Abstract
Retroviral particles contain a dimeric RNA genome, which serves as template for the generation of double-stranded DNA by reverse transcription. Transfer between RNA strands during DNA synthesis is governed by both sequence similarity between templates and structural features of the dimeric RNA. A kissing hairpin, believed to facilitate intermolecular recognition and dimer formation, was previously found to be a preferred site for recombination. To investigate if hairpin loop–loop-complementarity is the primary determinant for this recombination preference, we have devised a novel 5′ leader recombination assay based upon co-packaging of two wild-type or loop-modified murine leukemia virus vector RNAs. We found that insertion of an alternative palindromic loop in one of the two vectors disrupted site-directed template switching, whereas site-specificity was restored between vectors with complementary non-wild-type palindromes. By pairing vector RNAs that contained identical non-palindromic loop motifs and that were unlikely to interact by loop–loop kissing, we found no preference for recombination at the kissing hairpin site. Of vector pairs designed to interact through base pairing of non-palindromic loop motifs, we could in one case restore hairpin-directed template switching, in spite of the reduced sequence identity, whereas another pair failed to support hairpin- directed recombination. However, analyses of in vitro RNA dimerization of all studied vector combinations showed a good correlation between efficient dimer formation between loop-modified viral RNAs and in vivo cDNA transfer at the kissing hairpin. Our findings demonstrate that complementarity between wild-type or non-wild-type hairpin kissing loops is essential but not sufficient for site-specific 5′ leader recombination and lend further support to the hypothesis that a specific ‘kissing’ loop–loop interaction is guided by complementary sequences and maintained within the mature dimeric RNA of retroviruses.
INTRODUCTION
As a unique step of retrovirus replication, double-stranded DNA is generated by reverse transcription of an RNA genome consisting of two intermolecularly linked plus-strand RNAs. Transfer of nascent cDNA from one RNA template to the other during this process not only enhances genetic variability but also provides an efficient viral route of escape from RNA damage and fatal mutations. Generation of novel mosaic retroviruses by reverse transcriptase-mediated mixing of genetic determinants requires co-packaging of distinct viral RNA molecules into virus particles (1). Retroviral genomic RNA is selectively encapsidated into the budding virion, most likely as a dimer (2,3) linked by direct RNA–RNA interactions near the 5′-end of the transcripts (4). Whereas RNAs transcribed from a single locus or identical proviral loci form homodimers, RNA transcripts derived from different proviruses may associate to form heterodimeric genomes. Such heterodimers may be generated in cells that have been infected with two or more viruses or may alternatively consist of RNAs derived from a replicating virus (or a retroviral vector) and an endogenous retroviral sequence. Through a panel of studies (reviewed in 5), based primarily on in vitro template switching between synthetic RNAs and recombination between viral vector-derived RNAs, it has been well documented that sequence similarity between the acceptor template and the nascent DNA strand copied from the donor RNA is a major determinant for retroviral recombination. Moreover, accumulating in vitro evidence suggests that RNA secondary, and possibly tertiary, structures induce recombination by stalling reverse transcriptase during cDNA synthesis (6–11).
For most retroviruses studied, short palindromic sequences forming kissing hairpins appear to guide the initial linking of viral RNA through Watson–Crick basepair ‘kissing’ between the hairpin loops (12–20). Deletion or minor modifications of the hairpin loops abolish dimer formation in vitro, whereas restoration of loop–loop complementarity through insertion of either non-wild-type palindromic loop sequences or non-palindromic trans-complementary loop motifs restores in vitro dimer formation (12,19,21,22). However, conservation of kissing hairpin loop sequences and the fact that not all sets of complementary ‘kissing’ loops support dimer formation (12,21) suggest that constraints exist at the sequence level or at a higher structural level determined by the sequence.
In Moloney murine leukemia virus (Mo-MLV), in vitro RNA dimerization has identified two dimer-forming ‘kissing’ hairpins located at positions 204–228 and 278–303 (15,23–25). Each of these palindromic hairpins can alone support RNA dimerization but the presence of both appears to further enhance dimer formation and stability (24). Moreover, the core packaging signal, composed of two hairpins (located immediately downstream at positions 310–352 and 355–374, respectively) each exposing a GACG tetraloop (26,27), has been found to support and kinetically induce dimer linkage, possibly through an unusual two-basepair loop–loop interaction (28). In vivo studies showing reduced single-cycle transfer of kissing loop-modified MLV vectors (24,29) and reduced heat stability of hairpin-mutated MLV RNA dimers (24) provide further support for an important role of kissing hairpins, although full-length replicating viruses are not markedly inhibited by loop mutations (30).
Our current understanding of dimer structure and viral DNA synthesis suggests that dimerization and recombination are closely connected processes of the retroviral life cycle. Genetic modifications targeted to dimer-forming cis-elements may therefore influence not only intermolecular RNA interactions but also template switching during reverse transcription. Moreover, such changes are likely to change the affinity for other homologous or heterologous RNAs (19) and may, in the presence of other viral RNAs, affect the ratio of homodimers and heterodimers packaged in virions.
By mapping sites of cDNA transfer within the 5′ leader region of similar but not identical MLV RNAs we have previously shown that the proceeding reverse transcriptase during minus strand synthesis ‘jumps’ between co-packaged RNA templates preferentially within the kissing hairpin dimerization domain (kissing hairpin 2, KH2, see Fig. 1A) (31). Such site-specificity was seen in studies of recombination between mouse MLV-like endogenous sequences and (i) Akv-MLV vector RNA in single-cycle vector transfer (32) or (ii) replication-competent SL3-3 viruses injected in mice (33). Studies using vectors with point mutations in the KH2 loop suggested loop–loop basepairing to be crucial for KH2-directed template switching with the endogenous RNA partner, since as little as a single point mutation within the loop could disrupt specificity (29). However, this assay suffered from the fact that only one of the partners could be subject to mutational modification. Hence, the contribution from base pairing between loops as such could not be clearly distinguished from putative structural effects of loop-sequence or from effects of the length of sequence identity windows.
Figure 1.
