Pit cells were first described by Wisse et al.1 in 1976. Later, Kaneda et al.2 referred to this cell type as “natural killer (NK) cells” due to their resemblance to large granular lymphocytes that are known to possess NK cell activity. In 1984, Kaneda et al.3 also observed that in autoimmune hepatitis, these pit cells (now known as liver-specific NK cells) were frequently seen in close contact with hepatocytes that eventually showed degenerative or immature features, providing the first evidence that NK cells may contribute to hepatocellular damage in autoimmune hepatitis. Although more than 2 decades have since passed and the accumulating evidence indicates that NK cells are involved in the pathogenesis of many types of human autoimmune diseases,4 little is known about the role of NK cells in autoimmune liver disease specifically. To date, there are several studies reporting that NK cell functions are dysregulated in autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cirrhosis (PBC). These data show that in patients with PBC, there was increased cell-killing activity and decreased cytokine production in NK cells5 and that there appeared to be a genetic link between NK cell receptor ligand expression and susceptibility to primary sclerosing cholangitis.6 However, the mechanism by which NK cells are activated and contribute to the pathogenesis of autoimmune liver disease was largely unknown until Shimoda et al.7 published their recent data in this issue of Hepatology.
NK cells represent a small percentage of blood lymphocytes that have the ability to kill cancer cells and virus-infected cells through release of small cytoplasmic granules of perforin, granzymes, Fas ligand, or TRAIL (tumor necrosis factor–related apoptosis-inducing ligand). In contrast to the low percentage of NK cells in peripheral blood, liver lymphocytes are enriched in NK cells, accounting for 15%-30% of all liver lymphocytes that play an important role in immunosurveillance against tumor transformation and viral infection in the liver.8 It was originally thought that without requirement of activation, NK cells can kill target cells that are missing “self” markers of the major histocompatibility complex class I. It is now known that NK cells do require activation before killing target cells. Activation of NK cells is determined when there is an imbalance of signals from stimulatory and inhibitory receptors on the NK cells that interact with corresponding stimulatory and inhibitory ligands from target cells, respectively.9 If the stimulatory signal dominates over the inhibitory signal, NK cells become activated and kill target cells. NK cell stimulatory receptors include NKG2D, NKp46, NKp30, NKp44, and DNAC accessory molecule-1 (CD226). Among them, the NKG2D is the best characterized and is known to be activated by stimulatory ligands including RAE-1 (retinoic acid early inducible gene 1), histocompatibility 60, UL-16 binding protein-like transcript 1 expressed on mouse target cells, and MICA/B (major histocompatibility complex class I–related molecule A/B) and UL-16 binding proteins expressed on human target cells.9,10 In addition, NK cells are also activated by a variety of cytokines including interferons (IFNs), interleukin-2 (IL-2), IL-18, IL-12, and IL-15. Evidence has shown that type I IFNs play a key role in inducing NK cell activation, which in turn mediates death to virus-infected hepatocytes and inhibits hepatitis virus replication.11,12 Additionally, several Toll-like receptor (TLR) ligands can directly activate NK cells13 or stimulate surrounding antigen-presenting cells to produce cytokines that subsequently induce NK cell activation indirectly.14,15 TLRs are a group of proteins that recognize well-conserved microbial structures known as pathogen-associated molecular patterns. The TLR1, TLR2, TLR4, TLR5, TLR6, TLR10, and TLR11 proteins (TLR11 is present in mice, but not humans) are associated with plasma membranes and recognize bacterial cell wall components such as bacterial flagellin and viral particles. These proteins are distinct from TLR3, TLR7, TLR8, and TLR9, which are localized to the endosomes and recognize bacterial and viral nucleic acids. Many of the NK cell stimulatory ligands, cytokines, and TLR ligands have been implicated in NK cell activation, subsequently inducing liver injury, and inhibiting liver fibrosis and liver regeneration in animal models8 and in patients with viral hepatitis11,16 or nonalcoholic steatohepatitis.17
