Abstract
Objective
The use of selective cerebral perfusion with warmer temperatures during circulatory arrest has been increasingly utilized for arch replacement over concerns regarding the safety of deep hypothermic circulatory arrest (DHCA). However, little data actually exists on outcomes following arch replacement and DHCA. This study examines modern results with DHCA for proximal arch replacement to provide a benchmark for comparison against outcomes with lesser degrees of hypothermia.
Methods
Between 7/2005–6/2010, 245 proximal arch replacements (“hemi-arch”) were performed using deep hypothermia; mean minimum core and nasopharyngeal temperatures were 18.0±2.1°C and 14.1±1.6°C, respectively. Adjunctive cerebral perfusion was used in all cases. Concomitant ascending aortic replacement was performed in 41%, ascending plus aortic valve replacement in 23%, and aortic root replacement in 32%.
Results
Mean age was 58±14 years; 36% procedures were urgent/emergent. Mean duration of DHCA was 20.4±6.2 minutes. Thirty day/in-hospital mortality was 2.9%. Rates of stroke, renal failure, and respiratory failure were 4.1% (0.8% for elective cases), 1.2%, and 0.4%, respectively.
Conclusions
Deep hypothermia with adjunctive cerebral perfusion for circulatory arrest during proximal arch replacement affords excellent neurologic as well as non-neurologic outcomes. Centers utilizing lesser degrees of hypothermia for arch surgery, the safety of which remains unproven, should ensure comparable results.
Keywords: aortic surgery, hemi-arch, circulatory arrest, hypothermia, aortic arch
Introduction
Preventing cerebral injury and other end-organ dysfunction during aortic arch surgery remains a formidable challenge, which has led to the evolution of a number of circulatory management strategies over recent decades1–17. A unifying, central tenet of these various approaches is a perfusion algorithm encompassing some level of hypothermic circulatory arrest (HCA) to minimize cerebral metabolic consumption and simultaneously enable a bloodless operative field. Traditionally, HCA has been rendered in the setting of profound or deep hypothermia (DHCA), with core temperatures of ≤18°C. Further refinements in cerebral protection have included the advent of antegrade (ACP) and retrograde (RCP) cerebral perfusion adjuncts to attenuate cerebral injury during extended periods of HCA (beyond 30–45 minutes) often requisite in complex aortic arch operations 5, 9, 16.
Despite the modest diminution in adverse outcomes in more recent DHCA series, many investigators remain concerned over the potentially deleterious consequences of deep hypothermia6, 7, 10, 18, 19. Among these concerns is a heightened risk of postoperative bleeding secondary to the coagulopathy precipitated not only by the deep hypothermia itself, but also by the prolonged periods of cardiopulmonary bypass necessary for cooling and rewarming. Some evidence also suggests deep hypothermia may cause direct neuronal injury and lead to subtle neurocognitive deficits that may persist long-term20–22.
Citing these theoretical and empirically based concerns as justification, an increasing number of aortic surgeons are utilizing warmer temperatures during HCA (25–28°C), in conjunction with selective cerebral perfusion. This paradigm shift, however, has been predicated largely on adverse outcome data derived from outmoded DHCA series. Contemporary outcomes of aortic arch replacement with DHCA remain sparsely reported in the literature. As such, the objective of the current study was to analyze modern results with DHCA for proximal arch replacement to establish a point of reference for comparison of outcomes with moderate hypothermia.
Patients and Methods
Between July 2005 and June 2010, N=245 proximal arch replacement (“hemi-arch”) procedures (Figure 1) were performed at a single referral institution. Indications for hemi-arch replacement were as previously described23. Preoperative, intraoperative, and postoperative variables were abstracted from the Duke Thoracic Aortic Surgery Database, a prospectively maintained clinical registry of all patients who have undergone a thoracic aortic procedure at Duke University Medical Center (Durham, NC) since July, 2005. This study was reviewed and approved by the Institutional Review Board of Duke University and the need for individual patient consent was waived.
