68 (69.39) | 43 (66.15) |
| Brain injury | 30 (30.61) | 22 (33.85) |
| Dysphasia | 52 (53.06) | 42 (64.62) |
| Parenteral feeding | 34 (34.69) | 25 (38.46) |
| Urinary/intravascular catheters | 33 (33.67) | 20 (30.77) |
| Admission FIM | 34.25±16.44 | 37.03±15.78 |
| History of smoking/substance abuse | 14 (14.29) | 7 (10.77) |
| Cardiac disease | 18 (18.37) | 12 (18.46) |
| Hypertension | 69 (70.41) | 50 (76.92) |
| Renal and liver disease | 7 (7.14) | 8 (12.31) |
| Autoimmune disease | 1 (1.02) | 1 (1.54) |
| Thyroid disease | 11 (11.22) | 6 (9.23) |
| History of cancer | 9 (9.18) | 5 (7.69) |
| Mood/psychological disorder | 17 (17.35) | 10 (15.38) |
| Prior stroke/head injury | 21 (21.43) | 16 (24.62) |
| Invasive procedure performed during acute care | 51 (52.04) | 26 (40.00) |
| Antibiotics at acute care | 37 (37.76) | 32 (49.23) |
| Antibiotics at rehab hospital | 83 (84.69) | 60 (92.31) |
| Steroid/gastric-suppressing drugs at rehab hospital | 73 (74.49) | 51 (78.46) |
NOTE. Values are mean ± SD or n (%).
Abbreviation: rehab, rehabilitation.
Unrelated to the effects of LD-BIN on HAIs during inpatient rehabilitation, the side of the brain injury might influence other hospitalization events. However, no group differences were observed in the proportion of patients who had an invasive procedure performed during acute care (χ2=2.27, P=.13, NS), nor the proportion of patients receiving antibiotics (χ2=2.11, P=.15, NS) during their acute care stay (see table 2). Chi-square analysis revealed that there were also no significant group differences regarding the proportion of patients receiving antibiotics (χ2=2.11, P=.15, NS) and steroid/gastric-suppressing drugs (χ2=.34, P=.56, NS) during inpatient rehabilitation, before HAI diagnosis (see table 2). This reduces the likelihood that HAI differences might be related to different prescribing practices after left-sided versus right-sided brain injury.
We observed differences, however, based on the type of HAIs observed in the brain-injured HAI (n=163) sample (χ2=51.69, P<.001), with the highest proportion of infections classified as C difficile (38.03%), followed by MRSA (21.47%), ESBL (21.47%), and then VRE (16.56%). The lowest proportion of infections were those associated with urinary catheters (2.45%). As seen in table 3, there was a significantly higher proportion of C difficile (χ2=4.91, P=.03) and VRE (χ2=4.21, P=.04) cases among patients with left-sided relative to right-sided brain injury. Interestingly, patients with right-sided brain injury had a significantly higher proportion of HAIs classified as ESBL (χ2=18.51, P<.001) relative to those with left-sided brain injury. Although the occurrence of MRSA infection was greater in left-sided (n=22; 22.45%) relative to right-sided (n=13; 20%) brain-injured patients, differences between the groups were not statistically significant (χ2=0.14, P=.71, NS). Similarly, group differences (see table 3) for infections associated with urinary catheters were not statistically significant (χ2=2.14, P=.14, NS).
Table 3.
Proportion of HAIs by lateralization of lesion
| HAI | Left-Sided Brain Injury | Right-Sided Brain Injury |
|---|---|---|
| C difficile* | 44 (44.90) | 18 (27.69) |
| VRE* | 21 (21.43) | 6 (9.23) |
| ESBL† | 10 (10.20) | 25 (38.46) |
| MRSA | 22 (22.45) | 13 (20.00) |
| Infections associated with urinary catheters | 1 (1.02) | 3 (4.62) |
NOTE. Values are n (%).
