Abstract
Site-selective scission of ribonucleic acids (RNAs) has attracted considerable interest, since RNA is an intermediate in gene expression and the genetic material of many pathogenic viruses. Polyamine-imidazole conjugates for site-selective RNA scission, without free imidazole, were synthesized and tested on yeast phenylalanine transfer RNA. These molecules catalyze RNA hydrolysis non-randomly. Within the polyamine chain, the location of the imidazole residue, the numbers of nitrogen atoms and their relative distances have notable influence on cleavage selectivity. A norspermine derivative reduces the cleavage sites to a unique location, in the anticodon loop of the tRNA, in the absence of complementary sequence. Experimental results are consistent with a cooperative participation of an ammonium group of the polyamine moiety, in addition to it’s binding to the negatively charged ribose-phosphate backbone, as proton source, and the imidazole moiety as a base. There is correlation between the location of the magnesium binding sites and the RNA cleavage sites, suggesting that the protonated nitrogens of the polycationic chain compete with some of the magnesium ions for RNA binding. Therefore, the cleavage pattern is specific of the RNA structure. These compounds cleave at physiological pH, representing novel reactive groups for antisense oligonucleotide derivatives or to enhance ribozyme activity.
INTRODUCTION
Nucleic acids, although not endowed with the functional diversity of protein enzymes, are suited for sequence-specific recognition of RNAs through Watson–Crick base pairing. Molecules in development include antisense oligonucleotides and peptide nucleic acids (PNA) (1), RNA enzymes or ribozymes (2) and RNA-cleaving DNA enzymes (3). Combined strategies have recently emerged, using the substrate-recognition properties of nucleic acid enzyme together with the chemical functionality of proteins (4–6) or with various metal ions (7). Alternative strategies for site-selective RNA hydrolysis can be achieved by coupling small organic molecules that have affinity for the negatively charged ribose-phosphate backbone of RNAs to specific chemical functionalities of protein enzymes (8). At physiological pH, high concentrations of imidazole cleave preferentially RNA single strands (flexibility of the ribose-phosphate backbone required in the hydrolysis intermediate) and can be used to probe RNA structures (9). To improve selectivity, compounds with one or two imidazole residues conjugated to polycationic structures (10,11), intercalating dyes (12), oligonucleotides (13) or DNAzyme (4–6) were designed and tested on RNA models.
In this study, we have assessed the capacities of polyamine-imidazole conjugates for site-selective RNA scission. The influence of the polyamine length, the number of nitrogen atoms, the location of the imidazole group, on cleavage selectivity was assayed. One conjugate cleaves the target RNA at a unique location within one of its functional domains without using sequence-specific recognition through base pairing. The cleavage sites were mapped onto the RNA structure in which the position of a spermine and several magnesium ions is defined with accuracy. The specific location of the cleavage sites was rationalized according to the structure.
MATERIALS AND METHODS
Enzymes and RNAs
Alkaline phosphatase and T4 polynucleotide kinase are from New England Biolabs (Beverly, MA). RNases U2 and T1 are from Amersham-Pharmacia-Biotech (Orsay, France). [γ-32P]ATP (3000 mCi/mmol) is from Perkin Elmer (Boston, MA). Escherichia coli transfer-messenger (tm)RNA-tRNA- like domain (TLD) is over-expressed in E.coli cells and purified as described (14). Appropriate bands are electroeluted and pure RNAs are recovered by ethanol precipitation. Total tRNA from E.coli is from Sigma-Aldrich (Saint-Quentin, France). Partially purified yeast tRNAPhe was a gift from D. Rhodes (MRC, Cambridge, UK). The RNA mixture containing substantial amounts of precursor tRNAPhe was gel purified onto a 12% PAGE, electroeluted and ethanol precipitated.