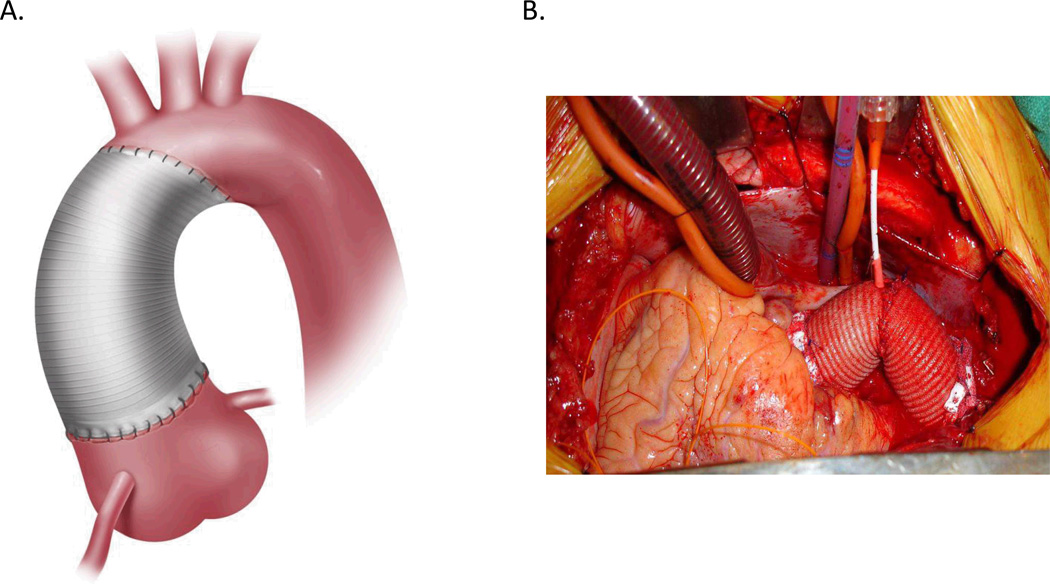
Figure1.
(A) Schematic illustration and (B) intraoperative photograph depicting ascending and hemi-arch aortic reconstruction.
All cases were performed via median sternotomy with intraoperative transesophageal echocardiographic (TEE) and invasive hemodynamic monitoring. Solumedrol (1gm, IV) was administered for pharmacologic neuroprotection to all patients preoperatively. Cardiopulmonary bypass with deep systemic hypothermia was utilized for all hemi-arch repairs, with the duration of cooling guided by neurocerebral monitoring with intraoperative electroencephalography (EEG) as previously described24 for all elective cases and when available for urgent/emergent cases for a total of n=201 cases (82%) monitored. In the unmonitored cases, patients were cooled for a minimum of 50 minutes or until the nasopharyngeal temperature was below 18 °C based on prior work by Stecker et al25. Mean minimum core and nasopharyngeal temperatures were 18.0 ± 2.1 °C and 14.1 ± 1.6 °C, respectively.
Upon completion of profound cooling and electrocerebral inactivity (ECI) by EEG, the circulation was halted, and the aortic arch was opened. Intraoperative assessment of aortic arch diameter was used to confirm preoperative imaging feasibility of proximal versus total arch replacement. Specifically, tapering of arch diameter to a normal caliber proximal to the left subclavian artery enabled hemi-arch replacement. This procedure was performed using the “peninsula” technique as described by Miller’s group at Stanford University26. Patients in whom the aortic arch remained dilated (≥ 40 mm) beyond the left subclavian artery required total arch replacement and are not included in this series.
Adjunctive ACP (n=218, 89%) or RCP (n=27, 11%) was used for cerebral protection in all circulatory arrest cases. ACP was the preferred adjunctive strategy with RCP being utilized in cases where the right axillary artery was not suitable for cannulation, generally because of a diameter <6 mm or dissection of the artery on preoperative computed tomography angiography (CTA). Dissection of the more proximal innominate artery was not a contraindication to right axillary cannulation. For ACP cases, the right axillary artery was cannulated using an 8 mm Dacron side graft technique. For RCP cases, the distal ascending aorta or, in cases of dissection involving the right axillary artery, the left axillary or femoral artery were chosen for arterial cannulation. If the distal ascending aorta or femoral artery were utilized, cannulation was switched to the hemi-arch graft after completion of this anastomosis. Perfusion data are summarized in Table 1.
Table 1.
