Abstract
ZO-1 is an actin filament (F-actin)–binding protein that localizes to tight junctions and connects claudin to the actin cytoskeleton in epithelial cells. In nonepithelial cells that have no tight junctions, ZO-1 localizes to adherens junctions (AJs) and may connect cadherin to the actin cytoskeleton indirectly through β- and α-catenins as one of many F-actin–binding proteins. Nectin is an immunoglobulin-like adhesion molecule that localizes to AJs and is associated with the actin cytoskeleton through afadin, an F-actin–binding protein. Ponsin is an afadin- and vinculin-binding protein that also localizes to AJs. The nectin-afadin complex has a potency to recruit the E-cadherin–β-catenin complex through α-catenin in a manner independent of ponsin. By the use of cadherin-deficient L cell lines stably expressing various components of the cadherin-catenin and nectin-afadin systems, and α-catenin–deficient F9 cell lines, we examined here whether nectin recruits ZO-1 to nectin-based cell-cell adhesion sites. Nectin showed a potency to recruit not only α-catenin but also ZO-1 to nectin-based cell-cell adhesion sites. This recruitment of ZO-1 was dependent on afadin but independent of α-catenin and ponsin. These results indicate that ZO-1 localizes to cadherin-based AJs through interactions not only with α-catenin but also with the nectin-afadin system.
INTRODUCTION
ZO-1 is an actin filament (F-actin)–binding protein, containing three PDZ domains, one SH3 domain, and one guanylate kinase (GK) domain in this order from the N terminus (Itoh et al., 1993; Willott et al., 1993). In epithelial cells, ZO-1 exclusively localizes to claudin-based tight junctions (TJs) and directly binds to claudin at the first PDZ domain (Itoh et al., 1999) and F-actin at the C-terminal half (Itoh et al., 1997; Fanning et al., 1998) and connects claudin to the actin cytoskeleton (Tsukita et al., 1999). Occludin localizes to TJs, and ZO-1 directly binds to it at the GK domain (Fanning et al., 1998), although the function of occludin at TJs is currently unknown. ZO-1 is excluded from cadherin-based adherens junctions (AJs) that are aligned at the basal side of TJs along the lateral membranes. In nonepithelial cells that have no TJs, however, ZO-1 localizes to cadherin-based AJs and directly binds to α-catenin (Itoh et al., 1991, 1993, 1997), which is associated with cadherin through β-catenin (Nagafuchi and Takeichi, 1989; Ozawa et al., 1989; Takeichi, 1991). α-Catenin is an F-actin–binding protein (Rimm et al., 1995) and furthermore binds two other F-actin–binding proteins, vinculin and α-actinin (Nieset et al., 1997; Weiss et al., 1998). As one of the many F-actin–binding proteins, ZO-1 may indirectly connect cadherin to the actin cytoskeleton (Itoh et al., 1997). Thus, the localization of ZO-1 is different between epithelial and nonepithelial cells, but the mechanism of this different localization is unknown.
In the initial stage during the formation of cell-cell junctions of epithelial cells, primordial spot-like cell-cell junctions are first formed at the tips of the cellular protrusions radiating from adjacent cells (Yonemura et al., 1995; Ando-Akatsuka et al., 1996; Adams et al., 1998; Vasioukhin et al., 2000). Cadherin and ZO-1 colocalize to the primordial junctions where occludin is not concentrated (Ando-Akatsuka et al., 1996). As cellular polarization proceeds, occludin gradually accumulates at the spot-like junctions to form TJs, and cadherin is sorted from occludin and ZO-1 to form AJs. To elucidate the mechanism of the formation of TJs and AJs, it is of importance to understand the mechanism of the different localization of ZO-1 between epithelial and nonepithelial cells.
We have recently found a novel cell-cell adhesion system at AJs that may regulate the formation of cadherin-based AJs and claudin-based TJs (Mandai et al., 1997; Asakura et al., 1999; Ikeda et al., 1999; Mandai et al., 1999; Sakisaka et al., 1999; Takahashi et al. 1999; Miyahara et al., 2000; Satoh-Horikawa et al., 2000; Tachibana et al., 2000). This system consists of at least three components, nectin, afadin, and ponsin. Nectin is a Ca2+-independent cell-cell adhesion molecule that belongs to the immunoglobulin superfamily (Takahashi et al., 1999). This adhesion molecule is identical to the poliovirus receptor-related protein and has recently been shown to serve as the α-herpesvirus entry and cell-cell spread mediator (Geraghty et al., 1998; Warner et al., 1998; Cocchi et al., 2000). Nectin comprises a family consisting of at least three members, nectin-1, -2, and -3, each of which has two or three splicing variants (Morrison and Racaniello, 1992; Aoki et al., 1994; Eberléet al., 1995; Lopez et al., 1995; Cocchi et al., 1998; Satoh-Horikawa et al., 2000). Most members have a C-terminal conserved motif of four amino acid (aa) residues (E/A-X-Y-V) that interacts with the PDZ domain of afadin (Takahashi et al., 1999; Satoh-Horikawa et al., 2000). Afadin has at least two splicing variants, l- and s-afadins. l-Afadin, a larger splicing variant, is an F-actin–binding protein with one PDZ domain and three proline-rich domains and connects nectin to the actin cytoskeleton (Mandai et al., 1997; Takahashi et al., 1999). s-Afadin, a smaller splicing variant, has one PDZ domain but lacks the F-actin–binding domain and the third proline-rich domain (Mandai et al., 1997). Human s-afadin is identical to the AF6 protein, the gene of which is originally found to be fused to the ALL-1 gene in acute leukemia (Prasad et al., 1993). In this study, we use “afadin” as l-afadin. Ponsin is an afadin-binding protein that colocalizes with nectin and afadin to cadherin-based AJs (Mandai et al., 1999; Tachibana et al., 2000). Furthermore, ponsin binds to vinculin, which is known to interact with both F-actin and α-catenin (Burridge and Feramisco, 1982; Menkel et al., 1994; Weiss et al., 1998) and colocalizes with vinculin to not only cadherin-based AJs but also focal contacts (Mandai et al., 1999). However, ponsin forms a binary complex with either afadin or vinculin and does not form a ternary complex (Mandai et al., 1999).
The nectin-afadin complex has a potency to recruit the E-cadherin-β-catenin complex through α-catenin in a ponsin- and vinculin-independent manner and is involved in the formation of AJs in cooperation with the cadherin-catenin complex (Tachibana et al., 2000). In epithelial cells of afadin (−/−) mice and (−/−) embryoid bodies, the proper organization of AJs and TJs is impaired (Ikeda et al., 1999). In spermatozoa of nectin-2 (−/−) mice, the nuclear and cytoskeletal morphology and mitochondrial localization are impaired (Bouchard et al., 2000). Nectin-1 has recently been determined by positional cloning to be responsible for cleft lip/palate-ectodermal dysplasia (Suzuki et al., 2000). Thus, evidence is accumulating that the nectin-afadin system plays an important role in the organization of AJs and TJs, although the function of ponsin is currently unknown.
Nectin-2, afadin, and ponsin colocalize with E-cadherin and ZO-1 to the primordial junctions during the formation of cell-cell junctions of MTD-1A epithelial cells (Yonemura et al., 1995; Ando-Akatsuka et al., 1996; Asakura et al., 1999). In MDCK epithelial cells, reduction of medium Ca2+ concentrations to a micromolar range causes disruption of AJs and TJs (Kartenbeck et al., 1991). Nectin-1α and afadin as well as ZO-1 are observed on the plasma membrane, whereas E-cadherin is not (Asakura et al., 1999; Sakisaka et al., 1999). Addition of a protein kinase C-activating phorbol ester induces the formation of a TJ-like structure (Balda et al., 1993), where nectin-1α and afadin as well as ZO-1, but not E-cadherin, are recruited (Balda et al., 1993; Asakura et al., 1999; Sakisaka et al., 1999). These observations have increased the possibility that the nectin-afadin system is involved in the localization of ZO-1.