Site-selective RNA hydrolysis
Labeling at the 5′-ends of yeast tRNAPhe and of tmRNA-TLD is performed with [γ-32P]ATP and phage T4 polynucleotide kinase on RNA dephosphorylated previously with alkaline phosphatase. After labeling, the RNAs are gel purified (12% PAGE), eluted and ethanol precipitated. Labeled RNAs (50 000 c.p.m./assay) supplemented with 1 µg of total tRNA from E.coli are heated for 3 min at 80°C in RNase-free water, cooled down for 5 min at room temperature, placed in a 10 mM Tris–HCl pH 7.5 (37°C), 1 mM EDTA and 12 mM MgCl2 buffer, and incubated for 10 min at room temperature. Reaction mixtures (20 µl) contain 200 000 c.p.m. of [32P]tRNAPhe or [32P]tmRNA-TLD, increasing amounts of the selected imidazole-polyamine conjugates (the pellet containing the purified compound was resuspended into a 100 mM Tris–HCl buffer pH 7.5 at 37°C). After an incubation of 3–4 h at 37°C, RNAs are ethanol precipitated, the pellets are washed twice with 80% EtOH, dried and counted. Then, the RNA fragments are submitted to either 8 or 12% PAGE. For the reaction of site-selected hydrolysis of labeled yeast tRNAPhe with increasing concentrations of imidazole (buffered at pH 7 using a 325 mM HCl solution), the reaction is carried out as described above, but the incubation time is 12 h at 37°C. The reaction is stopped by 20 µl 0.3 M Na acetate (pH 5.0), 400 µl of 2% lithium per chlorate in acetone. After centrifugation, the pellets are resuspended and washed with 300 µl acetone, dried, counted, supplemented with loading buffer and submitted to PAGE. Samples for the Gs and As ladders using RNAses T1 and U2, respectively, contain labeled RNAs (50 000 c.p.m./assay), 1 µg of total tRNA in a T1 (7 M urea, 25 mM Na citrate pH 4.5, 1 mM EDTA) or U2 (7 M urea, 30 mM Na citrate pH 3.5) buffers, incubated 10 min at 50°C and then placed on ice. 0.4 U of RNAse T1 (Gs) or 0.2 U of RNAse U2 (As) are added followed by an incubation of 10 min at 50°C, supplemented with loading buffer and submitted to PAGE. All the results are analyzed on a Phosphor Imager (Molecular Dynamics, Sunnyvale, CA). Computer modeling was achieved with Insight II (Mountain View, CA) on a 02 workstation.
RESULTS
Synthesis of the polyamines conjugates
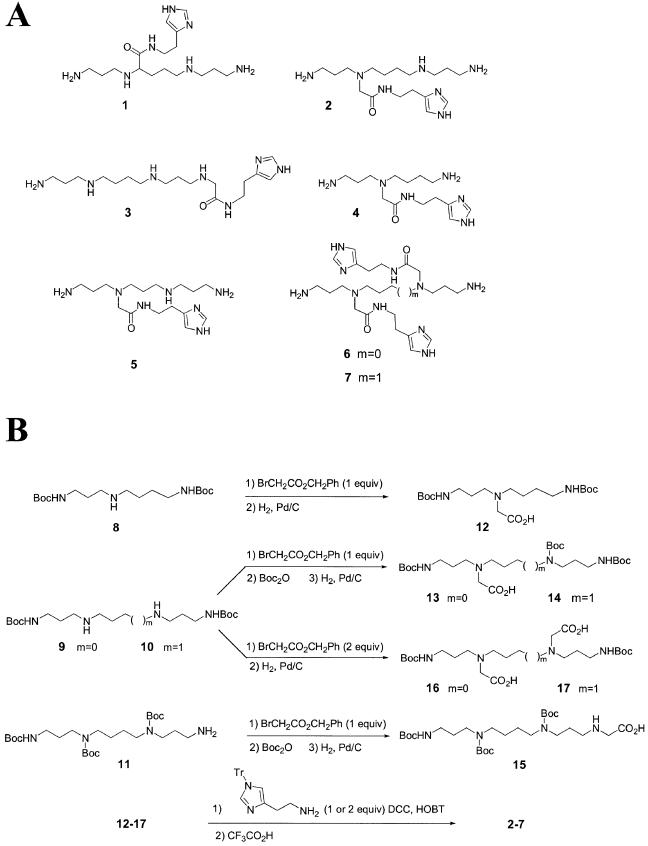
Structures of polyamine conjugates used in this study are presented in Figure 1A. Compound 1 was prepared according to the method reported by J.P.Behr and co-workers, i.e. biscyanoethylation of ornithine, hydrogenation, protection of the amino groups, condensation of the N-hydroxysuccinimide ester with histamine and final deprotection with trifluoroacetic acid (15). Although, as it was shown in this sequence, the free imidazole functionality was compatible with the coupling reaction, N-tritylhistamine (16) was preferred for the synthesis of 2–7 to increase solubility of the intermediates and to simplify purification steps. The histamine moiety was attached via a glycine linker directly to the amino group(s) of the polyamine core to avoid the presence of a stereogenic center, as in compound 1, and to make syntheses easier (Fig. 1B) (17). Regioselective diprotected tri- and tetra-amines 8, 9 and 10 were first synthesized, respectively, from spermidine, norspermine and spermine and Boc2O (18) while N1,N4,N9-triBoc spermine was prepared according to the multi-step protocol of Blagbrough and Geall (19). These di- or tri-protected polyamines were converted to their glycine derivatives 12–14 and 17 by reaction with one equivalent of benzyl bromoacetate followed by a catalytic hydrogenation (20). In the case of norspermine and spermine derivatives 13, 14 and 17, the cleavage of the benzyl ester was preceded by the protection of the last free amino group as tertiobutylcarbamate. Dialkylated norspermine and spermine derivatives 15 and 16 were similarly obtained by using two equivalents of alkylating agent. Imidazole conjugates 2–7 were then synthesized by coupling the corresponding mono- or diacids with N-tritylhistamine under standard conditions, followed by complete deprotection with trifluoroacetic acid.