Perfusion Data
| No. of Patients (%) N=245 |
|
|---|---|
| Cardiopulmonary Bypass Time, min (Mean±SD) | 212±50 |
| Aortic Cross-Clamp Time, min (Mean±SD) | 143±43 |
| DHCA Time, min (Mean±SD) | 20.4±6.2 |
| Antegrade Cerebral Perfusion | 218 (89) |
| - Duration, min (Mean±SD) | 16.3±6.3 |
| Retrograde Cerebral Perfusion | 27 (11) |
| - Duration, min (Mean±SD) | 18.1±5.1 |
DHCA, deep hypothermic circulatory arrest.
For performance of ACP, the base of the innominate and left common carotid arteries was clamped and perfusion was via the right axillary graft at a target flow rate of 5–15 cc/kg/min and an inflow temperature of 12°C to a target right radial arterial line pressure of 50–70 mm Hg. For RCP, flow (12°C) was retrograde via a long angled #26-French single stage venous cannula in the superior vena cava (SVC) with the SVC snared. RCP flows averaged 150–450 cc/min to a target central venous pressure of 25 mm Hg. Following completion of the hemi-arch anastomosis, CPB was reinstituted, and the patient re-warmed following a 5-minute period of cold reperfusion for free radical washout. During the rewarming phase other concomitant cardiac procedures were completed (Table 2).
Table 2.
Operative Details on N=245 Hemi-Arch Replacements
| No. of Patients (%) | |
|---|---|
| Ascending Aortic (AA) Replacement | 110 (45) |
| Wheat Procedure (AA + AVR) | 57 (23) |
| Aortic Root Replacement | 78 (32) |
| - Mechanical Composite | 25 (10) |
| - Pericardial Composite | 10 (4) |
| - Stentless Bioprosthetic | 28 (11) |
| - David Procedure (Valve-Sparing) | 15 (6) |
| Concomitant Cardiac Procedures | |
| - CABG | 52 (21) |
| - Maze Procedure | 6 (2) |
| - ASD Repair | 6 (2) |
| - Aortic Valve Repair | 9 (4) |
| - Mitral Valve Repair/Replacement | 4 (2) |
| - Other | 17 (7) |
AA, ascending aortic; AVR, aortic valve replacement
Antifibrinolytic therapy was routinely administered intraoperatively; prior to withdrawal from the U.S. market, aprotinin (Bayer Corporation, West Haven, CT) was used (2MU bolus and 0.5Mu/hr infusion until bleeding cessation). Subsequently, epsilon aminocaproic acid was administered as a 10g bolus followed by a 1g/hr infusion with an additional 5g bolus prior to separation from cardiopulmonary bypass. The return of washed, shed red blood cells (BRAT II cell saver, Cobe, Arvada, CO) to the patient was routine. Transfusion decisions in the perioperative period were aided by local guidelines and use of chest tube output, activated clotting time, platelet count, fibrinogen level, thromboelastogram, prothrombin, and partial thromboplastin time tests as recommended by the American Society of Anesthesiologists published guidelines27. Clopidogrel or other P2Y12 inhibitors, regardless of dose, were held 7 days prior to operation. Aspirin 325mg (but not 81mg) was held 5 days prior to operation. All antiplatelet agents were restarted at preoperative dose on postoperative day 1 unless active bleeding was noted.
Adverse perioperative events were prospectively documented and analyzed according to previously published guidelines for reporting morbidity and mortality after cardiac valvular operations28. All patients were re-examined with either gated MRA or CTA with transthoracic echocardiography at serial follow-up visits, 6–9 months post-surgery and then annually thereafter. The present report includes all data collected through the patients’ most recent follow-up visit. Deaths from all causes were included in the analysis. In addition, the social security death index was queried (http://ssdi.rootsweb.com/) to confirm all patient deaths.
Patient characteristics were reported as percentages for discrete variables. Means and standard deviations were provided for continuous variables. For comparisons, the Wilcoxon Rank Sum test was used for continuous variables, and the Chi-squared test for categorical variables, with an alternative hypothesis that the rates between groups were not different. All statistical analyses were computed using SAS version 9.1.3.
Results
A summary of pertinent preoperative patient variables is provided in Table 3. Mean patient age was 58 with a range of 19 to 87. Most of the patients were male (71%) and 37% presented with symptoms attributable to aortic pathology, including back pain, anterior chest pain, malperfusion, and syncope. A number of patients presented with acute aortic syndromes necessitating urgent/emergent operation (36%) including acute type A dissection (22%), aortic rupture (7%), and cardiac tamponade (2%). A sizeable proportion of patients (16%) had undergone previous cardiac operation via sternotomy. The maximal aortic diameter ranged from 4.5 cm to 9.5 cm with a mean of 5.6±0.9 cm.