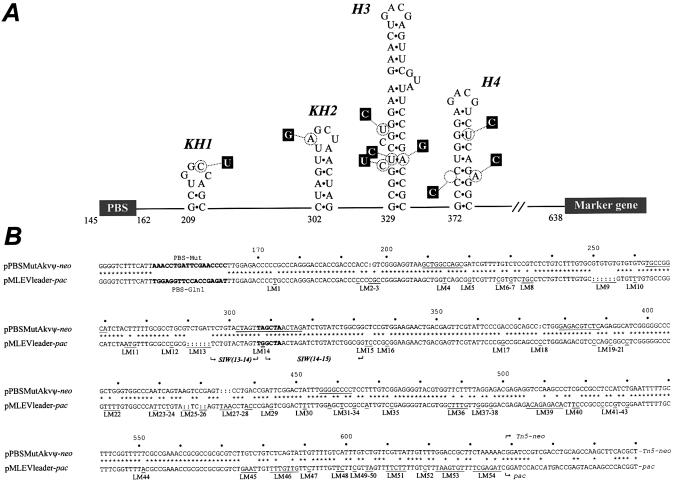
The 5′ leader region of Akv-MLV and MLV-like endogenous virus (MLEV). (A) Schematic representation of RNA hairpin structures involved in MLV RNA dimer formation and packaging. Kissing hairpins KH1 and KH2 are involved in RNA dimerization (15,23–25), whereas hairpins H3 and H4 function as the core packaging signal (26,27). Structure of the Akv hairpins is shown; nucleotides that differ in MLEV are shown in black boxes. (B) Alignment of 5′ leader regions of Akv and MLEV present in the PBS-Mut vector (PBSMutAkvΨ-neo) and the rescue vector (MLEVleader-pac), respectively. PBS sequences are in bold letters. Identical nucleotide positions are indicated by asterisks between the two sequences; nucleotide insertions are indicated by colons (:) inserted in the sequence. Single-base or clusters of genetic differences (leader markers, LM) are underlined in the MLEV sequence; marker numbers (LM1–LM54) are indicated below markers. Numbers indicated above alignment refer to position numbers in Akv. Akv position 638 corresponds to MLEV position 895 (DDBJ/EMBL/GenBank accession no. AF041383). The leader sequence of the MLEV vector differs from the previously published sequence at MLEV position 483 (LM4). Sequence identity windows SIW13–14 and SIW14–15, containing the KH2 sequence, are indicated by arrows. Palindromic sequences (8 nt or longer) in Akv are underlined.
We have therefore devised a new assay in which both recombination partners are exogenous and amenable to modification by mutation. By use of vector pairs with reduced or restored kissing loop–loop complementarity, we test the importance of kissing hairpins of various sequences in dimer formation and site-specific recombination. In addition, we support our in vivo data by an in vitro RNA dimerization assay. Our findings support the notion that recombination is facilitated by an interaction between complementary loop sequences of the KH2, but that the actual sequences of the complementary loops have an influence. Our results may be taken as indirect evidence that this interaction is maintained in the mature RNA dimer that serves as template for reverse transcription.
MATERIALS AND METHODS
Vector construction
A panel of transfer vectors harboring modifications of the kissing-loop dimerization sequence was derived from an Akv-MLV-based retroviral vector harboring the wild-type Akv-MLV 5′ leader region including the 476-bp packaging region, Ψ (the region between U5/PBS and leader/gag junctions). This vector, designated pPBSProAkvΨ-neo, contains the Akv-MLV long terminal repeat (LTR), a primer binding site (PBS) matching proline tRNA, and the neomycin resistance gene (neo) flanked upstream by the 5′ leader region and downstream by 480-bp Akv-MLV sequences including the 3′ untranslated region (UTR).
In brief, PBS knockout vectors harboring neo and rescue vectors harboring the puromycin resistance gene (pac) were generated as follows. The pac gene was PCR-amplified from pPUR (Clontech Laboratories) with primers containing BamHI and BsmI restriction sites, respectively. The amplified 634-bp fragment was inserted by standard cloning procedures into BamHI–BsmI-digested (restriction sites flanking neo) pPBSProAkvΨ-neo and pPBSMutAkvΨ-neo, the latter containing a defective PBS (PBS-Umu) as previously described (32), generating pPBSProAkvΨ-pac and pPBSMutAkvΨ-pac. The 465-bp packaging region of MLEV, a previously described MLV-like endogenous virus (31), was PCR-amplified from virion cDNA. The MLEV-Ψ fragment was connected by overlap extension and PCR with a fragment containing the Akv LTR, resulting in an Akv–MLEV chimeric fragment (the Akv–MLEV junction being in the U5 region) that was cloned into the appropriate position of pPBSProAkvΨ-pac, generating pMLEVleader-pac.
Alternative loop motifs were introduced into KH2-loop sequences of pPBSProAkvΨ-neo, pPBSMutAkvΨ-neo and pMLEVleader-pac by PCR-mediated mutagenesis using the following sense oligonucleotides matching Akv, or corresponding MLEV, positions 291–330 (Fig. 1B; 34) (altered loop sequences are underlined): ON1 (5′-CTGATTCTGTACTAGTATCGATACTAGATCTGTATCTGGC-3′) introducing the alternative palindrome 5′-ATCGAT-3′ (KLaltpal), ON2 (5′-CTGATTCTGTACTAGTTAGGATACTAGATCTGTATCTGGC-3′) and ON3 (5′-CTGATTCTGTACTAGTTACAATACTAGATCTGTATCTGGC-3′) introducing the non-palindromic sequences 5′-TAGGAT-3′ (KLnonpalA) and 5′-TACAAT-3′ (KLnonpalB), respectively, and ON4 (5′-CTGATTCTGTACTAGTATCCTAACTAGATCTGTATCTGGC -3′) and ON5 (5′-CTGATTCTGTACTAGTATTGTAACTAGATCTGTATCTGGC-3′) used for introduction of complementary non-palindromic loop sequences 5′-ATCCTA-3′ (KLmatchnonpalA) and 5′-ATTGTA-3′ (KLmatchnonpalB) into pMLEVleader-pac. Together with an antisense oligonucleotide matching the most 3′ part (positions 617–638) of either Akv-Ψ (ON6, 5′-CAGGTCGACGGATCCGTTTTTAGAAGCGGTCCAAAAC-3′) or MLEV-Ψ (ON7, 5′-CAGGTCGACGGATCCGATCTCGAAAACACTTAAAGAC-3′), 363-bp PCR fragments were generated, digested within flanking SpeI and BamHI restriction sites, and cloned into the appropriate sites of the respective vectors. To generate pPBSMutMLEVleader-pac, a BstBI–BamHI-digested PCR fragment containing PBSMut and 465 bp MLEV-Ψ was cloned into pPBSMutAkvΨ-pac containing BstBI within the PBSMut sequence and BamHI immediately downstream from Ψ. Based on computer-based estimations none of the introduced loop-modifications were found to interfere with folding of the hairpin and exposure of the loop sequence. All vector constructs were confirmed by sequence analysis.