P<.05.
P<.01.
Prior research suggested that prefrontal cortical regions might be particularly important in the LD-BIN.14,15 We hypothesized that cortical, rather than subcortical brain injury might be more associated with HAIs. In an exploratory analysis, we compared the proportion of HAIs in patients with cortical (ie, frontal, temporal, parietal, occipital lobes) versus subcortical (eg, basal ganglia, hippocampus, amygdala, thalamus, hypothalamus, cerebellum, pons, medulla) brain regions. In patients with left-sided brain injuries, 75% (49 of 65) of the total sample had brain-imaging records appropriate for analysis. Although there was a trend toward a higher proportion of cases with injuries in cortical (60.52%) relative to subcortical (39.48%) regions (χ2=3.36, P=.07, NS) in left-sided brain-injured patients, this did not reach significance.
Discussion
This retrospective study demonstrated variation in HAIs during inpatient rehabilitation, based on the side of brain injury. Specifically, we observed a significantly higher HAI proportion with left-sided versus right-sided brain injury. This finding is compatible with the previous metaanalysis19 reporting an increased risk of poor immune function after left-sided brain damage. The importance of an LD-BIN may not be restricted to the theoretic understanding of interactions between the nervous system and immune system, but may actually affect patient outcomes, quality care planning, and cost efficiency in the acute inpatient and inpatient rehabilitation settings. Specifically, we observed that both total infections and C difficile infection were more common after left-sided brain injury. Although previous research identified antibiotics, steroids, and gastric-suppressing drugs as risk factors for C difficile infection,23,24 our research suggests that left-sided brain injury after stroke or trauma may be an additional risk factor for this adverse consequence of hospitalization.
Cognitive deficits and neuropsychologic symptoms may occur as a result of focal brain dysfunction; this may result in aphasia, spatial neglect, limb apraxia, agnosia, and other behavioral syndromes. Thus, there may be an association between left-sided brain dysfunction, cognitive deficits, and infection. However, because assessment of cognitive function is performed on a case-by-case basis in our stroke rehabilitation setting, rather than being measured by a fixed battery of neuropsychologic tests, we are not able to comment on such a possible association. Research evaluating whether there may be an association between aphasia, object agnosia, limb apraxia, and other deficits associated with left-sided brain damage, and infection is needed, and should be collected in a future prospective study. Although we were unable to link the increased total infections or increased proportion of C difficile infections in patients with left-sided brain injury to other factors in our data, further research in larger patient samples is needed to examine whether the side of brain injury interacts with clinical or demographic factors.
In addition to C difficile infections, we also observed a significantly higher occurrence of VRE in patients with left-sided relative to right-sided brain injury. Although the mechanisms of LD-BIN modulation relevant to in-hospital infection are unclear, it is possible that the LD-BIN may interact with immunity in the intestinal tract systems (Toll-like receptor signaling).25–29 This suggests that interventions such as coadministration of probiotics to patients with left-sided brain injury may be especially important in HAI prevention.30–34
A surprising finding from our study was that patients with right-sided brain injury had a significantly higher proportion of infections caused by ESBL-producing bacteria relative to those with left-sided brain injury. Research has shown that previous antimicrobial drug exposure is a risk factor for colonization and subsequent positive clinical cultures for ESBL-producing bacteria.35 On this note, we performed a post hoc analysis on the 35 patients with infections caused by ESBL-producing bacteria, with the data showing a statistical trend (χ2=2.99, P=.08, NS) for a greater proportion of right-sided (52%) relative to left-sided brain-injured patients (20%) having received antibiotics while in acute care. Therefore, the higher prevalence of ESBL-producing bacteria in our sample of right-sided brain-injured patients may not relate to brain injury per se, but rather differences in antimicrobial drug exposure during initial acute care and before inpatient rehabilitation admission.