Table 3.
Preoperative Patient Characteristics
| No. of Patients (%) N=245 |
|
|---|---|
| Age, y (mean+SD) | 58±14 |
| - Range | 19–87 |
| Females | 70 (29) |
| Presenting Aortic Symptoms | 91 (37) |
| - Back Pain | 18 (7) |
| - Anterior Chest Pain | 73 (30) |
| - Malperfusion | 6 (2) |
| - Syncope | 9 (4) |
| Maximal Aortic Diameter, cm (Mean±SD) | 5.6±0.9 |
| - Range | 4.5–9.5 |
| Urgent/Emergency Operation | 89 (36) |
| - Acute Type A Dissection | 54 (22) |
| - Aortic Rupture | 16 (7) |
| - Cardiac Tamponade | 4 (2) |
| Redo Sternotomy | 39 (16) |
Procedures performed varied according to the extent of thoracic aortic and cardiac pathology and are summarized in Table 2. In addition to hemi-arch replacement, the majority of patients (>60%) required ascending aortic replacement either alone (45%) or in conjunction with aortic valve replacement (Wheat procedure, 23%). A significant proportion of patients also presented with disease of the aortic root meeting criteria for surgical repair (N=78, 32%). Aortic root replacement with performed with mechanical (N=30, 12%) or stented bovine pericardial (N=15, 6%) composite prostheses, commercially available stentless porcine full aortic roots (N=33, 13%), or with a valve-sparing David approach (N=15, 6%). Other concomitant cardiac procedures included coronary artery bypass grafting (N=52, 21%), Maze procedure for atrial fibrillation (N=6, 2%), atrial septal defect repair (N=6, 2%), aortic valve repair (N=9, 4%), mitral valve procedures (N=4, 2%), as well as other procedures (N=17, 7%).
Following hemi-arch replacement, the median postoperative length of stay was six days (Table 4). 30-day/in-hospital mortality was 2.9% (3.2% and 2.2% for elective and urgent/emergency cases, respectively); 365-day mortality was 3.3%. The overall rate of postoperative stroke was 4.1%; among elective cases (N=156), only one stroke occurred (0.8%). Seven patients (2.9%) required operative re-exploration for hemorrhage. Only one patient (0.4%) suffered postoperative respiratory failure necessitating tracheostomy. Renal failure requiring hemodialysis occurred in three patients (1.2%). During the median follow-up period of 21 months, 2 deaths occurred beyond hospitalization, yielding an actuarial survival rate of 99%.
Table 4.
Postoperative Outcomes
| No. (%) N=245 |
|
|---|---|
| Hospital Length of Stay, days (Median) | 6 |
| - 25th, 75th percentile | 5, 8 |
| 30-Day Mortality | 7 (2.9) |
| 30-Day Morbidity | |
| - Stroke | 10 (4.1) |
| - Elective Cases (N=156) | 1 (0.8) |
| - Re-Exploration for Bleeding | 7 (2.9) |
| - Renal Failure Requiring Dialysis | 3 (1.2) |
| - Respiratory Failure | 1 (0.4) |
Discussion
Central to the debate over the temperature nadir of HCA is the contention that deep hypothermia potentiates bleeding risk, is directly injurious to the brain and other organ systems, and therefore poses significant perioperative morbidity and mortality. The results from the present study would appear to refute these notions, however. With a rate of postoperative hemorrhage requiring re-exploration of only 2.9%, the reputed bleeding hazard imparted by DHCA may be overstated. The validity of this assertion is substantiated by a recent report by Kamiya and colleagues of HCA cases performed at moderate temperatures, where the rate of re-sternotomy was more than four times greater (14.1%)7 than the current series. This comparison is not entirely fair, however, as total arch replacements accounted for 25% of cases in the moderate hypothermia series, whereas only proximal arch replacements are included in this current analysis. Regardless, even with propensity matching, the study by Kamiya failed to demonstrate any discernible difference in bleeding or duration of cardiopulmonary bypass in moderate versus deep HCA cases7. These findings underscore the fact that other factors beside profound hypothermia, such as emergency surgery or preoperative hemoglobin, figure more prominently as predictors of hemorrhage following HCA29.