Cells, two-step- and co-transfections and virus infections
Ψ2 (35), BOSC23 (36) and NIH 3T3 cells were grown in Dulbecco modified Eagle medium with Glutamax-1 supplemented with 10% newborn calf serum (Ψ2, NIH 3T3) or 10% fetal bovine serum (BOSC23), 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were incubated at 37°C in 90% relative humidity and 5.7% CO2. Stably transfected Ψ2 packaging cells were generated by a two-step transfection protocol; briefly, 10 µg of PBSMut-vector plasmid DNA was transfected by calcium phosphate treatment into Ψ2 cells seeded at 5 × 103 cells/cm2 on the day prior to transfection. Two days after transfection, G418-containing medium (0.6 mg/ml) was added to select for the stably integrated neo-vector. G418-resistant colonies appearing after 12 days of selection were pooled. Subsequently, pooled G418-resistant cells (seeded at 5 × 103 cells/cm2 on the day prior to transfection) were transfected with 10 µg pac-containing rescue vector DNA. Puromycin-containing medium (1.5 µg/ml) was added after 2 days to select for cells with the inserted pac vector (37). Finally, combined G418–puromycin selection was retained for several days prior to virus harvest and vector transduction. BOSC23 packaging cells, seeded at 7.1 × 104 cells/cm2 on the day prior to transfection, were transiently transfected with a total of 21 µg plasmid DNA including 1 µg of pEGFP plasmid (Clontech Laboratories) used as transfection control. In one-vector transfers (PBSPro or PBSMut vectors transfected without rescue vector), 10 µg of vector DNA was utilized together with 10 µg of pUC19 DNA and 1 µg of pEGFP; in two-vector transfers, 10 µg of PBSMut vector DNA was co-transfected with 10 µg of PBS-functional rescue vector DNA and 1 µg of pEGFP. Transduction efficiencies were determined as follows: Ψ2 producer cells were seeded at maximum density (∼105 cells/cm2) and allowed to attach; the medium was renewed and left on the cells for 1 day. BOSC23-derived viruses were harvested 2 days after transfection. Filtered and serially diluted virus-containing medium samples were transferred to NIH 3T3 cells (seeded at 5 × 103 cells/cm2 the day prior to infection) in the presence of Polybrene (6 µg/ml). G418- or puromycin-containing medium (concentrations 0.6 mg/ml and 1.5 µg/ml, respectively) was added 2 days after infection and resistant colonies were counted and individually expanded after 10 days of selection. For Ψ2-based experiments, titers were normalized for the number of producer cells, as determined immediately after virus harvest.
Proviral DNA analysis and PCR-based screening for recombinant proviruses
Genomic DNA from G418-resistant clones and colony pools was prepared as previously described (38). Digestion or sequence analysis of individual transduced vector sequences was performed on PCR products encompassing part of the 5′-LTR, the PBS, the 5′-UTR and the upstream part of neo or pac depending on the marker gene present in the PBS-defective vector. The neo-PCR was performed with ON8 (5′-TTCATAAGGCTTAGCCAGCTAACTGCAG-3′) matching Akv-MLV positions 7838–7865 (34) and ON9 (5′-GGCGCCCCTGCGCTGACAGCCGGAACAC-3′) matching neo positions 1656–1683 (39). Resulting PCR products harboring the PBS-repaired 5′ leader were sequenced with sense ON10 (5′-TCCGAATCGTGGTCTCGCTGATCCTTGG-3′) matching positions 69–96 within the Akv U5 region (34) and with antisense ON11 (5′-CTTCCTTTAGCAGCCCTTGCGC-3′) matching neo positions 1223–1244. The pac-PCR was performed with ON8 and ON12 (5′-CGACGCGCGTGAGGAAGAGTTC-3′), the latter matching pac positions 582–603 (37; pPUR cloning vector, DDBJ/EMBL/GenBank accession no. U07648); sense ON10 and antisense ON13 (5′-GGCGCGTGGCGGGGTAGTCGGC-3′), the latter matching pac positions 513–534 (pPUR cloning vector), were used as sequence primers.
Statistical analysis of recombination data
Recombination events were split into two groups: A, recombination events within sequence identity windows (SIWs) 13–15; and B, recombination events outside SIWs 13–15, thus giving a binomial distribution of the data set. Statistical analysis of the data was performed with the χ2 test and for all determinations a P value <0.05 was considered significant.
RNA synthesis and preparation
Plasmids carrying MLEV 5′ leader and derived loop-modified leader sequences were used as templates in high-fidelity PCR amplification [using a mixture of Pfu (Promega) and Taq gold (Perkin Elmer)] to generate T7 polymerase templates for in vitro transcription. The sense primer contained a T7 promoter and the downstream primer was fully complementary to the template. For MLEV constructs the T7 promoter-containing primer ON14 (5′-CGGATTTCCGTAATACGACTCACTATAGGGAGATTGTGCCGGCATCTAATGTT-3′) and primers ON15 (5′-AGGCCGCTGGGAC GTCTCCCAGG-3′) and ON16 (5′-TACCCCTCGGACAGTGGCGG-3′) were used to amplify MLEV(523–654) and MLEV(523–738) templates, respectively. For the Akv-MLV constructs, the T7 promoter-containing primer ON17 (5′-CGGATTTCCGTAATACGACTCACTATAGGGAGATTGCGCCTGCGTCTGATTCTGT-3′) and primers ON18 (5′-CCCCGCTGCCTCTGAGAC-3′) and ON19 (5′-TACCCCTCCGACAAAGGAGG-3′) were used for generation of Akv-MLV(279–401) and Akv-MLV(279–481) templates, respectively. The PCR products were phenol–chloroform extracted and NaOAc–ethanol precipitated prior to T7 transcription. The RNA was transcribed in reaction volumes of 10 µl using a T7 Megascript kit (Ambion) and an equal volume of formamide buffer was added prior to denaturation (95°C, 2 min) and loading on an 8 M urea polyacrylamide gel. The RNA was visualized by UV-shadow paper and appropriately sized bands were excised and RNA extracted into a 0.25 M NaOAc (pH 6.0), 1 mM EDTA and phenol solution. After phenol–chloroform extraction, the RNA was NaOAc–ethanol precipitated, air-dried and finally dissolved in 20 µl ddH2O. The RNA concentration was determined by measuring absorbance at 260 nm.
For preparation of internally labelled RNA, T7 templates were transcribed using T7 Megascript (Ambion) including ∼15 µCi [α-32P]UTP (Amersham, 800 Ci/mmol) per nmol of UTP. RNA was purified as described above and the concentration was determined by scintillation counting. Integrity and purity of labelled and cold RNAs were analyzed on 7 M urea denaturing 6–8% polyacrylamide gel visualized by autoradiography and ethidium bromide, respectively.