On the basis of this assumption, we have examined here whether nectin has a potency to recruit ZO-1 to nectin-based cell-cell adhesion sites and have found that nectin has the potency to recruit not only α-catenin but also ZO-1 there. We discuss a possible role of the nectin-afadin system during the formation of AJs and TJs.
MATERIALS AND METHODS
Construction and Purification
Mammalian expression vectors were constructed with pPGKIH (Miyahara et al., 2000), pPGKIH-FLAG (Tachibana et al., 2000), pPGKIZ-Myc, and pEGFP-N1 (CLONTECH Laboratories, Palo Alto, CA) by the use of standard molecular biology methods (Sambrook et al., 1989). pPGKIH-FLAG was designed to express an N-terminal FLAG-tagged protein in which the preprotrypsin signal peptide precedes the FLAG tag. pPGKIZ-Myc was constructed to express an N-terminal Myc-tagged protein by replacing the hemagglutinin tag of pPGKIZ-HA (Tachibana et al., 2000) with the Myc tag. pEGFP-N1 was designed to express a C-terminal green fluorescent protein (GFP)-fusion protein. Mammalian expression vectors of mouse nectin-2α, α-catenin, and ponsin-2 contained the following aa: pPGKIH-nectin-2α, aa 1–467 (full length); pPGKIH-nectin-2α-ΔC, aa 1–463 (deletion of the C-terminal four aa residues); pPGKIH-FLAG-nectin-2α, aa 28–467; pPGKIZ-Myc-α-catenin, aa 1–906 (full length), and pEGFP-ponsin, 1–724 (full length).
Baculovirus transfer vectors were constructed with pFastBacHT (GIBCO-BRL, Grand Island, NY) and pFastBac1-Myc-His6 (Tachibana et al., 2000). pFastBacHT was designed to express an N-terminal His6-tagged protein. pFastBac1-Myc-His6 was designed to express a C-terminal Myc- and His6-tagged protein. Baculovirus transfer vectors of ZO-1 and afadin contained the following aa: pFastBacHT-ZO-1, aa 1–1745 (full length); and pFastBac1-Myc-His6-afadin, aa 1–1829 (full length). The His6-tagged protein of ZO-1 (His6-ZO-1) and the Myc- and His6-tagged protein of afadin (Myc-His6-afadin) were expressed in High Five insect cells (Invitrogen, San Diego, CA) and purified by the use of TALON metal affinity beads (CLONTECH Laboratories) as described (Tachibana et al., 2000).
Prokaryote expression vectors were constructed with pGEX-KG (Guan and Dixon, 1991) and pMal-C2 (New England Biolabs, Beverly, MA). The glutathione S-transferase (GST)-fusion or maltose-binding protein (MBP)-fusion vector of nectin-2α, afadin, and ZO-1 contained the following aa: GST-nectin-2α-CP, aa 387–467 (cytoplasmic region); MBP-afadin-PDZ, aa 1007–1125 (PDZ domain); MBP-ZO-1-PDZ1–2, aa 1–292 (first and second PDZ domains); and MBP-ZO-1-PDZ2–3, aa 181–503 (second and third PDZ domains). The GST- and MBP-fusion proteins were purified by the use of glutathione-Sepharose beads (Amersham-Pharmacia Biotech, Piscataway, NJ) and amylose resin beads (New England Biolabs), respectively.
Cell Culture and DNA Transfection
L, EL, and F9 cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. EL cells were cloned by introduction of the E-cadherin cDNA into L cells (Nagafuchi et al., 1987). EL cell lines stably expressing a FLAG-tagged protein of full-length nectin-2α (nectin-2α-EL cells) were prepared by the use of pPGKIH-FLAG-nectin-2α as described previously (Takahashi et al., 1999; Tachibana et al., 2000). L cell lines stably expressing full-length nectin-2α (nectin-2α-L cells) or C-terminal four-aa–deleted nectin-2α (nectin-2α-ΔC-L cells) were prepared as described before (Takahashi et al., 1999; Miyahara et al., 2000; Tachibana et al., 2000). The following L cell lines stably expressed the fusion molecules: nEα-L cells, the fusion molecule consisting of E-cadherin lacking its β-catenin–binding domain (aa 1–657) and full-length α-catenin (aa 1–906) (Nagafuchi et al., 1994; Imamura et al., 1999); nEαN-L cells, the fusion molecule consisting of E-cadherin lacking its β-catenin–binding domain and the N-terminal half of α-catenin (aa 1–508) (Nagafuchi et al., 1994; Imamura et al., 1999); and nEαC-L cells, the fusion molecule consisting of E-cadherin lacking its β-catenin–binding domain and the C-terminal half of α-catenin (aa 509–906) (Nagafuchi et al., 1994; Imamura et al., 1999). nEα-L, nEαN-L, and nEαC-L cells were obtained as described previously (Nagafuchi et al., 1994; Imamura et al., 1999). Nectin-2α-L and -2α-ΔC-L cells, both of which transiently expressed a Myc-tagged protein of full-length α-catenin (Myc-α-catenin), were prepared by the use of pPGKIZ-Myc-α-catenin as described by Tachibana et al. (2000). Nectin-2α-L cells transiently expressing a GFP-fusion protein of full-length ponsin (GFP-ponsin) were prepared by the use of pEGFP-ponsin as described by Tachibana et al. (2000). Where indicated, nectin-2α-L cells were cultured with 50 nM latrunculin A for 45 min or with 2 μM cytochalasin D for 15 min. F9 cells were cultured in gelatin-coated (0.1%) culture dishes. α-Catenin–deficient F9 cells [F9Dα(−/−) cells] and F9Dα(−/−) cells re-expressing α-catenin [αF9Dα(−/−) cells] were obtained as described by Maeno et al. (1999).
Antibodies
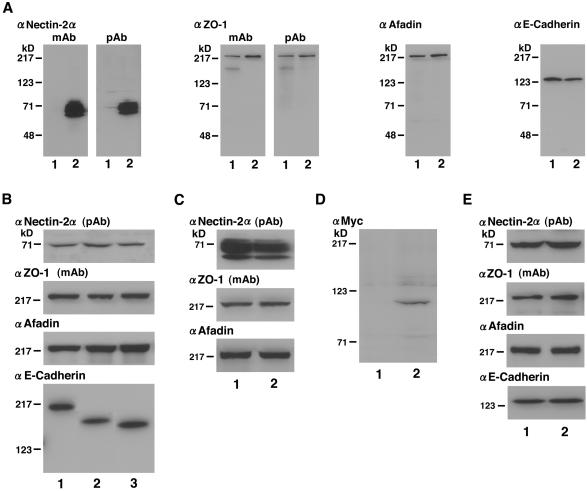
A rabbit anti-nectin-2α polyclonal antibody (pAb) was prepared as described by Takahashi et al. (1999). A rat anti-nectin-2 monoclonal antibody (mAb), which recognizes both nectin-2α and -2δ, was prepared as described by Takahashi et al. (1999). A mouse anti–ZO-1 mAb (Itoh et al., 1991) was kindly supplied by Drs. S. Tsukita and M. Itoh (Kyoto University, Kyoto, Japan). A rabbit anti–ZO-1 pAb was purchased from Zymed (San Francisco, CA). A mouse anti-afadin mAb was prepared as described by Sakisaka et al. (1999). A rat anti–E-cadherin mAb (ECCD-2) was kindly supplied by Dr. M. Takeichi (Kyoto University, Kyoto, Japan). A mouse anti-Myc mAb was from American Type Culture Collection (Manassas, VA). Mouse anti-vinculin and anti-GFP mAbs were purchased from Sigma Chemicals (St. Louis, MO) and CLONTECH, respectively. The specificity of each antibody was examined by Western blotting of various cell lines described above (Figure 1).
Figure 1.