Whatever the mechanisms of differences of increased vulnerability to HAIs in patients with left-sided brain injury, it is possible that management and treatment strategies tailored to the side of brain injury might alter the occurrence of HAIs. Creating special care pathways for patients with left-sided brain injury (to reduce exposure to virulent and antibiotic-resistant bacteria), and techniques to improve left cortical activation, such as behavioral training (eg, card games) that has been proposed to engage the left side of the brain,15 might decrease HAIs during inpatient rehabilitation.
If the left-right brain activity balance influences immunity, this is important to consider in planning noninvasive brain stimulation techniques such as transcranial magnetic stimulation36 or transcranial direct current electrical stimulation of the brain.37 Although noninvasive stimulation that increases left-sided brain activity and dominance might improve immune capacity and function, some noninvasive techniques may inhibit left-sided brain activity,38 and these might have adverse effects on health outcomes after stroke by increasing the incidence of HAIs. Both the overall patient benefits and cost-effectiveness of right-sided brain-stimulating treatments that might increase vulnerability to HAIs may require careful assessment.
Study limitations
Our study was conducted during inpatient rehabilitation. However, medical care of patients with brain injury is becoming structured around the complete hospital-to-home transition, including both the initial days of intensive care and hospital ward monitoring, as well as the inpatient rehabilitation period that follows. Because our study was conducted retrospectively, however, and most brain lesion data were not sufficient for lesion-volume analysis, our study findings are limited by our inability to control for the influence of lesion size on immune function. Future studies should evaluate whether larger lesions are positively correlated with more total and C difficile HAIs in brain-injured patients during either acute hospital care or inpatient rehabilitation.20 Although we were unable to identify clinical or demographic differences accounting for the higher proportion of HAIs among patients with left-sided brain injury, a prospective study would also need to exclude (or at least stratify against) patients in whom there had been significant medical events or surgical/invasive procedures during acute care (eg, operations, catheter placement, tracheostomy, PEG), as well as a history of health conditions leading to a greater risk of intercurrent infection (eg, immunosuppression). Unfortunately, in this retrospective study, we worked with data that were not collected to address the issue of risk for all types of infection, but were collected to improve the quality of medical care. Therefore, prospective studies should also take a range of infection types into account when examining hemispheric differences in infection risk after brain injury. In that way, a more robust case can be made for the association between brain lateralization and HAIs.
Finally, it is important to confirm our findings prospectively because infection may not be documented consistently, even if it is being administratively monitored to ensure quality control, as was the case in this study. A prospective study should set specific criteria for diagnosis of infection and for classification of type of infection. Additionally, this initial study classified patients as having left-sided versus right-sided brain injury based on their documented clinical syndrome and brain images that were read and interpreted as part of usual and standard care by an attending radiologist, who provided a written report. A prospective study would need to screen subjects carefully for signs of confounding brain processes in patients classified as having hemispheric injury, such as brainstem or cerebellar events or diffuse axonal injury in the other hemisphere. A prospective study would also need to confirm the radiologist’s readings by review of brain images by blinded independent raters.
Conclusions
The findings from our study are consistent with the hypothesis that an LD-BIN may modulate the immune system and influence an individual’s risk for clinically relevant in-hospital events. The LD-BIN may factor into appropriate stratification of patients in studies of stroke and brain injury outcome, strategies to manage and decrease acquired infections during the acute hospital and inpatient rehabilitation periods, and planning other behavioral and physiologic treatments to activate 1 side of the brain specifically, in order to translationally optimize both quality of care and neuroscience-based principles of neurologic treatment.
Acknowledgments
We thank Steven Kirshblum, MD, for his scientific advice and valuable suggestions related to the project and manuscript.
Supported by the Kessler Foundation and the National Institutes of Health (grant nos. R01NS055808, K24HD062647).