Figure 1.
Chemical structures (A) and scheme of synthesis (B) of seven polyamine derivatives for RNA hydrolysis.
RNA hydrolysis assays
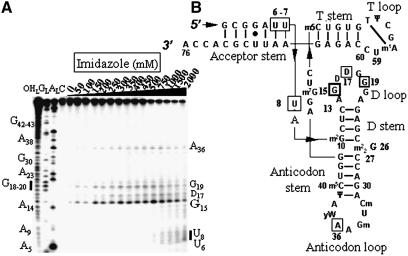
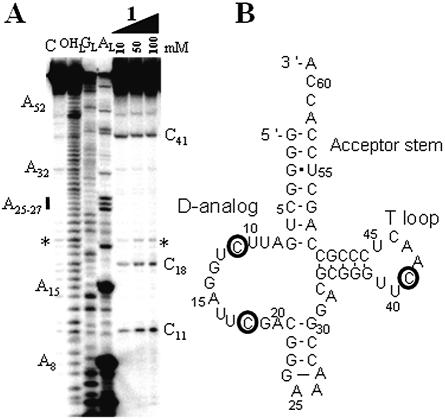
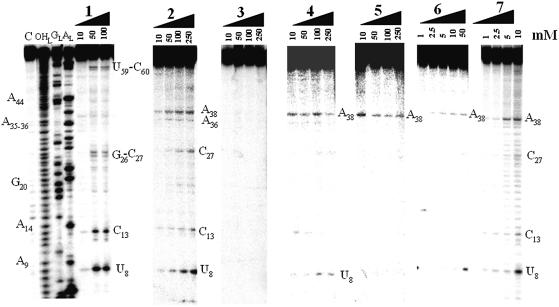
In the presence of an imidazole buffer, the RNA is cleaved at seven locations (Fig. 2), in the acceptor stem (U6–U8, only at high concentration of imidazole), in the D-loop (a strong cleavage site is at G15, weaker ones at D17 and G19) and in the anticodon loop (A36). Free imidazole hydrolyzes RNA at concentrations above 100 mM. The T stem–loop, as well as the D- and anticodon-stems, are not cleaved. Figure 3 brings the experimental support that compound 1 is able to hydrolyze RNA by itself at selective sites, in the absence of free imidazole. The RNA model is native yeast tRNAPhe whose structure has been solved recently at ∼2 Å resolution with a molecule of spermine and magnesium ions (21,22). There are six main cleavage sites, two strong ones, U8 at the junction between the acceptor and D-stems and C13 in the D-stem, and four weaker ones, G26–C27 at the junction between the D and anticodon-stems and U59–C60 in the T-loop. Cleavage occurs at and above 50 mM concentration of compound 1. The experimental controls carried out in the presence of either spermine or spermidine at concentrations of 10, 50, 100 and 250 mM have been performed on tRNAPhe. Both spermine and spermidine do not cleave tRNAPhe by themselves from 10 to 100 mM concentrations, but two cuts appear at positions 37 and 47 for a 250 mM concentration for both polyamines (not shown).
Figure 2.