Specificity of antibodies and expression levels of various constructs. The cell lysates of various L cell lines and F9 cell lines (each 20 μg of protein) were subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting with the anti–nectin-2 mAb, the anti–nectin-2α pAb, the anti–ZO-1 mAb, the anti–ZO-1 pAb, the anti-afadin mAb, the anti–E-cadherin mAb, or the anti-Myc mAb. (A) EL and nectin-2α-EL cells. Lane 1, EL cells; lane 2, nectin-2α-EL cells. (B) nEα-L, nEαN-L, and nEαC-L cells. Lane 1, nEα-L cells; lane 2, nEαN-L cells; lane 3, nEαC-L cells. (C) Nectin-2α-L and -2α-ΔC-L cells. Lane 1, nectin-2α-L cells; lane 2, nectin-2α-ΔC-L cells. (D) Nectin-2α-L cells transfected with pPGKIZ-Myc-α-catenin or the empty vector. Lane 1, empty vector; lane 2, pPGKIZ-Myc-α-catenin. (E) αF9Dα(−/−) and F9Dα(−/−) cells. Lane 1, αF9Dα(−/−) cells; lane 2, F9Dα(−/−) cells. The results shown are representative of three independent experiments.
Subcellular Fractionation and Immunoprecipitation
Confluent cells cultured on a 10-cm dish were washed with phosphate-buffered saline and scraped. The cells were again washed with phosphate-buffered saline and sonicated in a buffer (10 mM HEPES/NaOH at pH 7.5, 100 mM KCl, 1 mM MgCl2, and 25 mM NaHCO3) on ice for 15 s five times at 3-min intervals. The homogenate was subjected to centrifugation at 100,000 × g for 30 min. A comparable amount of each fraction (each 20 μg of protein) was subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting.
Immunoprecipitation was performed as described previously (Takahashi et al., 1999; Miyahara et al., 2000). Briefly, nectin-2α-L or -2α-ΔC-L cells were sonicated in buffer A (10 mM HEPES/NaOH at pH 7.5, 100 mM KCl, 1 mM MgCl2, and 25 mM NaHCO3, 1% Triton X-100, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/ml pepstatin A), followed by centrifugation at 100,000 × g for 30 min. The supernatant (2 mg of protein) was incubated with the anti–nectin-2 mAb at 4°C for 2 h. Anti-rat immunoglobulin beads (American Qualex International, San Clemente, CA; 20 μl of wet volume) were added to this sample, and incubation was further performed at 4°C overnight. After the beads were extensively washed with buffer A, the bound proteins were eluted by boiling the beads in an SDS sample buffer (60 mM Tris/Cl at pH 6.7, 3% SDS, 2% [vol/vol] 2-mercaptoethanol, and 5% glycerol), and subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting.
Affinity Chromatographies
To examine the interaction of full-length ZO-1 with full-length afadin, Myc-His6-afadin (20 μg of protein) was immobilized on anti-Myc mAb-coupled beads (20 μl of wet volume) prepared as described by Takahashi et al. (1999). His6-ZO-1 (100 μg of protein) was applied to the Myc-His6-afadin–immobilized beads equilibrated with buffer B (20 mM Tris/Cl at pH 7.5, 150 mM NaCl, and 0.1% Triton X-100). After the beads were extensively washed with buffer B, the bound proteins were eluted by boiling the beads in the SDS sample buffer. The sample was then subjected to SDS-PAGE (8% polyacrylamide gel), followed by staining with Coomassie brilliant blue.
To examine the interaction of ZO-1 or afadin with nectin-2α, MBP-ZO-1-PDZ1–2, MBP-ZO-1-PDZ2–3, or MBP-afadin-PDZ (each 20 μg of protein) was immobilized on amylose resin beads (20 μl of wet volume). His6-ZO-1 (20 μg of protein) was also immobilized on TALON metal affinity beads (20 μl of wet volume). GST-nectin-2α-CP (100 μg of protein) was applied to the MBP-fusion protein-immobilized beads equilibrated with buffer B. After the beads were extensively washed with buffer B, elution was performed with buffer B containing 10 mM maltose. GST-nectin-2α-CP (100 μg of protein) was also applied to the His6-ZO-1–immobilized beads equilibrated with buffer B. After the beads were extensively washed with buffer B, elution was performed with buffer B containing 100 mM imidazole/Cl at pH 7.5. Each eluate was subjected to SDS-PAGE (10 or 13% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue.
Other Procedures
Immunofluorescence microscopy of cultured cells was done as described previously (Mandai et al., 1997; Takahashi et al., 1999). Protein concentrations were determined with bovine serum albumin as a reference protein (Bradford, 1976). SDS-PAGE was done as described by Laemmli (1970).
RESULTS
Colocalization of ZO-1 with Nectin-2 and Afadin at E-Cadherin–based AJs
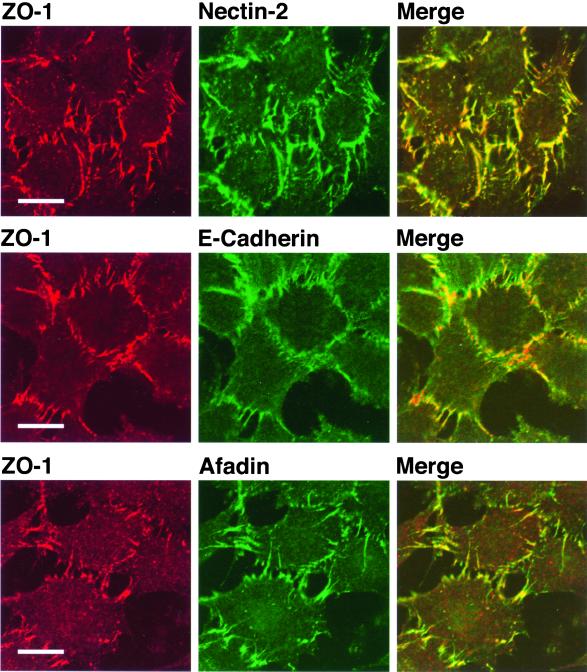
It has been shown that ZO-1 localizes to E-cadherin–based AJs in EL cells (Itoh et al., 1993). L cells are cadherin-deficient mouse fibroblasts, and EL cells are an L cell line stably expressing E-cadherin (Nagafuchi et al., 1987). We have previously shown that endogenous nectin-2 and afadin localize to E-cadherin–based cell-cell AJs in EL cells (Mandai et al., 1997; Takahashi et al., 1999). We first examined the localization of ZO-1 in an EL cell line stably expressing a FLAG-tagged protein of full-length nectin-2α (nectin-2α-EL cells). The expression level of the nectin-2α protein in nectin-2α-EL cells was much higher than that in EL cells, but the expression level of the ZO-1, afadin, or E-cadherin protein in nectin-2α-EL cells was similar between these two types of EL cell lines (Figure 1A). ZO-1 colocalized with nectin-2α and afadin to E-cadherin–based cell-cell AJs in nectin-2α-EL cells (Figure 2).
Figure 2.
Colocalization of ZO-1 with nectin-2α and afadin at E-cadherin–based AJs. Nectin-2α-EL cells were doubly stained with various combinations of the anti–ZO-1 pAb, the anti–nectin-2 mAb, the anti–E-cadherin mAb, and the anti-afadin mAb. Bars, 10 μm. The results shown are representative of three independent experiments.