In this issue of Hepatology, Shimoda et al.7 provide evidence that in vitro activation of hepatic NK cells from patients with PBC required both TLR4 (direct stimulation) and TLR3 (indirect stimulation by activating monocytes to produce IFN-α, which then directly simulates NK cells). They also reported findings that in vitro treatment with TLR ligands and/or IFN-α did not affect the expression of NK cell stimulatory and inhibitory receptors on NK cells. However, it is not clear whether the expression of these NK cell receptors and their corresponding ligands on biliary epithelial cells were up-regulated in vivo in patients with PBC. A previous study reported that expression of NK cell stimulatory ligands was up-regulated in the livers of infants with biliary atresia and contributed to activation of NK cell–mediated ductal injury in these patients.18 Moreover, given the fact that inflammatory cytokines and several TLR ligands, which are elevated in the livers of patients with PBC, have been shown to augment the expression of NK cell stimulatory receptors and their ligands in liver injury models,8,19 it is plausible that the expression of these receptors and their ligands are up-regulated and contribute to the pathogenesis of PBC via activation of NK cells. Further studies are required to confirm this speculation.
Activated NK cells can participate in the pathogenesis of liver diseases directly by killing liver cells or by producing cytokines that affect liver cells.8 It has been shown that NK cells are able to kill autologous hepatocytes, stellate cells, and biliary epithelial cells in animal models of liver injury8,19; however, there are few studies that have examined the cytotoxicity of human NK cells against autologous liver cells because it is often not feasible to obtain liver NK cells and liver parenchyma or nonparenchyma cells from the same individual. However, Shimoda et al.7 took advantage of their ability to isolate primary human biliary epithelial cells and liver lymphocytes from the same patient, and perform cytotoxicity assessments with NK cells against autologous biliary epithelial cells. Based on their results, it was clearly demonstrated that only the specific combination of TLR4 and TLR3 was able to activate liver mononuclear cells to kill autologous biliary epithelial cells. It was also found that the liver mononuclear cells from patients with PBC had higher cytotoxicity after stimulation with TLR4 plus TLR3 than those from patients with viral hepatitis or alcoholic liver disease; however, why NK cells from patients with PBC had increased killing activity against autologous epithelial cells is not clear.
So, by what mechanism do NK cells kill biliary epithelial cells? It has been well-documented that activated NK cells kill target cells by releasing perforin, granzymes, Fas ligand, and TRAIL under various conditions. In the liver, NK cells express higher basal levels of TRAIL and have higher cytotoxic activity than peripheral NK cells. Additionally, TRAIL expression on liver NK cells is up-regulated by a range of factors (such as IFN-α, IFN-γ, TLR3 ligand, and so forth) and contributes to liver NK cell killing of activated stellate cells, stressed hepatocytes, and biliary epithelial cells in animal models of liver injury and in patients with liver disease.8 Interestingly, Shimoda et al.7 also confirmed that IFN-α treatment up-regulated TRAIL expression in liver NK cells and that TRAIL was a major factor contributing to the cytotoxicity of NK cells against autologous biliary epithelial cells.