Electrophoregram of the cleavage pattern of labeled tRNAPhe by an imidazole buffer. (A) Lane C, incubation control; lane OHL, alkaline hydrolysis ladder; lane GL, guanine ladder; lane AL, adenine ladder. Sequence is indexed on the left side. Nucleotides indexed on the right side are the cleavage sites. (B) Yeast tRNAPhe secondary structure with imidazole induced cleavage points. Thickness of the symbols (rectangles) is proportional to the intensity of the cuts. Nucleotides at the 5′-boundary of the cleavage sites are indicated onto tRNAPhe secondary structure, the cleavage sites are at the 3′-phosphate. This molecule has 11 modified bases: N2-methylguanosine (m2G), dihydrouridine (D), N2,N2-dimethylguanosine (m22G), 2′-O-methylcytidine (Cm), 2′-O-methylguanosine (Gm), wybutosine (yW), pseudouridine (Ψ), 5-methylcytidine (m5C), 7-methylguanosine (m7G), 5-methyluridine (m5U) and 1-methyladenosine (m1A).
Figure 3.
Yeast tRNAPhe hydrolysis by compound 1. (A) Electrophoregram of the cleavage pattern. The indications provided are as in Figure 2A. (B) Cleavage points (circles, with a thickness that is proportional to the intensity of the cuts) mapped onto yeast tRNAPhe secondary structure.
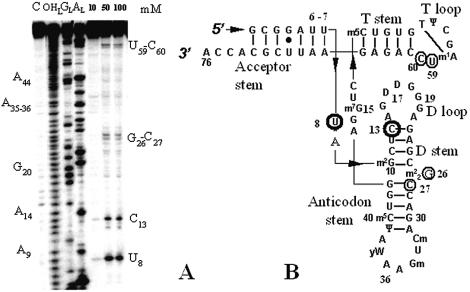
The tRNA portion of tmRNA, a small RNA that interacts with selected translating ribosomes to target the nascent polypeptides for degradation (23), is also subjected to specific hydrolysis in the presence of 1 without free imidazole (Fig. 4). There are three main cleavage sites, two in the D-analog (C11 and C18) and one in the T-loop (C41), indicating that various RNAs can be hydrolyzed by compound 1 without free imidazole. The solution conformation of tmRNA-TLD was recently monitored by structural probes, with evidence indicating that both C11 and C41 are accessible towards structural probes specific of single-stranded RNA sequences (14). Therefore, 2 nt out of the 3 that are cleaved by compound 1 are accessible. Their cleavage by 1 is probably a consequence of the intrinsic structure of tmRNA-TLD.
Figure 4.
Hydrolysis of the tRNA portion of tmRNA by compound 1. (A) Electrophoregram of the cleavage pattern. The indications provided are as in Figure 2A. The star shows an example of a cleavage site that was not retained since it is already present in the control lane. (B) Cleavage points mapped onto the RNA secondary structure (27).
Varying the position of the imidazole with respect to the polycationic chain was achieved with compounds 1, 2 and 3 (Fig. 5). The imidazole-containing lateral chain is branched on an internal carbon (1), nitrogen (2) or terminal nitrogen (3), respectively. 2 cleaves tRNAPhe at three positions similar to those observed with 1 (U8, C13 more weakly and C27). Two additional sites are in the anticodon loop, at A36 and A38. 3 does not cleave the RNA, even at a 250 mM concentration (Fig. 5).
Figure 5.
Site-selected hydrolysis of yeast tRNAPhe by compounds 1–7. The indications provided are as in Figure 2A.
Next, the structure of the polycationic chain was modified, without affecting the imidazole-containing lateral branch (compounds 4 and 5; Fig. 1). For conjugate 4, a spermidine derivative, cleavage sites become restricted to U8 and A38 (Fig. 5). Compared with 1, the lateral branch of compound 4 connects an internal amine and not an internal carbon, modifying the positioning of the lateral branch with respect to the polycationic chain. Interestingly, in the case of 5, only one cleavage site remains, at position 38 in the anticodon loop (Fig. 5). Di-imidazole derivatives (compounds 6 and 7) trigger RNA cleavage at a much lower concentration compared with their mono-imidazole counterparts, since cleavage is observed at 5 mM. For 6, cleavage occurs at U8 and A38, and U8, C13, C27 and A38 for 7, but with a loss of selectivity around 10 mM for 7.