In vitro RNA dimerization, gel electrophoresis and analysis
Labelled RNA was used at a fixed concentration of 2 nM and titrated with increasing amounts of non-labelled RNA (from 1 to 100 nM). The dimerization reactions were mixed in thin-walled PCR tubes on ice, and subsequently heated for 2 min at 95°C and chilled on ice for 2 min. After addition of 2 ml 5× dimerization buffer (10 mM Tris–HCl pH 7.5, 40 mM NaCl, 5 mM MgCl2 final concentration), the reactions were incubated for 30 min at 45°C. Following incubation the reactions were stopped on ice and 2 µl of a 6× loading buffer (80% glycerol, 0.025% bromophenolblue, 1 mM EDTA) was added. The reactions were loaded on 2.5% agarose gels with 1× TBE running buffer. Gels were run at 7 V/cm at 4°C for ∼4 h. After electrophoresis the gels were dried in a Bio-Rad gel vacuum drier at 60°C. RNA monomers and dimers were visualized by phosphoimaging (Bio-Rad Personnel FX, Kodak phosphoimager storage screens). Bio-Rad software, Quantity One, was used for analysis and quantification.
RESULTS
Site-specific recombination between co-packaged vector RNAs
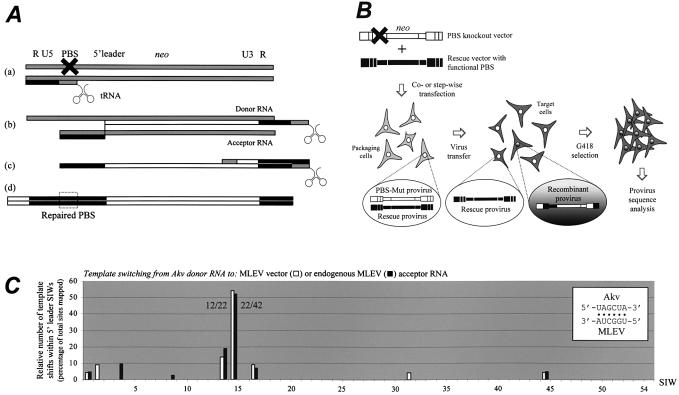
We have previously shown that PBS-defective Akv-MLV vectors packaged in murine packaging cells are repaired through recombination with MLEV, an endogenous MLV-like retroviral sequence (31) that shares 78% sequence identity with Akv within the 5′ leader region (Fig. 1B). Co-packaging of vector RNA with MLEV RNA allows a glutamine tRNA annealed to the PBS of MLEV to initiate minus-strand DNA synthesis, strong-stop DNA is subsequently transferred intermolecularly and cDNA synthesis is continued through the selective marker gene (Fig. 2A, steps a and b). To obtain perfect PBS complementarity during second strand transfer, the proceeding reverse transcriptase must switch from the vector RNA template to the MLEV template within a 5′ leader recombination window flanked upstream by the MLEV PBS and downstream by the neo or gag start codon (Fig. 2A, step b). The resulting provirus contains the glutamine PBS (PBS-Gln) flanked by MLEV-derived R-U5 and leader sequences and a chimeric 3′-LTR with MLEV R and U5 regions (Fig. 2A, step d) (32). Genetic marker differences (leader markers, LMs) dispersed throughout the Akv and MLEV packaging regions (Fig. 1B) allow detailed mapping of Akv–MLEV junction sites indicative of template shifts from donor to acceptor template.
Figure 2.
Forced recombination between MLV vector RNAs. (A) Patch-repair of PBS-Mut vector by co-packaging with PBS-functional viral rescue RNA. (a) Initiation of reverse transcription from PBS of rescue RNA; (b) cDNA synthesis through marker gene of PBS-Mut RNA and template switch from PBS-Mut donor RNA to rescue acceptor RNA; (c) plus strand DNA synthesis copying PBS sequence from tRNA leading to plus strand transfer facilitated by annealing of complementary PBS sequences copied from tRNA and acceptor RNA, respectively; (d) recombinant provirus with chimeric 5′ leader (R, U5 and 5′ Ψ derived from rescue sequence, the remaining part of 5′ leader derived from PBS-Mut vector). Grey, black and white bars indicate RNA, DNA derived from PBS-functional sequence, and DNA derived from PBS knockout vector, respectively. Hatched box indicates repaired PBS. (B) Experimental design for studies of template switching between vector RNAs. Pairs of vectors (in the example shown a PBS-Mut vector with an Akv-derived 5′ leader sequence flanked downstream by neo and a rescue vector harboring PBS-Gln, MLEV leader sequence, and pac) were either step-wise transfected into Ψ2 murine packaging cells or co-transfected into human BOSC23 virus-producing cells. Viruses were transferred to NIH3T3 target cells, G418-resistant (or alternatively in experiments with a pac-containing PBS-Mut vector, puromycin-resistant) colonies were isolated and expanded, and recombinant proviruses were sequenced. (C) Distribution of junction sites within the 5′ leader recombination window. Each column represents the number of template shifts mapped within a specific SIW presented as percentage of total number of analyzed proviruses. Molecular markers LM1–LM54 (x-axis) define a total of 54 SIWs intervening individual marker differences (further details in Fig. 1B). White and black columns represent patterns of template switching from vector RNA (PBSMut) to vector RNA (MLEVleader-containing ‘rescue’ vector) and from vector RNA (PBSMut) to endogenous viral RNA (MLEV), respectively. RNAs were stably expressed in Ψ2 packaging cells. Data represented by black columns include data previously presented (28).
We set out to establish an assay system based on the interaction and template switching between (i) vector RNA with a PBS-knockout mutation and (ii) rescue vector RNA harboring a functional PBS sequence, both expressed in murine or human packaging cells (Fig. 2B). This set-up would allow genetic manipulation of cis-elements in both recombination partners. First, however, we established, by step-wise transfection of vector plasmids (containing no modifications in leader sequence located between PBS and marker gene), murine ψ2 producer cell lines expressing the PBS-defective vector (PBSMutAkvΨ-neo), the PBS-functional rescue vector (MLEVleader-pac) or both. Also, vectors in which the marker genes were swapped were expressed in Ψ2 cells to test whether the marker genes had any effect on the distribution of junction sites. In both cases, viruses were transferred to murine fibroblasts, which were subsequently selected for expression of the selective marker gene contained within the PBS-mutated vector (Fig. 2B). With both vector combinations, titers of PBS-Mut vectors were found to increase slightly upon co-expression of the rescue vector; for PBSMutAkvΨ-neo we could register a 3-fold increase (from 10 to 30 c.f.u./ml) when MLEVleader-pac was present, whereas with swapped marker genes the PBSMut pac-titer was increased 6-fold (from 15 to 90 c.f.u./ml). This finding correlates with the fact that all analyzed proviruses transduced from double-transfected Ψ2s, except one that retained the PBS-Mut sequence, were recombinants (22 out of a total 23). For vector combinations PBSMutAkvΨ-neo + MLEVleader-pac and PBSMutAkvΨ-pac + MLEVleader-neo, six of nine and nine of 13 junctions sites, respectively, were mapped between LMs 13 and 15. We refer to these patches of sequence identity between LMs as SIWs. Of these events, three and 12 junction sites were located in SIW13–14 and SIW14–15, respectively (white columns, Fig. 2C).