List of abbreviations
- ESBL
extended-spectrum β-lactamase
- HAI
hospital-acquired infection
- LD-BIN
left-dominant brain immune network
- MRSA
methicillin-resistant Staphylococcus aureus
- NS
not significant
- PEG
percutaneous endoscopic gastrostomy
- TBI
traumatic brain injury
- VRE
vancomycin-resistant enterococcus
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 2.Griffin DG. The injured brain: TBI, mTBI, the immune system, and infection: connecting the dots. Mil Med. 2011;176:364–8. doi: 10.7205/milmed-d-10-00021. [DOI] [PubMed] [Google Scholar]
- 3.Helling TS, Evans LL, Fowler DL, Hays LV, Kennedy FR. Infections complications in patients with severe head injury. J Trauma. 1988;28:1575–7. doi: 10.1097/00005373-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Fassbender K, Dempfle CE, Mielke O, et al. Proinflammatory cytokines: indicators of infection in high-risk patients. J Lab Clin Med. 1997;130:535–9. doi: 10.1016/s0022-2143(97)90131-1. [DOI] [PubMed] [Google Scholar]
- 5.Hilker R, Poetter C, Findeisen N, et al. Nosocomial pneumonia after acute stroke. Implications for neurological intensive care medicine. Stroke. 2003;34:975–81. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 6.Mylotte JM, Graham R, Kahler L, Young L, Goodnough S. Epidemiology of nosocomial infection and resistant organisms in patients admitted for the first time to an acute rehabilitation unit. Clin Infect Dis. 2000;30:425–32. doi: 10.1086/313708. [DOI] [PubMed] [Google Scholar]
- 7.Dziedzic T, Slowik A, Szczudlik A. Nosocomial infections and immunity: lesson from brain-injured patients. Crit Care. 2004;8:266–70. doi: 10.1186/cc2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardos P, Degenne D, Lebranchu Y, Biziere K, Renoux G. Neocortical lateralization of NK activity in mice. Scand J Immunol. 1981;13:609–11. doi: 10.1111/j.1365-3083.1981.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 9.Renoux G, Biziere K, Renoux M, Guillaumin JM, Degenne D. A balanced brain asymmetry modulates T cell-mediated events. J Neuroimmunol. 1983;5:227–38. doi: 10.1016/0165-5728(83)90043-7. [DOI] [PubMed] [Google Scholar]
- 10.Barneoud P, Neveu PJ, Vitiello S, Le Moal M. Functional heterogeneity of the right and left cerebral neocortex in the modulation of the immune system. Physiol Behav. 1987;41:525–30. doi: 10.1016/0031-9384(87)90306-4. [DOI] [PubMed] [Google Scholar]
- 11.Neveu PJ, Barneoud P, Georgiades O, Vitiello S, Vincendeau P, Le Moal M. Brain neocortex influence on the mononuclear phagocyte system. J Neurosci Res. 1989;22:188–93. doi: 10.1002/jnr.490220212. [DOI] [PubMed] [Google Scholar]
- 12.Vlajković S, Nikolić V, Nikolić A, Milanović S, Janković BD. Asymmetrical modulation of immune reactivity in left- and right-biased rats after ipsilateral ablation of the prefrontal, parietal and occipital brain neocortex. Int J Neurosci. 1994;78:123–34. doi: 10.3109/00207459408986051. [DOI] [PubMed] [Google Scholar]
- 13.Członkowska A, Korlak J, Kuczyňska A. Lymphocyte subsets after stroke. Ann N Y Acad Sci. 1988;540:608–10. doi: 10.1111/j.1749-6632.1988.tb27188.x. [DOI] [PubMed] [Google Scholar]
- 14.RosenKranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100:11148–52. doi: 10.1073/pnas.1534743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond MC, Weidner J, Schow P, Grell S, Everett M. Mental stimulation increases circulating CD4-positive lymphocytes: a preliminary study. Brain Res Cogn Brain Res. 2001;12:329–31. doi: 10.1016/s0926-6410(01)00059-3. [DOI] [PubMed] [Google Scholar]
- 16.Gruzelier J, Burgess A, Baldeweg T, et al. Prospective associations between lateralised brain function and immune status in HIV infection: analysis of EEG, cognition and mood over 30 months. Int J Psychophysiol. 1996;23:215–24. doi: 10.1016/s0167-8760(96)00064-5. [DOI] [PubMed] [Google Scholar]