DISCUSSION
Site-specific RNA cleavage by imidazole-containing polyamine compounds is described in the absence of free imidazole. Varying the position of the imidazole and the structure of the polycationic chain could lead to a novel compound that triggers a unique and specific cleavage of the target RNA. When two imidazoles are present, cleavages occur at a concentration one order of magnitude lower than when there is only one imidazole. Adding an imidazole buffer to the mono-imidazole derivatives might reduce their respective concentrations required for cleavage.
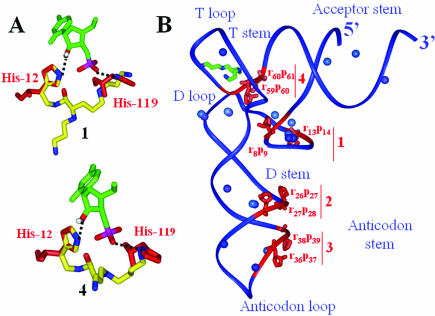
The imidazole rings of catalytic residues His12 and His119 in the catalytic site of RNase A serve, respectively, as base and acid catalysts and the protonated side chain of Lys41 stabilizes the pentacoordinated phosphorous intermediate during transition state (24). Our experimental results are consistent with a cooperative participation of one of the amino group of the polyamine chain, predominantly protonated at physiological pH, as proton source, and the imidazole moiety, which is partially unprotonated, as base. The polycationic moiety is also involved in binding the negatively charged ribose-phosphate backbone. Indeed, when the imidazole ring of conjugate 1 is superimposed onto His12 from the active site of RNase A crystal structure in complex with cytidine 3′-monophosphate (25), the polycationic backbone has sufficient length and flexibility to superimpose one of its protonated amino groups on a nitrogen atom of the imidazole ring of His119 (Fig. 6A). The same observations have been noticed with compounds 2 and 5–7. For 3, although the bond requirement is fulfilled, binding of the polycationic chain onto the RNA may place the imidazole ring too far away from the ribose-phosphate backbone. Alternatively, the longer distance between the imidazole and the protonable nitrogens might prevent RNA catalysis. The distance between one of the two protonated nitrogens of the spermidine chain and the imidazole ring of 4 is minimal, but still sufficient to trigger RNA hydrolysis. The number of cleavages is probably reduced because a shorter polyamine with fewer protonated nitrogens binds the RNA at fewer locations, including sites 1 and 3, but not 2 and 4 (Fig. 6B). When norspermine replaces spermidine (compound 5), only A38 from site 3 is cleaved, indicating that both the position of the polyamine moiety and the distance between a protonated nitrogen and an imidazole have been optimized to induce a single cleavage site. Nevertheless, intra-molecular catalysis is not proven, and inter-molecular catalysis cannot be excluded, especially at such concentrations. 15N NMR spectroscopy was used to map the interactions between natural polyamines and total tRNA from E.coli and have shown that the internal -NH2+- groups bind more strongly to tRNA than the external -NH3+ moieties (26). Thus, substitutions of the secondary amines in conjugates 4, 6 and 7 might affect their binding to tRNAPhe.
Figure 6.
(A) Superimposing 1 and 4 (yellow-blue) onto RNaseA active site (red) in complex with cytidine 3′-monophosphate (green). (B) tRNAPhe tertiary structure (blue backbone following the ribose-phosphate chain) with the cleavage sites (red), magnesium ions (blue), spermine (green-blue). The four main cleavage sites are denoted 1–4. Conjugate 1 cleaves sites 1, 2 and 4, conjugate 2 cleaves sites 1–3, conjugates 4 and 6 cleave sites 1 and 3 and conjugate 5 cleaves only at site 3.
Yeast tRNAPhe structure has density for a spermine in the major groove of the TΨC stem, as well as accurate information on the location of Mg2+ binding sites. The location of the cleavage sites triggered by conjugates 1–7 is indicated [Fig. 6; the 2′-OH from the attacked riboses (r) and the hydrolyzed phosphates (p) are displayed]. The cleavage sites are clustered into four areas (sites 1–4). In site 1, two cleavage sites are opposite since the sugar-phosphate backbone loops back between U8 and C13; in that cleft, there are Mg2+ binding sites that could be occupied by some of the protonated nitrogens of the polycationic chain. Nearby sites 2 and 3, Mg2+ binding sites could be favored anchoring points for the protonated nitrogens of the conjugates. Site 4 is close to the binding site of spermine, suggesting that the triamine moiety might bind at a similar location; alternatively, there are Mg2+ binding sites within the T- and D-loops that could be displaced by protonated nitrogens of the polycationic chain. Three additional Mg2+ binding sites are in the acceptor stem that prevents hydrolysis intermediate state formation.