Considering the high acuity (36% non-elective) and procedural complexity of the patients in the present series, the operative mortality was quite low (2.9%) and compares very favorably with other large published series of DHCA for aortic arch replacement4, 8, 9, 15, 16, 30–32. More specifically, in a review of 20 published series including over 2000 patients and spanning the time period 1989–2007, Elefteriades calculated an overall operative mortality of 9.6% following aortic arch surgery with DHCA17. Unpublished work from the authors’ institution evaluating outcomes for ascending/arch replacement in North America (N = 45,894) using the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD) demonstrate an overall operative mortality of 8.9%, including 3.4% and 15.4% for elective and non-elective cases, respectively. Finally, the 15–30% operative mortality reported by studies from the International Registry for Acute Aortic Dissection (IRAD) for acute Type A dissection repair was nearly 5–10 times that observed in the present study33, 34.
Relative to that reported in recent series of moderate hypothermia5, 7, 18, 19, the significantly reduced operative mortality and morbidity presented here further supports the safety and efficacy of DHCA approaches. For example, in a retrospective comparison of moderate HCA with selective ACP (N=205) versus DHCA alone (N=66) at Emory University, the operative mortality in the moderate HCA group was 8.8% (and 22.7% in the DHCA alone group)5, which is significantly higher than the 2.9% reported herein. Further, the results of the Emory study, if taken at face value, are misleading given that a significantly greater proportion of the DHCA patients were emergencies (61% versus 32% in the moderate hypothermia group). In addition, no adjunctive cerebral perfusion was utilized in the DHCA group, an adjunct we deem to be a key facet of an optimal neuroprotective strategy. In one of the largest series ever published of aortic arch reconstruction with DHCA and RCP (N=682) and moderate HCA with ACP (N=94), Bavaria’s group from the University of Pennsylvania reported uniformly excellent outcomes with operative mortalities of 2.8% and 3.2%, respectively12. An important caveat to consider when comparing the Penn results to those of the current study, however, is that all of the cases in the Penn series were elective. Last year, the Emory group published an update on their outcomes with moderate hypothermia and unilateral selective ACP in N=412 patients19. The operative mortality in that study, which included a very similar patient cohort to that in the present report, was 7.7% with a 4.6% incidence of renal failure requiring dialysis and a postoperative length of stay averaging 10 days.
The relatively high rate of postoperative renal failure in this latter study raises important concerns about the susceptibility to visceral ischemia and other end-organ dysfunction implicit in HCA performed with warmer temperatures. This effect becomes especially pronounced during more complex cases when aortic arch reconstruction times extend beyond thirty minutes12. Therefore, profound hypothermia may be an indispensable modality during such aortic cases where the anticipated duration of HCA may be prolonged, and preservation of end-organ viability beyond the brain is at a premium. As demonstrated by the current results, proximal arch replacement under deep hypothermia can minimize ischemic insult to the periphery with resultant low rates of renal (1.2%) and respiratory failure (0.4%), along with 0% rate of paraplegia. To date, published data5, 7, 12, 19 do not support moderate hypothermia approaches utilizing selective ACP as providing an equal degree of protection to non-brain organs. On the contrary, in vivo studies in large animal models of moderate HCA have revealed that the spinal cord may be particularly susceptible to ischemic injury under such conditions with an alarmingly high incidence of irreversible paraplegia at 60 minutes35. The enhanced cytoprotective effects ascribed to deep versus moderate HCA may, at the molecular level, entail upregulation of the small-ubiquitin-like modifier (SUMO) conjugation pathway36. Further delineation of these molecular mechanisms are hopefully forthcoming, but these and other data already available provide a compelling argument for deep hypothermia as the preferred perfusion strategy for HCA.
Formal assessment for subtle neurocognitive deficits following DHCA was not performed in this study. Notwithstanding, the rates of permanent neurologic dysfunction such as stroke were relatively low and virtually nonexistent among electively performed cases (0.8%). In the previously mentioned large series of electively performed arch procedures reported by Milewski12 and colleagues from the University of Pennsylvania, the rate of permanent neurologic dysfunction was approximately 3% for both DHCA and moderate HCA approaches. Proponents of moderate hypothermia have cited a higher incidence of postoperative delirium in procedures utilizing DHCA18, along with other more subtle indices of neurocognitive impairment20–22. An in-depth psychometric analysis of DHCA patients recently performed at Yale discounts these claims, however, documenting complete preservation of cognitive capacity, even among high-cognitive patients37.