Another interesting finding from this publication was that the TLR4 ligand, lipopolysaccharide (LPS), in synergy with IFN-α, was able to activate NK cells, which may have significant implications on the pathogenesis of other liver diseases. It is well known that gut bacteria–derived LPS is central to the pathogenesis of various types of liver disorders, including steatohepatitis,20,21 fibrosis,22,23 and liver cancer.24 Early studies suggested that LPS activation of TLR4 on Kupffer cells plays a central role in pathogenesis of these liver disorders.25 Recent studies report that LPS also activates TLR4 on hepatic stellate cells,22 sinusoidal endothelial cells,23 and hepatocytes,26 contributing to fibro-genesis and hepatocellular damage. The study by Shimoda et al.7 highlight an unappreciated mechanism by which LPS may contribute to the pathogenesis of liver diseases by activation of NK cells. Although Shimoda et al.7 reported that LPS alone did not induce the cytotoxicity of NK cells against autologous biliary epithelial cells, previous studies have shown that human NK cells express TLR4, and that LPS treatment stimulated human CD56+ NK cells to produce IFN-γ.27 This suggests that LPS alone is sufficient to stimulate NK cell production of cytokines, but is not able to enhance NK cell cytotoxicity. In addition, LPS can also stimulate macrophages, dendritic cells, and mast cells to produce cytokines that activate NK cells indirectly. Because hepatic LPS levels are elevated in alcoholic liver disease,20 it would be interesting to examine whether elevated LPS can activate NK cells, thereby contributing to the pathogenesis of this disease.
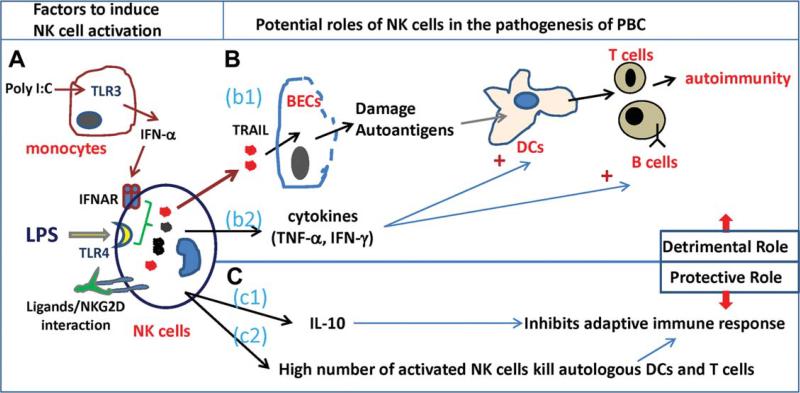
In summary, Shimoda et al.7 provided convincing in vitro evidence that NK cells kill autologous biliary epithelial cells, and that NK cells from patients with PBC have higher activity than those from patients with other liver diseases. However, the exact role of NK cells in the pathogenesis of PBC still remains unsolved. Figure 1 summarizes the potential roles of NK cell activation in the pathogenesis of PBC. NK cells likely play a detrimental role in PBC by killing biliary epithelial cells, which can lead to bile duct damage and abnormal exposure to autoantigens, and by their interaction with antigen-presenting cells and T cells, thereby enhancing adaptive immune responses. However, NK cells have also been implicated in suppression of certain types of autoimmune diseases by producing the anti-inflammatory cytokine (IL-10) that inhibits adaptive immune response, or by directly killing autologous dendritic cells and T cells.4 Thus, it appears that NK cells play a complex (either detrimental or protective) role in the pathogenesis of PBC and can modulate the initiation, maintenance, or the progression of the disease. Greater understanding of the role of NK cells in PBC may help us identify novel therapeutic strategies for the treatment of this disorder.
Fig. 1.
Potential roles of NK cells in the pathogenesis of PBC. (A) Several factors may contribute to NK cell activation, including TLR4 ligand (LPS) and IFN-α produced by TLR3 ligand (polyinosinic:polycytidylic acid [poly I:C])-activated monocytes, and the interaction of NK cell stimula-tory ligands and NKG2D on NK cells. (B) Detrimental roles of NK cells in the pathogenesis of PBC: (b1) activated NK cells kill biliary epithelial cell (BECs) by producing TRAIL, leading to bile duct damage and autoantigen release. Dendritic cells (DCs) present autoantigens to T cells, leading to autoimmunity, and (b2) activated NK cells produce cytokines that enhance functions of antigen-presenting cells and adaptive immunity. (C) Protective effects of NK cells in the pathogenesis of PBC: (c1) activated NK cells produce IL-10 that inhibits adaptive immune response, and (c2) activated NK cells kill autologous DCs and T cells, thereby inhibiting adaptive immune response.