For comparison, proviruses that were generated through viral transfer from singly transfected Ψ2 cells were sequenced to map recombination with the endogenous MLEV (black columns, Fig. 2C). For PBSMutAkvΨ-neo, two of four junction sites were mapped within SIW14–15. We have previously mapped 16 of 24 junction sites within SIW13–14 and SIW14–15 for this vector (32). For PBSMutAkvΨ-pac, nine of a total of 14 mapped junction sites were mapped to the same windows. Hence, equal frequencies of recombination within the kissing-loop region were found with RNA donor templates carrying either neo (18/28 ≈ 64%) or pac (9/14 ≈ 64%), demonstrating that the context of the marker gene does not influence the recombination pattern.
In summary, we find identical junction site distribution patterns for the two assay systems, providing further evidence that template switching between Akv and MLEV RNAs occurs site-specifically within the narrow region containing the proposed dimer-forming kissing hairpin.
Site-specific recombination between transiently co-transfected vectors
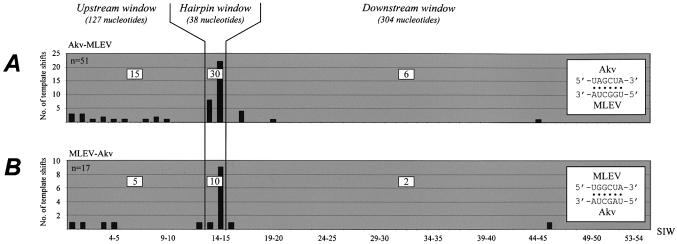
To easily analyze various combinations of recombination partners in a non-murine background we tested recombination between PBS-Mut and rescue vectors that were transiently co-transfected into human BOSC23 packaging cells. To compare distribution patterns, the 5′ leader was divided in three major windows, ‘upstream’, ‘hairpin’ and ‘downstream’ (Fig. 3). Up to a 40-fold titer increase upon co-transfection with the rescue vector (data not shown) indicated a high level of rescue-vector-dependent transduction, confirmed by the finding that all transduced proviruses were recombinants harboring a repaired PBS. We believe this prominent level of vector rescue to be a result of a favorable ratio between PBS-Mut and rescue vectors established by efficient co-transfection in the 293-derived packaging cells. For the vector pairs PBSMutAkvΨ-neo + MLEVleader-pac and PBSMutAkvΨ-pac + MLEVleader-neo a total of 20 and 31 recombinants, respectively, were analyzed by sequencing of the 5′ leader. In the majority of the analyzed clones (30 out of 51), junction sites were located within the 38-nt kissing-loop-containing ‘hairpin’ window between LM13 and LM15 (Fig. 3A). In support of this finding we examined recombinants that were generated by template switching from MLEV donor RNA to Akv acceptor leader RNA. To do so, PBS-Mut was introduced into an MLEVleader-containing pac-vector generating PBSMutMLEVleader-pac. RNA from this vector was allowed to co-package with RNA derived from PBSProAkvΨ-neo leading to the generation of PBSPro-containing recombinant proviruses integrated in the transduced NIH 3T3 cells. Again, recombination sites clustered within KH2; 10 of 17 events of template switching were mapped between LM13 and LM15 (Fig. 3B). We conclude that recombination occurs site-specifically within KH2 between Akv and MLEV donor and acceptor RNA templates without preference for a specific donor–acceptor combination. The remaining experiments were carried out, therefore, by transient co-transfections in BOSC23 cells.
Figure 3.
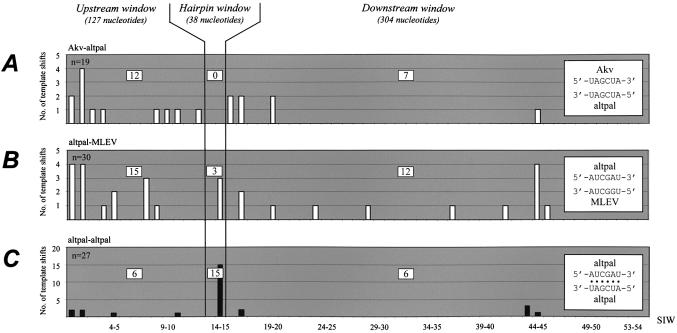
Site-specific template switching between pairs of vectors harboring wild-type Akv and MLEV palindromic loop motifs. Panels illustrate the junction site distribution observed for each vector pair transiently co-transfected into BOSC23 cells. (A) The vector pairs PBSMutAkvΨ-neo + MLEVleader-pac or PBSMutAkvΨ-pac + MLEVleader-neo were transfected, allowing template switching during reverse transcription from an Akv donor to a MLEV acceptor RNA template (according to model, Fig. 2A). (B) The donor–acceptor template relationship was inversed by co-transfecting PBSMutMLEVleader-pac and PBSProAkvΨ-neo containing MLEV and Akv 5′ leaders, respectively. The predicted base pairing between interacting hairpin loops is indicated by black dots in diagrams inset at the right of each panel. Columns represent the number of template shifts mapped within specific SIWs; the total number of analyzed proviruses (n) is indicated in the upper left corner of each panel. Molecular markers LM1–LM54 are evenly distributed at the x-axis despite differences in length of the SIWs intervening individual marker differences. To classify each mapped junction site and compare distribution patterns, the 5′ leader was divided into three major windows, ‘upstream’, ‘hairpin’ and ‘downstream’, as indicated. The number of recombination sites mapped within each major window is listed in open squares.