Study Limitations
The authors acknowledge that even slight neurocognitive deficits following any cardiac operation have important prognostic implications38. Consequently, one criticism of the current study is the lack of comprehensive neurocognitive evaluation both in the perioperative period and in long-term follow-up. Future studies will therefore need to incorporate such formal evaluations. Moreover, the current study was retrospective in design and did not include a comparison group, such as a patient cohort in whom moderate HCA was utilized. Prospective, randomized studies directly comparing deep and moderate HCA, in conjunction with selective cerebral perfusion techniques, would undoubtedly provide valuable and accurate insight into which circulatory management strategy maximizes neuroprotective effects while preserving peripheral organ function.
Conclusions
In summary, the use of deep hypothermia with adjunctive cerebral perfusion for circulatory arrest during proximal arch replacement affords excellent neurologic as well as non-neurologic outcomes. Centers utilizing lesser degrees of hypothermia for arch surgery, the safety of which remains unproven, should ensure comparable results.
References
- 1.Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral perfusion with cold blood: A 13-year experience. Ann Thorac Surg. 1999;67:1874–1878. doi: 10.1016/s0003-4975(99)00411-7. discussion 1891-1874. [DOI] [PubMed] [Google Scholar]
- 2.Cooley DA, Mahaffey DE, De Bakey ME. Total excision of the aortic arch for aneurysm. Surg Gynecol Obstet. 1955;101:667–672. [PubMed] [Google Scholar]
- 3.Crawford ES, Saleh SA, Schuessler JS. Treatment of aneurysm of transverse aortic arch. J Thorac Cardiovasc Surg. 1979;78:383–393. [PubMed] [Google Scholar]
- 4.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–1063. [PubMed] [Google Scholar]
- 5.Halkos ME, Kerendi F, Myung R, et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg. 2009;138:1081–1089. doi: 10.1016/j.jtcvs.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Harrington DK, Lilley JP, Rooney SJ, Bonser RS. Nonneurologic morbidity and profound hypothermia in aortic surgery. Ann Thorac Surg. 2004;78:596–601. doi: 10.1016/j.athoracsur.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: A propensity score analysis. J Thorac Cardiovasc Surg. 2007;133:501–509. doi: 10.1016/j.jtcvs.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Kazui T, Washiyama N, Muhammad BA, et al. Total arch replacement using aortic arch branched grafts with the aid of antegrade selective cerebral perfusion. Ann Thorac Surg. 2000;70:3–8. doi: 10.1016/s0003-4975(00)01535-6. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 9.Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg. 2007;83:S796–S798. doi: 10.1016/j.athoracsur.2006.10.082. discussion S824-731. [DOI] [PubMed] [Google Scholar]
- 10.Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: A risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–914. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 11.Kouchoukos NT, Masetti P. Total aortic arch replacement with a branched graft and limited circulatory arrest of the brain. J Thorac Cardiovasc Surg. 2004;128:233–237. doi: 10.1016/j.jtcvs.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: Results in short elective arch reconstructive times. Ann Thorac Surg. 2010;89:1448–1457. doi: 10.1016/j.athoracsur.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 13.Minakawa M, Fukuda I, Yamauchi S, et al. Early and long-term outcome of total arch replacement using selective cerebral perfusion. Ann Thorac Surg. 2010;90:72–77. doi: 10.1016/j.athoracsur.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Strauch JT, Spielvogel D, Lauten A, et al. Technical advances in total aortic arch replacement. Ann Thorac Surg. 2004;77:581–589. doi: 10.1016/S0003-4975(03)01342-0. discussion 589–590. [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106:19–28. discussion 28–31. [PubMed] [Google Scholar]
- 16.Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg. 2002;74:2040–2046. doi: 10.1016/s0003-4975(02)04023-7. [DOI] [PubMed] [Google Scholar]