Acknowledgments
This work was supported by the intramural program of National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health (NIH).
Abbreviations
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- NK
natural killer
- PBC
primary biliary cirrhosis
- TLR
toll-like receptor
- TRAIL
tumor necrosis factor–related apoptosis-inducing ligand
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Wisse E, van't Noordende JM, van der Meulen J, Daems WT. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173:423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- 2.Kaneda K, Dan C, Wake K. Pit cells as natural killer cells. Biomed Res. 1983;4:567–576. [Google Scholar]
- 3.Kaneda K, Kurioka N, Seki S, Wake K, Yamamoto S. Pit cell-hepatocyte contact in autoimmune hepatitis. HEPATOLOGY. 1984;4:955–958. doi: 10.1002/hep.1840040529. [DOI] [PubMed] [Google Scholar]
- 4.Schleinitz N, Vely F, Harle JR, Vivier E. Natural killer cells in human autoimmune diseases. Immunology. 2010;131:451–458. doi: 10.1111/j.1365-2567.2010.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, Eric Gershwin M. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Hov JR, Lleo A, Selmi C, Woldseth B, Fabris L, Strazzabosco M, Karlsen TH, Invernizzi P. Genetic associations in Italian primary sclerosing cholangitis: heterogeneity across Europe defines a critical role for HLA-C. J Hepatol. 2010;52:712–717. doi: 10.1016/j.jhep.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, Tsuneyama K, Nakanuma Y, Leung P, Ansari A, Gershwin M, Akashi K. The interaction between Toll like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. HEPATOLOGY. 2011;53:1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 10.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 11.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an inter-feron-alfa-dependent manner. Gastroenterology. 2010;138:325–335. e1–2. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, Schlaphoff V, Fytili P, Cornberg M, Manns MP, Geffers R, Pietschmann T, Guzman CA, Ljunggren HG, Wedemeyer H. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Lauzon NM, Mian F, MacKenzie R, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–112. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. HEPATOLOGY. 2009;49:940–949. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zhang S, Zou Z, Shi J, Zhao J, Fan R, Qin E, Li B, Li Z, Xu X, Fu J, Zhang J, Gao B, Tian Z, Wang FS. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. HEPATOLOGY. 2011;53:73–85. doi: 10.1002/hep.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahraman A, Schlattjan M, Kocabayoglu P, Yildiz-Meziletoglu S, Schlensak M, Fingas CD, Wedemeyer I, Marquitan G, Gieseler RK, Baba HA, Gerken G, Canbay A. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. HEPATOLOGY. 2010;51:92–102. doi: 10.1002/hep.23253. [DOI] [PubMed] [Google Scholar]
- 18.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest. 2009;119:2281–2290. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao B. NKG2D, its ligands, and liver disease — good or bad? HEPATOLOGY. 2010;51:8–11. doi: 10.1002/hep.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. HEPATOLOGY. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff S, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. HEPATOLOGY. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 22.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 23.Jagavelu K, Routray C, Shergill U, O'Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angio-genesis in the liver. HEPATOLOGY. 2010;52:590–601. doi: 10.1002/hep.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. HEPATOLOGY. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 25.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. HEPATOLOGY. 2010;52:1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 26.Raetzsch CF, Brooks NL, Alderman JM, Moore KS, Hosick PA, Klebanov S, Akira S, Bear JE, Baldwin AS, Mackman N, Combs TP. Lipopolysaccharide inhibition of glucose production through the Toll-like receptor-4, myeloid differentiation factor 88, and nuclear factor kappa b pathway. HEPATOLOGY. 2009;50:592–600. doi: 10.1002/hep.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mian MF, Lauzon NM, Andrews DW, Lichty BD, Ashkar AA. FimH can directly activate human and murine natural killer cells via TLR4. Mol Ther. 2010;18:1379–1388. doi: 10.1038/mt.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]