In conclusion, novel RNaseA mimics that do not depend upon free imidazole for RNA cleavage were designed, synthesized and tested on an RNA whose detailed atomic structure is known. One conjugate cleaves the RNA target at a unique location within one of its main functional domains, without using sequence-specific recognition through base pairing. There is correlation between the location of the magnesium binding sites from the RNA target and the cleavages sites. Further investigations of other imidazole derivatives are currently in progress in our laboratories.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr J. P. Behr (Strasbourg, France) for providing us with some of 1, Dr D. Rhodes (MRC, UK) for a partially purified yeast tRNAPhe and the ‘Conseil Régional de Bretagne’ for financial support (PRIR no. 691).
REFERENCES
- 1.Braasch D.A. and Corey,D.R. (2002) Novel antisense and peptide nucleic acid strategies for controlling gene expression. Biochemistry, 41, 4503–4510. [DOI] [PubMed] [Google Scholar]
- 2.Doudna J.A. and Cech,T.R. (2002) The chemical repertoire of chemical ribozymes. Nature, 418, 222–228. [DOI] [PubMed] [Google Scholar]
- 3.Cairns M.J., Saravolac,E.G. and Sun,L.Q. (2002) Catalytic DNA: a novel tool for gene suppression. Curr. Drug Targets, 3, 269–279. [DOI] [PubMed] [Google Scholar]
- 4.Santoro S.W., Joyce,G.F., Sakthivel,K., Gramatikova,S. and Barbas,C.F. (2000) RNA Cleavage by a DNA enzyme with extended chemical functionality. J. Am. Chem. Soc., 122, 2433–2439. [DOI] [PubMed] [Google Scholar]
- 5.Perrin D.M., Garestier,T. and Helene,C. (2001) Bridging the gap between proteins and nucleic acids: a metal-independent RNAseA mimic with two protein-like functionalities. J. Am. Chem. Soc., 123, 1556–1563. [DOI] [PubMed] [Google Scholar]
- 6.Lermer L., Roupioz,Y., Ting,R. and Perrin,D.M. (2002) Toward an RNaseA mimic: a DNAzyme with imidazoles and cationic amine. J. Am. Chem. Soc., 124, 9960–9961. [DOI] [PubMed] [Google Scholar]
- 7.Trawick B.N., Daniher,A.T. and Bashkin,J.K. (1998) Inorganic mimics of ribonucleases and ribozymes: from random cleavage to sequence-specific chemistry to catalytic antisense drugs. Chem. Rev., 98, 939–960. [DOI] [PubMed] [Google Scholar]
- 8.Oivanen M., Kuusela,S. and Lonnberg,H. (1998) Kinetics and mechanisms for the cleavage and isomerization of the phosphodiester bonds of RNA by bronsted acids and bases. Chem. Rev., 98, 961–990. [DOI] [PubMed] [Google Scholar]
- 9.Vlassov V.V., Zuber,G., Felden,B., Behr,J.P. and Giegé,R. (1995) Cleavage of tRNA with imidazole and spermine imidazole constructs: a new approach for probing RNA structure. Nucleic Acids Res., 23, 3161–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinozuka K., Nakashima,Y., Shimizu,K. and Sawai,H. (2001) Synthesis and characterization of polyamine-based biomimetic catalysts as artificial ribonuclease. Nucl. Nucl. Nucleic Acids, 201, 137–130. [DOI] [PubMed] [Google Scholar]
- 11.Verbeure B., Lacey,C.J., Froyen,M., Rozenski,J. and Herdewijn,P. (2002) Synthesis and cleavage experiments of oligonucleotide conjugates with a diimidazole-derived catalytic center. Bioconjug. Chem., 13, 333–350. [DOI] [PubMed] [Google Scholar]
- 12.Podyminogin M.A., Vlassov,V.V. and Giegé,R. (1993) Synthetic RNA-cleaving molecules mimicking ribonuclease A active center. Design and cleavage of tRNA transcripts. Nucleic Acids Res., 21, 5950–5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beloglazova N.G., Sil’nikov,V.N., Zenkova,M.A. and Vlassov,V.V. (2000) Cleavage of yeast tRNAPhe with complementary oligonucleotide conjugated to a small ribonuclease mimic. FEBS Lett., 481, 277–280. [DOI] [PubMed] [Google Scholar]