- 17.Elefteriades JA. What is the best method for brain protection in surgery of the aortic arch? Straight dhca. Cardiol Clin. 2010;28:381–387. doi: 10.1016/j.ccl.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Cook RC, Gao M, Macnab AJ, et al. Aortic arch reconstruction: Safety of moderate hypothermia and antegrade cerebral perfusion during systemic circulatory arrest. J Card Surg. 2006;21:158–164. doi: 10.1111/j.1540-8191.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 19.Leshnower BG, Myung RJ, Kilgo PD, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: A contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg. 2010;90:547–554. doi: 10.1016/j.athoracsur.2010.03.118. [DOI] [PubMed] [Google Scholar]
- 20.Kumral E, Yuksel M, Buket S, et al. Neurologic complications after deep hypothermic circulatory arrest: Types, predictors, and timing. Tex Heart Inst J. 2001;28:83–88. [PMC free article] [PubMed] [Google Scholar]
- 21.Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg. 1999;117:156–163. doi: 10.1016/s0022-5223(99)70481-2. [DOI] [PubMed] [Google Scholar]
- 22.Welz A, Pogarell O, Tatsch K, et al. Surgery of the thoracic aorta using deep hypothermic total circulatory arrest. Are there neurological consequences other than frank cerebral defects? Eur J Cardiothorac Surg. 1997;11:650–656. doi: 10.1016/s1010-7940(96)01129-3. [DOI] [PubMed] [Google Scholar]
- 23.Lima B, Williams JB, Bhattacharya SD, Shah AA, Andersen N, Wang A, Harrison JK, Hughes GC. Individualized thoracic aortic replacement for the aortopathy of bicuspid aortic valve disease. Journal of Heart Valve Disease. 2011 (in press) [PMC free article] [PubMed] [Google Scholar]
- 24.Husain AM, Ashton KH, Hughes GC. Thoracic aortic surgery. In: Husain AM, editor. A practical approach to neurophysiologic intraoperative monitoring. New York: Demos Medical Publishing; 2008. pp. 227–259. [Google Scholar]
- 25.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 26.Itoh A, Fischbein M, Arata K, Miller DC. "Peninsula-style" transverse aortic arch replacement in patients with bicuspid aortic valve. Ann Thorac Surg. 2010;90:1369–1371. doi: 10.1016/j.athoracsur.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the american society of anesthesiologists task force on perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg. 2008;135:732–738. doi: 10.1016/j.jtcvs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Williams JB, Phillips-Bute B, Bhattacharya SD, et al. Predictors of massive transfusion with thoracic aortic procedures involving deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg. 2007;84:1955–1964. doi: 10.1016/j.athoracsur.2007.07.017. discussion 1955–1964. [DOI] [PubMed] [Google Scholar]
- 31.Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: Experience with seventy patients. J Thorac Cardiovasc Surg. 2000;119:558–565. doi: 10.1016/s0022-5223(00)70136-x. [DOI] [PubMed] [Google Scholar]
- 32.Numata S, Ogino H, Sasaki H, et al. Total arch replacement using antegrade selective cerebral perfusion with right axillary artery perfusion. Eur J Cardiothorac Surg. 2003;23:771–775. doi: 10.1016/s1010-7940(03)00090-3. discussion 775. [DOI] [PubMed] [Google Scholar]
- 33.Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type a aortic dissection: The international registry of acute aortic dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type a aortic dissection: The international registry of acute aortic dissection experience. J Thorac Cardiovasc Surg. 2005;129:112–122. doi: 10.1016/j.jtcvs.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Etz CD, Luehr M, Kari FA, et al. Selective cerebral perfusion at 28 degrees c--is the spinal cord safe? Eur J Cardiothorac Surg. 2009;36:946–955. doi: 10.1016/j.ejcts.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 36.Yang W, Ma Q, Mackensen GB, Paschen W. Deep hypothermia markedly activates the small ubiquitin-like modifier conjugation pathway; implications for the fate of cells exposed to transient deep hypothermic cardiopulmonary bypass. J Cereb Blood Flow Metab. 2009;29:886–890. doi: 10.1038/jcbfm.2009.16. [DOI] [PubMed] [Google Scholar]
- 37.Percy A, Widman S, Rizzo JA, et al. Deep hypothermic circulatory arrest in patients with high cognitive needs: Full preservation of cognitive abilities. Ann Thorac Surg. 2009;87:117–123. doi: 10.1016/j.athoracsur.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Hammon JW, Stump DA, Butterworth JB, Moody DM. Approaches to reduce neurologic complications during cardiac surgery. Semin Thorac Cardiovasc Surg. 2001;13:184–191. doi: 10.1053/stcs.2001.24079. [DOI] [PubMed] [Google Scholar]