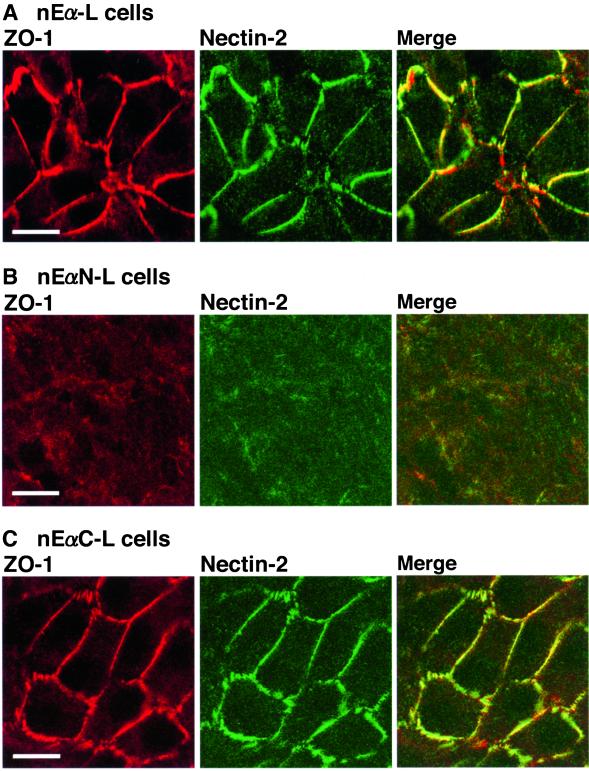
It has been shown by the use of three types of L cell lines, nEα-L, nEαC-L, and nEαN-L cells, that the localization of ZO-1 to E-cadherin–based AJs is mediated through the C-terminal half of α-catenin (Nagafuchi et al., 1994; Imamura et al., 1999). nEα-L cells express a chimeric protein (nEα) of β-catenin–binding domain-deleted E-cadherin fused with full-length α-catenin; nEαN-L cells express a chimeric protein (nEαN) of β-catenin–binding domain-deleted E-cadherin fused with the N-terminal half of α-catenin; and nEαC-L cells express a chimeric protein (nEαC) of β-catenin–binding domain-deleted E-cadherin fused with the C-terminal half of α-catenin. The expression level of the nectin-2α, ZO-1, afadin, or chimeric protein was similar among these three types of L cell lines (Figure 1B). We have previously shown that nectin-2 and afadin localize to nEα- and nEαC-based cell-cell adhesion sites but not to nEαN-based adhesion sites (Tachibana et al., 2000). Ponsin and vinculin localize to nEα-based and nEαN-based cell-cell adhesion sites but not to nEαC-based adhesion sites. These results indicate that the localization of nectin-2 and afadin at E-cadherin–based AJs is mediated through the C-terminal half of α-catenin (Tachibana et al., 2000). They furthermore indicate that the localization of nectin-2 and afadin at E-cadherin–based AJs is not mediated through ponsin or vinculin. Consistent with these earlier observations, ZO-1 colocalized with nectin-2 to nEα-based and nEαC-based cell-cell adhesion sites, whereas neither ZO-1 nor nectin-2 was concentrated at nEαN-based adhesion sites (Figure 3). Taken together, these results indicate that ZO-1 colocalizes with nectin-2 and afadin to E-cadherin–based AJs and that the colocalization of these proteins there is mediated through the C-terminal half of α-catenin. The localization of ZO-1 to E-cadherin–based AJs is, furthermore, independent of vinculin and ponsin.
Figure 3.
Localization of ZO-1 and nectin-2 at E-cadherin–based AJs through the C-terminal half of α-catenin. nEα-L, nEαN-L, and nEαC-L cells were doubly stained with the anti–ZO-1 mAb and the anti–nectin-2 mAb. (A) nEα-L cells. (B) nEαN-L cells. (C) nEαC-L cells. Bars, 10 μm. The results shown are representative of three independent experiments.
Afadin-dependent Recruitment of ZO-1 to Nectin-2α–based Cell-Cell Adhesion Sites
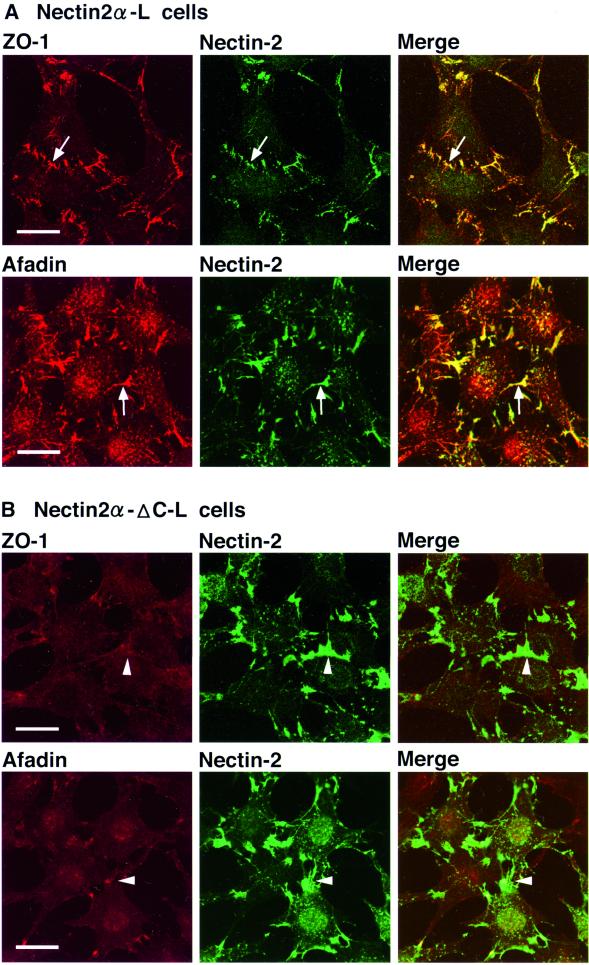
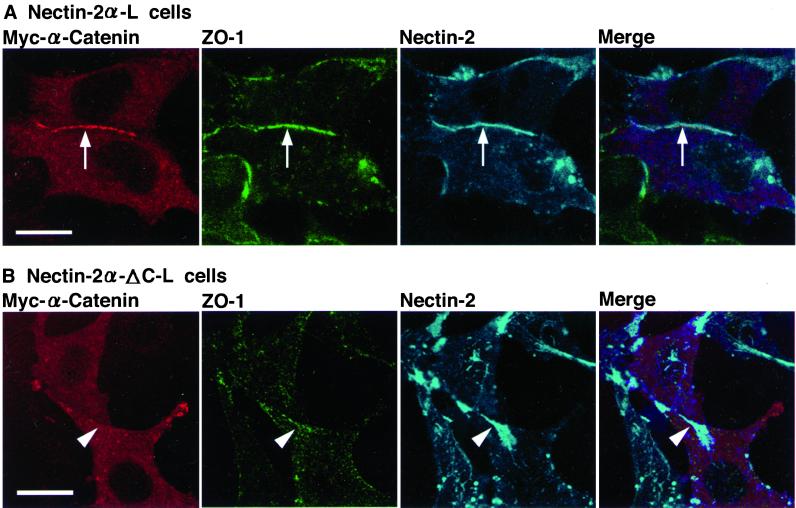
We have previously shown that L cells expressing full-length nectin-2α (nectin-2α-L cells) form nectin-2α–based cell-cell adhesion sites, where afadin is recruited through its direct interaction with full-length nectin-2α, whereas L cells expressing C-terminal four-aa–deleted nectin-2α (nectin-2α-ΔC; nectin-2α-ΔC-L cells) also form nectin-2α-ΔC–based cell-cell adhesion sites, but afadin does not interact with nectin-2α-ΔC or is not recruited to the adhesion sites (Miyahara et al., 2000; Tachibana et al., 2000). The expression level of the nectin-2α protein in nectin-2α-L cells was similar to that of the nectin-2α-ΔC protein in nectin-2α-ΔC-L cells (Figure 1C). The expression level of the ZO-1 or afadin protein was also similar between these L cell lines. In these cell lines, cadherin was not expressed (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). Endogenous α-catenin was expressed but the amount was remarkably less than that in EL cells and negligible (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). We have shown that transiently expressed α-catenin is recruited to nectin-2α–based, but not nectin-2α-ΔC–based, cell-cell adhesion sites (Tachibana et al., 2000). We examined whether ZO-1 is recruited to nectin-2α–based cell-cell adhesion sites even in the absence of the cadherin-catenin system. In nectin-2α-L cells, ZO-1 and afadin colocalized to nectin-2α–based cell-cell adhesion sites (Figure 4A), whereas in nectin-2α-ΔC-L cells, neither ZO-1 nor afadin localized to nectin-2α-ΔC–based cell-cell adhesion sites (Figure 4B). These results indicate that ZO-1 is recruited to nectin-2α–based cell-cell adhesion sites in an afadin-dependent manner even in the absence of the cadherin-catenin system.