Non-wild-type palindromic loops support site-specific template switching
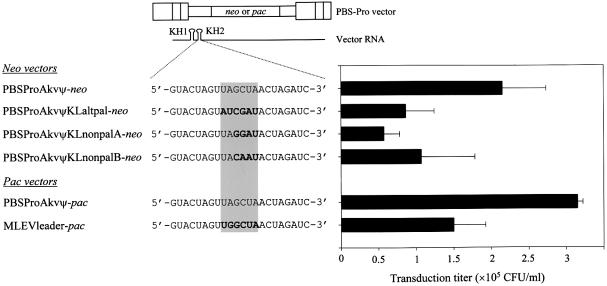
To investigate whether putative base pairing between non-wild-type palindromic loop motifs can support site-specific template switching, the wild-type palindromic loop sequence, 5′-UAGCUA-3′, was replaced by the inverted palindromic sequence 5′-AUCGAU-3′ with expected functional properties as in the wild-type. Titer assays showed a slight reduction in transfer efficiency of PBSProAkvΨKLaltpal-neo compared to the wild-type vector (Fig. 4). Three vector combinations were tested by transient co-expression in BOSC cells; in combinations (i) PBSMutAkvΨ-neo + MLEVleaderKLaltpal-pac and (ii) PBSMutAkvΨKLaltpal-neo + MLEVleader-pac, there was no complementarity or similarity between loop motifs, whereas in (iii) PBSMutAkvΨKLaltpal-neo + MLEVleaderKLaltpal-pac both loop–loop complementarity and identity was maintained by compensatory modifications. For each vector pair 5′ leader recombination sites were mapped. As depicted in Figure 5A and B, switching of template between RNAs containing the alternative palindrome and the wild-type loop motif (derived from Akv or MLEV) did not occur site-specifically. None of 19 or three of 30 junction sites, respectively, were mapped within markers LM13 and LM15, and for both vector combinations the remaining junction sites were dispersed throughout the ‘upstream’ and ‘downstream’ recombination windows. In contrast, when both interacting RNAs contained the KLaltpal sequence, thereby regenerating perfect 6/6 loop–loop complementarity, site-specific template switching within KH2 (15 of 27 identified junction sites) was restored, generating an overall site distribution similar to the ‘wild-type’ distribution of sites (P > 0.75) (Fig. 5C). These findings demonstrate that the recombination site-specificity can be modulated by genetically altering sites of intermolecular linkage but, in addition, that the lack of putative loop–loop base pairing does not exclude RNA co-packaging and subsequent recombination.
Figure 4.
Transduction efficiencies of vectors harboring modified kissing hairpin loop sequences. Three vector constructions with altered loop sequences in KH2 were generated from PBSProAkvΨ-neo. MLEVleader-pac was generated from and compared to PBSProAkvΨ-pac. KH1 was not modified. Bold letters indicate nucleotide substitutions within loop (light grey box). Transduction titers were measured by counting the G418- or puromycin-resistant colonies appearing per millilitre of virus-containing supernatant transferred from transiently transfected BOSC23 packaging cells to NIH 3T3 cells. Titer values are based on at least three individual experiments. Standard deviations are indicated by error bars.
Figure 5.
Site-specific template switching is restored between pairs of vectors harboring non-wild-type palindromic loop motifs. Panels illustrate the junction site distribution observed for each vector pair transiently co-transfected into BOSC23 cells. For vector pairs (A) PBSMutAkvΨ-neo + MLEVleaderKLaltpal-pac and (B) and PBSMutAkvΨKLaltpal-neo + MLEVleader-pac there was no complementarity, putative base pairing (see inset diagrams on right) or sequence similarity between loop motifs, whereas the loop–loop complementarity and identity was maintained by compensatory modifications in the vector pair PBSMutAkvΨKLaltpal-neo + MLEVleaderKLaltpal-pac (C). Loop–loop base pairs possibly generated between interacting hairpin loops are indicated by black dots in diagrams inset at the right of each panel; missing dots indicate that conventional loop–loop base pairing cannot be predicted. Columns represent the number of template shifts mapped within specific SIWs (white and black columns were used for vector pairs with absent and full loop–loop complementarity, respectively); the total number of analyzed proviruses (n) is indicated in the upper left corner of each panel. Molecular markers LM1–LM54 are evenly distributed at the x-axis despite differences in length of the SIWs intervening individual marker differences. The 5′ leader recombination window is divided into three major windows, ‘upstream’, ‘hairpin’ and ‘downstream’, as indicated. The number of recombination sites mapped within each major window is listed in open squares. Due to the similarity of the loop sequences, LM14 does not exist as a marker between vectors PBSMutAkvΨKLaltpal-neo and MLEVleaderKLaltpal-pac.
Palindromic loop sequences facilitate RNA heterodimer formation in vitro
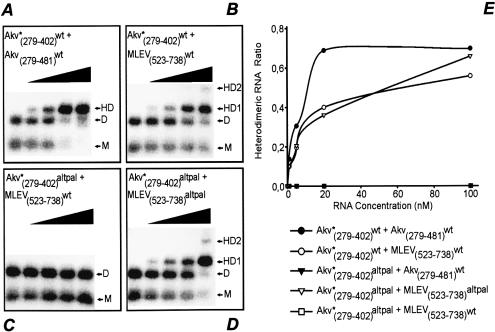
To correlate differences in patterns of template switching with dimer-forming capacity of the kissing hairpin mutants, we studied in vitro RNA heterodimerization between RNAs in vitro-transcribed from wild-type or kissing-loop-modified Akv and MLEV templates. All experiments were done by titration of a fixed amount of radioactively labelled Akv RNA (124 nt RNA encompassing kissing hairpin and core packaging sequence) with increasing amounts of unlabelled 203-nt Akv RNA or 216-nt MLEV RNA. Formation of a heterodimer complex between the shorter labelled RNA and the longer unlabelled RNA will give rise to a shift towards a heterodimer band with lower mobility than the homodimer band. It should be noted that heterodimers are formed in a competition with homodimerization of the labelled and unlabelled RNA. As expected, unmodified Akv RNAs were found to dimerize efficiently (Fig. 6A). Similarly, wild-type Akv and MLEV RNA formed heterodimers although with slightly reduced efficiency (Fig. 6B). This result could reflect the fact that the Akv and MLEV RNAs harbor several sequence differences (Fig. 1), including a difference in KH2, which predicts that loop–loop basepairing in an Akv–MLEV heterodimer involves a G:U wobble basepair. When the KLaltpal sequence was inserted into one RNA partner we could not detect any heterodimer complexes (Fig. 6C and E). However, insertion of the palindromic sequence into both Akv and MLEV RNAs resulted in efficient dimer formation (Fig. 6D), mimicking the complex formation seen for wild-type Akv and MLEV RNAs. We conclude that a kissing loop–loop interaction is crucial for in vitro dimerization between tested Akv and MLEV RNAs. In summary, our findings demonstrate dual roles of cis-elements in RNA dimerization and in vivo recombination and provide indirect evidence that restoration of optimal dimer formation between complementary loops promotes site-preferential template switching within the KH2 region.