- 14.Gaudin C., Nonin-Lecomte,S., Tisné,C., Corvaisier,S., Bordeau,V., Dardel,F. and Felden,B. (2003) The tRNA-like domains of E. coli and A. aeolicus transfer-messenger RNA: structural and functional studies. J. Mol. Biol., 331, 457–471. [DOI] [PubMed] [Google Scholar]
- 15.Zuber G., Sirlin,C. and Behr,J.P. (1993) Enhanced ligation of DNA with a synthetic effector molecule. J. Am. Chem. Soc., 115, 4939–4940. [Google Scholar]
- 16.Nguyen D.N., Stump,C.A., Walsh,F., Fernandes,C., Davide,J.P., Ellis-Hutchings,M.R., Robinson,R.G., Williams,T.M., Lobell,R.B., Huber,H.E. and Buser,C.A. (2002) Potent inhibitors of farnesyltransferase and geranylgeranyltransferase-I. Bioorg. Med. Chem. Lett., 12, 1269–1273. [DOI] [PubMed] [Google Scholar]
- 17.Byk G., Dubertret,C., Escriou,V., Frederic,M., Jaslin,G., Rangara,R., Pitard,B., Crouzet,J., Wils,P., Schwartz,B. and Scherman,D. (1998) Synthesis, activity, and structure-activity relationship studies of novel cationic lipids for DNA transfer. J. Med. Chem., 41, 229–235. [DOI] [PubMed] [Google Scholar]
- 18.Cohen G.M., Cullis,P.M., Hartley,J.A., Mather,A. and Symmons,M.C. (1992) Targeting of cytotoxic agents by polyamines: synthesis of a chlorambucil-spermidine conjugate. J. Chem. Soc. Chem. Commun., 298–300. [Google Scholar]
- 19.Blagbrough I.S. and Geall,A.J. (1998) Practical synthesis of unsymmetrical polyamine amides. Tetrahedron Lett., 39, 439–442. [Google Scholar]
- 20.Muller D., Zelster,I., Bitan,G. and Gilon,C. (1997) Building units for N-backbone cyclic peptides. 3. Synthesis of protected Nα-(ω-aminoalkyl)amino acids and Nα-(ω-carboxyalkyl)amino acids. J. Org. Chem., 62, 411–416. [DOI] [PubMed] [Google Scholar]
- 21.Shi H. and Moore,P.B. (2000) The crystal structure of yeast phenylalanine tRNA at 1.93 Å resolution: a classic structure revisited. RNA, 6, 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jovine L., Djordjevic,S. and Rhodes,D. (2000) The crystal structure of yeast phenylalanine tRNA at 2.0 Å resolution: cleavage by Mg2+ in 15-year old crystals. J. Mol. Biol., 301, 401–414. [DOI] [PubMed] [Google Scholar]
- 23.Gillet R. and Felden,B. (2001) Emerging views on tmRNA-mediated protein tagging and ribosome rescue. Mol. Microbiol., 42, 879–885. [DOI] [PubMed] [Google Scholar]
- 24.Breslow R. (1991) How do imidazole groups catalyze the cleavage of RNA in enzyme models and in enzymes? Evidence from ‘negative catalysis’. Acc. Chem. Res., 24, 317–324. [Google Scholar]
- 25.Zegers I., Maes,D., Dao-Thi,M.H., Poortmans,F., Palmer,R. and Wyns,L. (1994) The structures of RNase A complexed with 3′-CMP and d(CpA): active site conformation and conserved water molecules. Protein Sci., 3, 2322–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frydman L., Rossomando,P.C., Frydman,V., Fernandez,C.O., Frydman,B. and Samejima,K. (1992) Interactions between natural polyamines and tRNA: an 15N NMR analysis. Proc. Natl Acad. Sci. USA, 89, 9186–9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corvaisier S., Bordeau,V. and Felden,B. (2003) Inhibition of transfer messenger RNA aminoacylation and trans-translation by aminoglycoside antibiotics. J. Biol. Chem., 278, 14788–14797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.