Figure 4.
Afadin-dependent recruitment of ZO-1 to nectin-2α–based cell-cell adhesion sites. Nectin-2α-L and -2α-ΔC-L cells were doubly stained with various combinations of the anti–ZO-1 mAb, the anti–nectin-2 mAb, and the anti-afadin mAb. (A) Nectin-2α-L cells. (B) Nectin-2α-ΔC-L cells. Arrows, nectin-2α–based cell-cell adhesion sites; arrowheads, nectin-2α-ΔC–based cell-cell adhesion sites. Bars, 10 μm. The results shown are representative of three independent experiments.
Afadin-dependent Association of ZO-1 with Nectin-2α
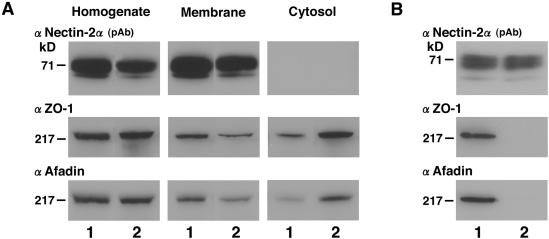
We next examined whether ZO-1 is associated with nectin-2α through afadin. For this purpose, we performed subcellular fractionation and immunoprecipitation analyses of nectin-2α-L and -2α-ΔC-L cells. When these cells were sonicated, followed by ultracentrifugation, similar amounts of nectin-2α and -2α-ΔC were recovered only in the membrane fraction (Figure 5A). However, ZO-1 and afadin were recovered in both the membrane and cytosol fractions, but the amounts of ZO-1 and afadin recovered in the membrane fraction in nectin-2α-L cells were more than those in nectin-2α-ΔC-L cells. When the cell extracts of nectin-2α-L and -2α-ΔC-L cells were subjected to immunoprecipitation by the use of the nectin-2 mAb, similar amounts of nectin-2α and -2α-ΔC were precipitated (Figure 5B). ZO-1 and afadin were coimmunoprecipitated with nectin-2α, whereas they were not coimmunoprecipitated with nectin-2α-ΔC. The reason why some amounts of ZO-1 and afadin were recovered in the membrane fraction in nectin-2α-ΔC-L cells is not known.
Figure 5.
Afadin-dependent association of ZO-1 with nectin-2α. (A) Subcellular fractionation analysis. Nectin-2α-L and -2α-ΔC-L cells were sonicated, followed by ultracentrifugation. A comparable amount of each fraction (each 20 μg of protein) was subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting with the anti–nectin-2α pAb, the anti–ZO-1 mAb, and the anti-afadin mAb. (B) Immunoprecipitation analysis. The cell extracts of nectin-2α-L and -2α-ΔC-L cells (each 2 mg of protein) were separately subjected to immunoprecipitation with the anti–nectin-2 mAb. The immunoprecipitate was then subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting with the anti–nectin-2α pAb, the anti–ZO-1 mAb, and the anti-afadin mAb. Lane 1, nectin-2α-L cells; lane 2, nectin-2α-ΔC-L cells. The results shown are representative of three independent experiments.
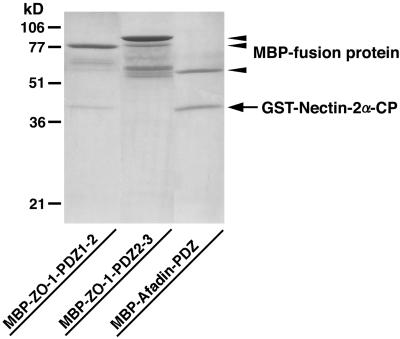
We have previously shown by affinity chromatography with the use of the N-terminal and C-terminal fragments of ZO-1 and the N-terminal, middle, and C-terminal fragments of afadin that ZO-1 does not directly interact with afadin (Sakisaka et al., 1999). We confirmed here this conclusion by affinity chromatography with the use of full-length ZO-1 and full-length afadin. A His6-tagged protein of full-length ZO-1 (His6-ZO-1) did not bind to a Myc- and His6-tagged protein of full-length afadin (Myc-His6-afadin) immobilized on affinity beads (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). We next examined whether ZO-1 directly interacts with nectin-2α. A GST-fusion protein of the cytoplasmic region of nectin-2α (GST-nectin-2α-CP) bound to an MBP-fusion protein of the first and second PDZ domains of ZO-1 (MBP-ZO-1-PDZ1–2) immobilized on amylose resin beads. However, the stoichiometry of the interaction of GST-nectin-2α-CP with MBP-ZO-1-PDZ1–2 was ∼0.1:1, whereas that of GST-nectin-2α-CP with a MBP-fusion protein of the PDZ domain of afadin (MBP-afadin-PDZ) was ∼1:1 (Figure 6). GST-nectin-2α-CP did not bind to a MBP-fusion protein of the second and third PDZ domains of ZO-1 (MBP-ZO-1-PDZ2–3). A similar result was obtained with full-length ZO-1 (His6-ZO-1; Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). These results indicate that ZO-1 does not directly interact with nectin-2α or afadin, although ZO-1 is recruited to nectin-2α–based cell-cell adhesion sites in an afadin-dependent manner.
Figure 6.
Inability of ZO-1 to directly interact with nectin-2α. MBP-ZO-1-PDZ1–2, MBP-ZO-1-PDZ2–3, or MBP-afadin-PDZ (each 20 μg of protein) was immobilized on amylose resin beads. GST-nectin-2α-CP (100 μg of protein) was applied to the affinity beads. After the beads were extensively washed, elution was performed with 10 mM maltose. The eluate was subjected to SDS-PAGE (13% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue. The results shown are representative of three independent experiments.
Ponsin- and Vinculin-independent Recruitment of ZO-1 to Nectin-2α–based Cell-Cell Adhesion Sites
Ponsin and vinculin colocalize to nEαN-based cell-cell adhesion sites but not to nEαC-based adhesion sites (Tachibana et al., 2000). Conversely, ZO-1 colocalized with nectin-2 and afadin to nEαC-based cell-cell adhesion sites but not to nEαN-based adhesion sites (Figure 3, B and C). As described above, these results indicate that ZO-1 is recruited to cell-cell adhesion sites, where nectin-2 and afadin colocalize, in a manner independent of ponsin and vinculin. To further confirm these results, we prepared nectin-2α-L cells where a GFP-fusion protein of full-length ponsin (GFP-ponsin) was transiently expressed. In these cells, neither GFP-ponsin nor vinculin localized to nectin-2α–based cell-cell adhesion sites, although both proteins colocalized to focal contacts (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). These results provide additional evidence that neither ponsin nor vinculin is necessary for the recruitment of ZO-1 to nectin-2α–based cell-cell adhesion sites.
Afadin-dependent Corecruitment of ZO-1 and α-Catenin to Nectin-2α–based Cell-Cell Adhesion Sites
We have previously shown that α-catenin is recruited to nectin-2α–based cell-cell adhesion sites (Tachibana et al., 2000). We next examined whether ZO-1 and α-catenin are corecruited. For this purpose, a Myc-tagged protein of full-length α-catenin (Myc-α-catenin) was transiently expressed in nectin-2α-L cells (Figure 1D). In these cells, both ZO-1 and Myc-α-catenin localized to nectin-2α–based cell-cell adhesion sites (Figure 7A). When Myc-α-catenin was transiently expressed in nectin-2α-ΔC-L cells, neither ZO-1 nor Myc-α-catenin localized to nectin-2α-ΔC–based cell-cell adhesion sites (Figure 7B). The expression level of the Myc-α-catenin protein was similar between these two types of L cell lines (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). These results indicate that ZO-1 and α-catenin are corecruited to nectin-2α–based cell-cell adhesion sites in an afadin-dependent manner.