Figure 6.
In vitro homodimer and heterodimer formation between wild-type and kissing hairpin-modified vector RNAs with palindromic loop motifs. Radioactively labelled Akv RNA (indicated by *) carrying wild-type or other loop motifs was titrated with increasing concentrations (no RNA, 1, 5, 20 and 100 nM) of non-labelled Akv- or MLEV-derived RNA, as indicated in each diagram by a black triangle. RNAs were mixed on ice, heated to 95°C, snap-cooled on ice, allowed to dimerize in dimerization buffer, and then loaded on agarose gels (see Materials and Methods for details). Each pair of RNAs is listed above the gels; Akv numbers refer to numbers in Figure 1B, whereas MLEV numbers refer to the published sequence of MLEV (32; DDBJ/EMBL/GenBank accession no. AF041383). Monomer, homodimer and heterodimer bands are indicated by M, D and HD, respectively. For some RNA combinations two heterodimer complexes (HD1 and HD2) were detected as indicated. Quantifications of band intensities are presented in (E) in which the relative amount of radioactivity present in the heterodimer bands (heterodimeric RNA ratio) is plotted as a function of titrating RNA concentration (nM). Combinations Akv*(279–402)altpal + Akv(279–481)wt (closed inverted triangles) and Akv*(279–402)altpal + MLEV(523–738)wt (open squares) did not form heterodimers at any RNA concentration, resulting in overlap of the two graphs. Data for the ‘Akv*(279–402)altpal + Akv(279–481)wt’ combination are included in graphical format only.
Loop–loop complementarity and loop primary sequence composition are crucial for kissing-loop-directed template switching
Based on the interaction between two identical alternative palindromes it is not possible to distinguish between effects from loop–loop complementarity and sequence similarity at the preferred junction site. We therefore set out to test recombination between vectors with non-palindromic loop motifs. Two PBSMutAkvΨ-neo-derived vectors harboring the non-palindromic loop motifs KLnonpalA (5′-TAGGAT-3′) and KLnonpalB (5′-TACAAT-3′) were generated. For both PBSPro counterparts of these vectors, titers were reduced 2–3-fold compared to the wild-type vector (Fig. 4). In addition, we constructed the MLEVleader-pac-derived counterparts harboring (i) loop sequences (KLnonpalA and KLnonpalB) with retained intermolecular loop similarity but no complementarity and (ii) matching loop sequences [KLmatchnonpalA (5′-ATCCTA-3′) and KLmatchnonpalB (5′-ATTGTA-3′)] which restore complementarity but have no similarity to the non-palindromic loop motifs. These vector constructs were tested in appropriate combinations for in vivo template switching and in vitro RNA dimerization.
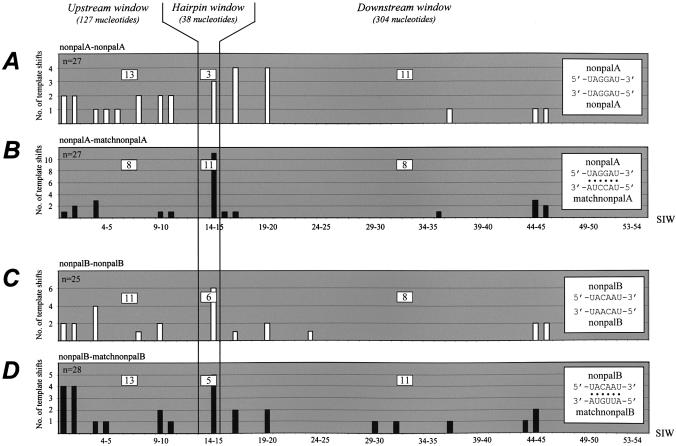
For the combinations PBSMutAkvΨKLnonpalA-neo + MLEVleaderKLnonpalA-pac (Fig. 7A) and PBSMutAkvΨ KLnonpalA-neo + MLEVleaderKLmatchnonpalA-pac (Fig. 7B), we observed a clear difference in the pattern of recombination. Three of 27 (∼11%) and 11 of 27 (∼41%) junction sites, respectively, were mapped within the ‘hairpin’ window. These patterns differ statistically from each other (P < 0.015) and, moreover, are distinct (in the case of KLnonpalA–KLnonpalA) (P < 0.0001) or comparable (in the case of KLnonpalA–KLmatchnonpalA) (P > 0.12) to the patterns observed for pairs with the wild-type or the alternative palindromic loop motif. We conclude that loop–loop complementarity rather than similarity in the context of the KLnonpalA base composition is crucial for kissing-loop-mediated recombination, suggesting that possible intermolecular recognition at the recombination site raises the site preference well above background levels.
Figure 7.
Distribution of recombination sites between vector RNAs containing complementary or non-complementary non-palindromic kissing hairpin loop sequences. Panels illustrate the junction site distribution observed for each vector pair transiently co-transfected into BOSC23 cells. For vector pairs (A) PBSMutAkvΨKLnonpalA-neo + MLEVleaderKLnonpalA-pac and (C) PBSMutAkvΨKLnonpalB-neo + MLEVleaderKLnonpalB-pac intermolecular loop similarity but no complementarity was retained (white columns), whereas there was full loop–loop complementarity but no similarity between non- palindromic loop motifs in the vector pairs (B) PBSMutAkvΨKLnonpalA-neo + MLEVleaderKLmatchnonpalA-pac and (D) PBSMutAkvΨKLnonpalB-neo + MLEVleaderKLmatchnonpalB-pac (black columns). Predicted loop–loop base pairings are indicated by black dots in diagrams inset at the right of each panel; missing dots indicate that conventional loop–loop base pairing cannot be predicted. Columns represent the number of template shifts mapped within specific SIWs; the total number of analyzed proviruses (n) is indicated in the upper left corner of each panel. The number of recombination sites mapped within the three major windows, ‘upstream’, ‘hairpin’ and ‘downstream’, is listed in open squares.
For the combinations PBSMutAkvΨKLnonpalB-neo + MLEVleaderKLnonpalB-pac (Fig. 7C) and PBSMutAkvΨ KLnonpalB-neo + MLEVleaderKLmatchnonpalB-pac (Fig. 7D) a total of 25 and 28 recombinants, respectively, were obtained. The junction site distribution patterns for the two vector combinations were statistically similar (P > 0.58) but showed no specific preference for the kissing-loop region and were significantly different from the distribution seen for the wild-type vector pair (P < 0.005). These data suggest that rescue of the KLnonpalB vector occurred independently of the kissing-loop sequence, possibly due to a weak interaction between the loop motifs (see below).