- 17.Koch HJ, Uyanik G, Bogdahn U, Ickenstein GW. Relation between laterality and immune response after acute cerebral ischemia. Neuroimmunomodulation. 2006;13:8–12. doi: 10.1159/000092108. [DOI] [PubMed] [Google Scholar]
- 18.Meador KJ, Loring DW, Ray PG, Helman SW, Vazquez BR, Neveu PJ. Role of cerebral lateralization in control of immune processes in humans. Ann Neurol. 2004;55:840–4. doi: 10.1002/ana.20105. [DOI] [PubMed] [Google Scholar]
- 19.Summer RC, Parton A, Nowicky AV, Kishore U, Gidron Y. Hemispheric lateralization and immune function: a systematic review of human research. J Neuroimmunol. 2011;240:1–12. doi: 10.1016/j.jneuroim.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Minnerup J, Wersching H, Brokinkel B, et al. The impact of lesion location and lesion size on post-stroke infection frequency. J Neurol Neurosurg Psychiatry. 2010;81:198–202. doi: 10.1136/jnnp.2009.182394. [DOI] [PubMed] [Google Scholar]
- 21.American Medical Association. International Classification of Diseases, 9th Revision, Clinical Modification. Chicago: American Medical Association; 2008. [Google Scholar]
- 22.Hamilton BB, Granger CV, Sherwin FS, Zielezny M, Tashman JS. A uniform national data system for medical rehabilitation. In: Fuhrer MJ, editor. Rehabilitation outcomes: analysis and measurement. Baltimore: Brooks; 1987. [Google Scholar]
- 23.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acidesuppressive agents and the risk of community acquired Clostridium difficile associated disease. JAMA. 2005;294:2984–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 24.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33–8. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins PA, Sriskandan S. Mammalian Toll-like receptors: to immunity and beyond. Clin Exp Immunol. 2005;140:395–407. doi: 10.1111/j.1365-2249.2005.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandl K, Plitas G, Mihu CN, et al. Vancomycin-resistant enterococci exploit antibiotic induced innate immune deficits. Nature. 2008;455:804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201:534–43. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun. 2011;79:1498–503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000;95(1 Suppl):S11–3. doi: 10.1016/s0002-9270(99)00809-6. [DOI] [PubMed] [Google Scholar]
- 31.Goldin BR, Gorbach SL. Clinical indications for probiotics: an overview. Clin Infect Dis. 2008;46(2 Suppl):S96–100. doi: 10.1086/523333. [DOI] [PubMed] [Google Scholar]
- 32.Kim AS. Using the good to beat out the bad: probiotics for eliminating vancomycin-resistant enterococci colonization. J Clin Gastroenterol. 2011;45:844–5. doi: 10.1097/MCG.0b013e31823336cd. [DOI] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Lan JG, Cruickshank SM, Singh JC, et al. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J Gastroenterol. 2005;11:3375. doi: 10.3748/wjg.v11.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris AD, McGregor JC, Johnson JA, et al. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis. 2007;13:1144–9. doi: 10.3201/eid1308.070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galletta EE, Rao PR, Barrett AM. Transcranial magnetic stimulation (TMS): potential progress for language improvement in aphasia. Top Stroke Rehabil. 2011;18:87–91. doi: 10.1310/tsr1802-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 38.Koch G, Bonnì S, Giacobbe V, et al. θ-Burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology. 2012;78:24–30. doi: 10.1212/WNL.0b013e31823ed08f. [DOI] [PubMed] [Google Scholar]