Figure 7.
Afadin-dependent corecruitment of ZO-1 and α-catenin to nectin-2α–based cell-cell adhesion sites. Nectin-2α-L and -2α-ΔC-L cells were transfected with pPGKIZ-Myc-α-catenin and triply stained with the anti-Myc mAb, the anti–ZO-1 pAb, and the anti–nectin-2 mAb. (A) Nectin-2α-L cells. (B) Nectin-2α-ΔC-L cells. Arrows, nectin-2α–based cell-cell adhesion sites; arrowheads, nectin-2α-ΔC–based cell-cell adhesion sites. Bars, 10 μm. The results shown are representative of three independent experiments.
α-Catenin–independent Colocalization of ZO-1 with Nectin-2 and Afadin
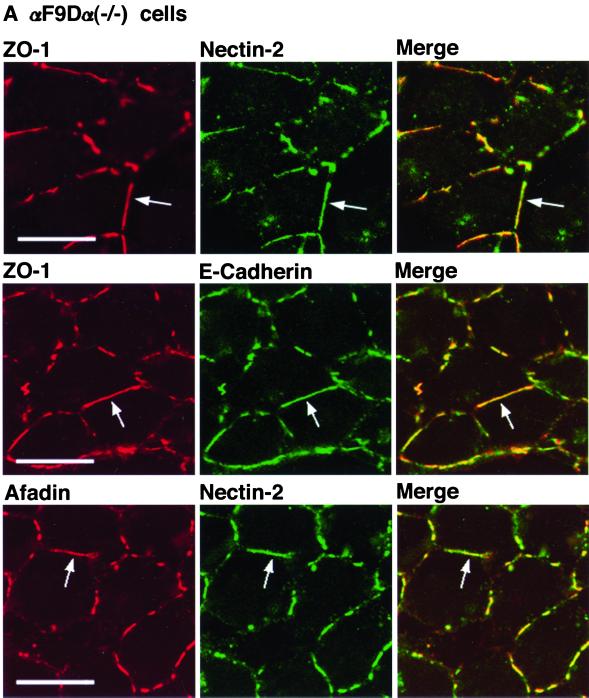
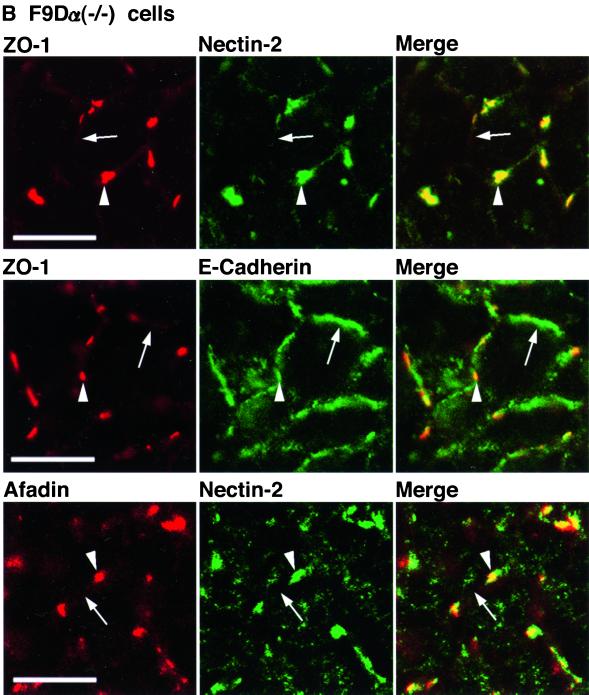
We have previously shown that nectin-2 and E-cadherin colocalize to cell-cell AJs in an α-catenin-dependent manner and that nectin-2 and E-cadherin exhibit different localization in an α-catenin–deficient F9 cell line, F9Dα(−/−) cells (Tachibana et al., 2000). The F9 cell line is derived from mouse teratocarcinoma-derived embryonal carcinoma cells. F9Dα(−/−) cells have been generated by disruption of both of the α-catenin alleles with a targeting vector (Maeno et al., 1999). We then examined whether ZO-1 is recruited to nectin-2–based or E-cadherin–based cell-cell adhesion sites in F9Dα(−/−) cells. As a control, we used αF9Dα(−/−) cells, which have been generated by introduction of an expression vector encoding full-length α-catenin into F9Dα(−/−) cells (Maeno et al., 1999). It has been shown that αF9Dα(−/−) cells re-express α-catenin in an amount similar to that of wild-type F9 cells (Maeno et al., 1999). The expression level of the ZO-1, afadin, E-cadherin, and nectin-2α protein was similar between these two types of F9 cell lines (Figure 1E). In αF9Dα (−/−) cells, E-cadherin localized to belt-like cell-cell adhesion sites where ZO-1 colocalized with nectin-2 and afadin (Figure 8A). In F9Dα(−/−) cells, E-cadherin showed a similar staining pattern, but ZO-1 was hardly concentrated at cell-cell adhesion sites between two cells where E-cadherin was concentrated (Figure 8B). ZO-1 was concentrated with nectin-2 and afadin at spot-like cell-cell adhesion sites where more than two cells adhered to each other. These results indicate that ZO-1 is recruited to E-cadherin–based cell-cell AJs in an α-catenin–dependent manner, whereas it is recruited to nectin-2–based cell-cell adhesion sites in an α-catenin–independent manner.
Figure 8.
α-Catenin-independent colocalization of ZO-1 with nectin-2 and afadin. αF9Dα(−/−) and F9Dα(−/−) cells were doubly stained with various combinations of the anti–ZO-1 mAb, the anti–nectin-2 mAb, the anti–E-cadherin mAb, and the anti-afadin mAb. (A) αF9Dα(−/−) cells. (B) F9Dα(−/−) cells. Arrows, cell-cell adhesion sites between two cells; arrowheads, cell-cell adhesion sites where more than two cells adhere to each other. Bars, 10 μm. The results shown are representative of three independent experiments.
Recruitment of ZO-1 to Nectin-2α–based Cell-Cell Adhesion Sites in a Manner Insensitive to F-Actin–disrupting Agents
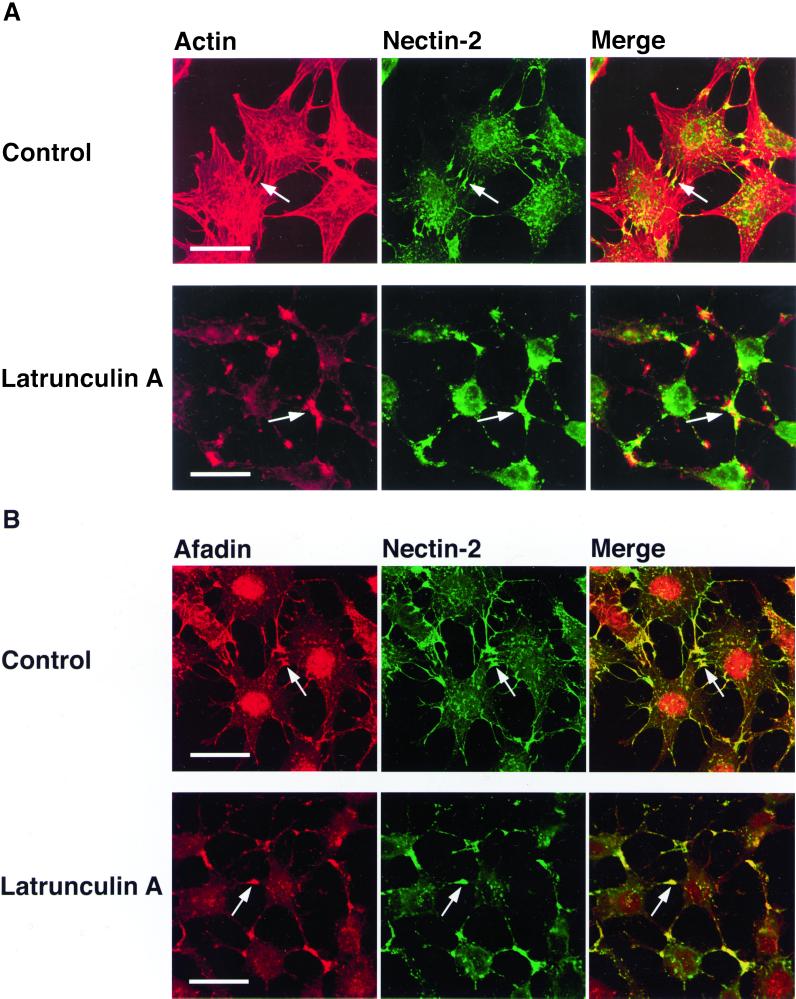
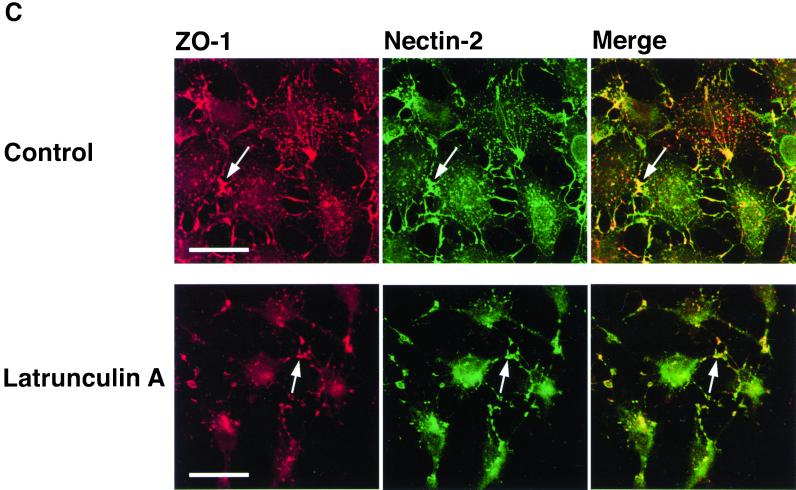
Both afadin and ZO-1 are F-actin–binding proteins (Itoh et al., 1997; Mandai et al., 1997; Fanning et al., 1998). In the last set of experiments, therefore, we examined by the use of F-actin–disrupting agents, latrunculin A and cytochalasin D, whether actin cytoskeletal structures are involved in the recruitment of ZO-1 to nectin-2α–based cell adhesion sites. In nectin-2α-L cells, F-actin was associated with nectin-2α–based cell-cell adhesion sites where ZO-1 and afadin colocalized (Figure 9). When these cells are incubated with latrunculin A for 45 min, the cells became round and most of actin cytoskeletal structures were disrupted (Figure 9A). Under these conditions, nectin-2α formed cell adhesion sites where ZO-1 and afadin colocalized (Figure 9, B and C). When the cells were cultured with the agent for a longer time, the cells detached from dishes (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). Essentially similar results were obtained with cytochalasin D (Yokoyama, Tachibana, Nakanishi, Yamamoto, Irie, Mandai, Nagafuchi, Monden, and Takai, unpublished results). These results suggest that latrunculin A- or cytochalasin D-sensitive actin cytoskeletal structures are not essential for the recruitment of ZO-1 to nectin-2α–based cell-cell adhesion sites, although it could not be concluded from these results that any actin cytoskeletal structure is not involved in this recruitment.
Figure 9.
Recruitment of ZO-1 to nectin-2α–based cell-cell adhesion sites in a manner insensitive to latrunculin A. Nectin-2α-L cells were incubated in the presence or absence of 50 nM latrunculin A for 45 min, followed by double staining with various combinations of rhodamine-phalloidin, the anti–nectin-2 mAb, the anti-afadin mAb, and the anti–ZO-1 mAb. (A) F-actin and nectin-2. (B) Afadin and nectin-2. (C) ZO-1 and nectin-2. Arrows, nectin-2α–based cell-cell adhesion sites. Bars, 10 μm. The results shown are representative of three independent experiments.
DISCUSSION
We have previously shown that nectin has a potency to recruit α-catenin to nectin-based cell-cell adhesion sites in an afadin-dependent manner in L cells (Tachibana et al., 2000). We have shown here that nectin has a potency to recruit not only α-catenin but also ZO-1 to nectin-based cell-cell adhesion sites in L cells. This recruitment of ZO-1 is dependent on the interaction of nectin with afadin, because full-length nectin capable of interacting with afadin recruits ZO-1 but nectin-ΔC incapable of interacting with afadin does not. It has been shown that ZO-1 directly interacts with α-catenin (Itoh et al., 1997; Imamura et al., 1999). It is therefore possible that the recruitment of ZO-1 to nectin-based cell-cell adhesion sites is mediated through α-catenin, but this possibility is unlikely, because in F9Dα(−/−) cells that are deficient of α-catenin, ZO-1 still colocalizes with nectin.
It is of crucial importance to clarify the molecular mechanism of the linkage between afadin and ZO-1. It has previously been reported that the AF6 protein, a smaller splicing variant of afadin, directly binds ZO-1 (Yamamoto et al., 1997). However, our present results, together with our previous reports (Sakisaka et al., 1999; Tachibana et al., 2000), indicate that afadin does not directly binds ZO-1 to a significant extent. We cannot currently exclude the possibility that the posttranslationally modified forms of these proteins directly interact with each other or that these proteins interact with each other in the presence of an unidentified cofactor.
The second possible molecular mechanism of the linkage between afadin and ZO-1 is that ponsin and/or vinculin are involved in it. We have previously shown that ponsin and vinculin colocalize with nectin-2 and afadin to nEα-based cell-cell adhesion sites (Tachibana et al., 2000). We have shown here that ZO-1 colocalizes there with nectin-2 and afadin. However, ponsin or vinculin does not localize to nEαC-based cell-cell adhesion sites where nectin-2 and afadin colocalize (Tachibana et al., 2000), although ZO-1 colocalizes there with nectin-2 and afadin. Furthermore, neither ponsin nor vinculin is recruited to nectin-2α–based cell-cell adhesion sites where afadin and ZO-1 colocalize. Thus, the second possibility is negated.
The third possible molecular mechanism of the linkage is that actin cytoskeletal structures are involved in it, because afadin (Mandai et al., 1997) and ZO-1 (Itoh et al., 1997; Fanning et al., 1998) are F-actin–binding proteins. However, we have shown here that afadin and ZO-1 colocalize to nectin-based cell-cell adhesion sites where actin cytoskeletal structures are mostly disrupted by an inhibitor of actin polymerization, latrunculin A or cytochalasin D, indicating that at least these agent-sensitive actin cytoskeletal structures are not involved in the linkage between afadin and ZO-1. Nectin forms cell-cell adhesion sites even under the conditions where actin cytoskeletal structures are disorganized by latrunculin A or cytochalasin D. This result is consistent with our earlier observation that full-length nectin and nectin-ΔC exhibit similar cell adhesion activity (Miyahara et al., 2000). It is likely that the connection of nectin to the actin cytoskeleton through afadin is not essential for cell adhesion activity. It has recently been shown that disorganization of actin cytoskeletal structures shifts cadherin-based cell adhesion activity from the strong to the weak state (Imamura et al., 1999). By analogy with cadherin, the connection of nectin to the actin cytoskeleton may be required for stronger cell adhesion activity. Thus, further studies are necessary for our understanding of the molecular mechanism of the linkage between afadin and ZO-1.
In EL cells, endogenous nectin-2, afadin, and ZO-1 all colocalize with E-cadherin and α- and β-catenins at the same cell-cell adhesion sites (Itoh et al., 1993; Mandai et al., 1997; Imamura et al., 1999; Takahashi et al., 1999). This colocalization of the two cell-cell adhesion systems is mediated through at least afadin and α-catenin (Tachibana et al., 2000). ZO-1 has been shown to be associated with E-cadherin through direct interaction with α-catenin (Itoh et al., 1997; Imamura et al., 1999). This result, together with our present results, suggests that, in cell-cell adhesion sites where both nectin and cadherin colocalize, ZO-1 may localize there through interactions with both afadin and α-catenin. We cannot currently exclude the possibility that the recruitment of α-catenin to nectin-based cell-cell adhesion sites is mediated through ZO-1, but one possible function of ZO-1 is a connector between the nectin-afadin and cadherin-catenin systems. Another possible function of ZO-1 is that it connects these two cell-cell adhesion systems to the actin cytoskeleton, because ZO-1 as well as afadin and α-catenin is an F-actin–binding protein (Rimm et al., 1995; Itoh et al., 1997; Mandai et al., 1997).
In well-polarized epithelial cells, ZO-1 is not associated with the cadherin-catenin system or the nectin-afadin system but is associated with the claudin-occludin system at TJs (Stevenson et al., 1986; Itoh et al., 1993; Mandai et al., 1997, 1999; Asakura et al., 1999; Sakisaka et al., 1999; Takahashi et al., 1999; Tsukita et al., 1999). During the formation of cell-cell junctions including AJs and TJs, E-cadherin, β- and α-catenins, nectin-2, afadin, and ZO-1 first accumulate at the spot-like junctions, followed by the recruitment of occludin and probably claudin (Yonemura et al., 1995; Ando-Akatsuka et al., 1996; Asakura et al., 1999). Once TJs are separated from AJs, ZO-1 is translocated to TJs. Our present results have increased the possibility that nectin has a potency to recruit claudin and occludin to nectin-based cell-cell adhesion sites through afadin and ZO-1. However, our preliminary analysis has revealed that nectin-2α does not show this activity in L cells expressing both nectin-2α and claudin-1, but this may be simply because many other components of TJs, such as ZO-2 (Jesaitis and Goodenough, 1994), ZO-3 (Haskins et al., 1998), JAM (Marîn-Padura et al., 1998), cingulin (Citi et al., 1988), MAGI (Dobrosotskaya et al., 1997; Ide et al., 1999), symplekin (Keon et al., 1996), and 7H6 antigen (Zhong et al., 1993), may be absent in nonepithelial cells, such as L cells. It is of crucial importance to identify such components for our understanding of the mechanism of the formation of the junctional complex in epithelial cells.
ACKNOWLEDGMENTS
We thank Dr. M. Takeichi (Kyoto University, Kyoto, Japan) for helpful discussion and for providing us with the anti–E-cadherin mAb. We also thank Drs. S. Tsukita and M. Itoh (Kyoto University, Kyoto, Japan) for helpful discussion and for the anti–ZO-1 mAb and MBP-ZO-1-PDZ1–2 and -ZO-1-PDZ2–3. This work at the Department of Molecular Biology and Biochemistry, Osaka University Graduate School of Medicine/Faculty of Medicine, was supported by Grants-in-Aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, Sports, and Culture, Japan (1999, 2000).
Abbreviations used:
- aa
amino acid(s)
- AJs
adherens junctions
- F-actin
actin filament(s)
- GFP
green fluorescent protein
- GK
guanylate kinase
- GST
glutathione S-transferase
- mAb
monoclonal antibody
- MBP
maltose-binding protein
- pAb
polyclonal antibody
- TJs
tight junctions
REFERENCES
- Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1996;179:115–125. doi: 10.1002/(SICI)1097-4652(199905)179:2<115::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- Asakura T, Nakanishi H, Sakisaka T, Takahashi K, Mandai K, Nishimura M, Sasaki T, Takai Y. Similar and differential behavior between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells. 1999;4:573–581. doi: 10.1046/j.1365-2443.1999.00283.x. [DOI] [PubMed] [Google Scholar]
- Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Dong Y, McDermott BM, Jr, Lam D, Brown KR, Shelanski M, Bellvé AR, Racaniello VR. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherens junctions. Mol Cell Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Feramisco JR. α-Actinin and vinculin from non-muscle cells:calcium sensitive interactions with actin. Cold Spring Harbor Symp Quant Biol. 1982;46:587–597. doi: 10.1101/sqb.1982.046.01.055. [DOI] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Dubreuil P, Lopez M, Campadelli-Fiume G. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB) J Virol. 2000;74:3909–3917. doi: 10.1128/jvi.74.8.3909-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attribute of a bona fide receptor for herpes simplex virus type 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosotskaya I, Guy RK, James GL. MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J Biol Chem. 1997;272:31589–31597. doi: 10.1074/jbc.272.50.31589. [DOI] [PubMed] [Google Scholar]
- Eberlé F, Dubreuil P, Mattei M-G, Devilard E, Lopez M. The human PRR2 gene, related to the poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alpha herpes viruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Nishimori H, Tokino T, Nakamura Y, Takai Y. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) at tight junctions of epithelial cells. Oncogene. 1999;18:7810–7815. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka K, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y. Afadin: a key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1131. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno M, Tsukita S, Nagafuchi A. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelia cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Yonemura S, Nagafuchi A, Tsukita S, Tsukita S. A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol. 1991;115:1449–1462. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila disc-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Schmelz M, Franke WW, Geiger B. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J Cell Biol. 1991;113:881–892. doi: 10.1083/jcb.113.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keon BH, Schafer SS, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez M, Eberlé F, Mattei M-G, Gabert J, Birg F, Birdin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- Maeno Y, Moroi S, Nagashima H, Noda T, Shiozaki H, Monden M, Tsukita S, Nagafuchi A. α-Catenin-deficient F9 cells differentiate into signet ring cells. Am J Pathol. 1999;154:1323–1328. doi: 10.1016/s0002-9440(10)65385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Takahashi T, Satoh K, Nishioka H, Mizoguchi A, Takai Y. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J Cell Biol. 1999;144:1001–1017. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marîn-Padura I, Lostagio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel AR, Kroemker M, Bubeck P, Ronsiek M, Nikolai G, Jockusch BM. Characterization of an F-actin-binding domain in the cytoskeletal protein vinculin. J Cell Biol. 1994;126:1231–1240. doi: 10.1083/jcb.126.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Morrison ME, Racaniello VR. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-α-catenin fusion molecules. J Cell Biol. 1994;127:235–247. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:3679–3684. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset JE, Redfield AR, Jin F, Knudson KA, Johnson KR, Wheelock MJ. Characterization of the interactions of α-catenin with α-actinin and β-catenin/plakoglobin. J Cell Sci. 1997;110:1013–1022. doi: 10.1242/jcs.110.8.1013. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Barbault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Gu Y, Alder H, Nakamura T, Canaani O, Saito H, Huebner K, Gale RP, Nowell PC, Kuriyama K, Miyazaki Y, Croce CM, Canaani E. Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 1993;53:5624–5628. [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Nakanishi H, Takahashi K, Mandai K, Miyahara M, Satoh A, Takaishi K, Takai Y. Different behavior of l-afadin and neurabin-II during the formation and destruction of cell-cell adherens junction. Oncogene. 1999;18:1609–1617. doi: 10.1038/sj.onc.1202451. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Takai K. Nectin-3: a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpes virus receptor, in cleft lip/plate ectodermal dysplasia. Nat Genet. 2000;25:427–429. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1171. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi H, Miyahara M, Mandai M, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junction through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Structural and signaling molecules come together at tight junctions. Curr Opin Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Direct actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. J Virol. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between α-catenin and vinculin. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson BB, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologue to the Drosophila disc-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similar and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995;108:127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin, and ZO-2. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]