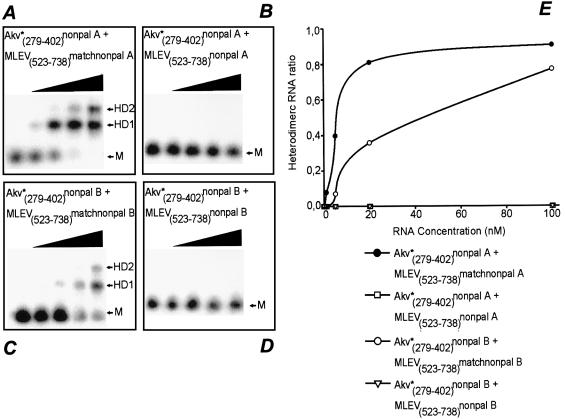
To correlate these findings with possible differences in RNA dimer formation, we again used in vitro heterodimer complex formation as a measure for loop–loop interaction and dimerization. As shown in Figure 8A–E, KLnonpalA and B in fact differed in their ability to support dimer formation. For both sequences, neither homodimers nor heterodimers could be detected when KLnonpal loop motifs were matched in Akv and MLEV RNAs (Fig. 8B and D). For the KLnonpalA–KLmatchnonpalA combination efficient dimer formation was observed even for the lower RNA concentrations (Fig. 8A) (the concentration of unlabelled RNA at which 50% of labelled RNA is bound in heterodimeric complexes, H50% = 7 nM). This level was similar to the wild-type level, although the two experiments are not directly comparable as the competition for RNA homodimerization is significantly reduced when using constructs carrying non-palindromic sequences. In contrast, heterodimeric complexes were obtained only with the highest concentrations of unlabelled RNA when KLnonpalB- and KlmatchnonpalB-containing RNAs were combined in vitro (Fig. 8C) (H50% = 50 nM). These results together demonstrate a parallel influence of the mutations on the efficiency of loop–loop base pairing between non-palindromic loop motifs and on the KH2 preference for template switching in vivo, suggesting that intermolecular loop–loop complementarity and interaction is a driving force for retroviral recombination.
Figure 8.
In vitro homodimer and heterodimer formation between wild-type and kissing hairpin-modified vector RNAs with non-palindromic loop motifs. Radioactively labelled Akv RNA (indicated by *) carrying non-palindromic loop motifs was titrated with increasing concentrations (no RNA, 1, 5, 20 and 100 nM) of non-labelled Akv- or MLEV-derived RNA, as indicated in each diagram by a black triangle and described in the legend to Figure 6. Each pair of RNAs is listed above the gels; Akv numbers refer to numbers in Figure 1B, whereas MLEV numbers refer to the published sequence of MLEV (32; DDBJ/EMBL/GenBank accession no. AF041383). Monomer and heterodimer bands are indicated by M and HD, respectively. For RNA combinations with matching hairpins two heterodimer complexes (HD1 and HD2) were detected as indicated. Quantifications of band intensities are presented in (E) in which the relative amount of radioactivity present in the heterodimer bands (heterodimeric RNA ratio) is plotted as a function of titrating RNA concentration (nM). Combinations Akv*(279–402)nonpalA + MLEV(523–738)nonpalA and Akv*(279–402)nonpalB + MLEV(523–738)nonpalB did not form heterodimers at any RNA concentration, resulting in overlap of the two graphs.
DISCUSSION
Our earlier work on 5′ leader recombination between an exogenous Akv-MLV-based vector and an endogenous virus (MLEV) led to the hypothesis that loop-complementarity of the kissing hairpin (KH2) causes a recombinational preference at this site (29,31). To test this hypothesis, we developed an assay for forced minus-strand recombination in the 5′ leader of MLV based upon co-expression in human packaging cells and viral co-packaging of two retroviral vectors harboring the dispersed genetic marker differences of the Akv and MLEV 5′ leaders. In summary, our results show that mutations in the 6-nt KH2 loop abolish the recombination site preference for KH2 but that site preference can be restored between (i) vectors harboring the same alternative palindrome (AUCGAU as opposed to the Akv wt palindrome UAGCUA) and (ii) between a pair of vectors with complementary non-palindromic sequences of the KH2 loop (UAGGAU and AUCCUA). By analyzing recombination between pairs of vectors with non-palindromic loop motifs, we could differentiate between roles played by loop–loop complementarity and sequence similarity of the recombination window. The nonpalA vector (AUGGAU loop) showed higher KH2 recombination-preference with its matching partner matchnonpalA (AUCCUA loop) than with its partner with KH2 identity (AUGGAU loop), in spite of the 6 nt disruption of sequence identity in matchnonpalA. Although this finding strongly indicates that loop–loop complementarity can facilitate KH2-directed strand transfer, pairing of complementary non-palindromic nonpalB–matchnonpalB sequences did not restore KH2-specific template switching, suggesting that the actual nucleotide sequence along with complementarity determines the loop–loop-directed RNA linkage.
By quantitative studies of dimerization of the same 5′ leader RNAs in vitro, we confirmed the existence of a KH2 sequence-dependent variation in RNA dimerization. Importantly, our data reveal a direct correlation between efficient RNA dimer formation and in vivo site-preference for recombination at the dimer linkage site. Hence, pairs of palindromic and non-palindromic loop sequences that supported site-directed recombination also facilitated efficient in vitro RNA dimerization, whereas pairs of vectors that were found to recombine at randomly distributed sites in the 5′ leader did not easily form heterodimers. Moreover, the preference for alternative recombination sites (like SIW1–2 and SIW44–45) was to some extent unaffected by loop modifications, indicating that template switching around the kissing hairpin was exclusively affected by reduced or restored loop–loop complementarity. We conclude that hairpin-directed template switching of the reverse transcriptase during viral DNA synthesis depends on loop–loop base pairing at the KH2 site.
Based upon our in vitro studies we may speculate that a reduced interaction between nonpalB and matchnonpalB loops may, in vivo, result in a less stable heterodimer, perhaps due to a deficiency in formation of the extended duplex in the mature RNA dimer. It is a well-known phenomenon, based on studies of MLV, HaSV and HIV-1 and -2 in vitro RNA dimerization, that KH complementarity is crucial but not in itself adequate for dimerization of RNAs (12,18,19,21, 22,25,40). Such variations between palindromes may reflect differences in loop–loop binding and RNA duplex formation or, alternatively, formation of RNA secondary structures that do not support dimer formation.
Our data provide examples that intermolecular association rather than sequence similarity may play a crucial role for kissing hairpin-directed strand transfer. When RT advances along the highly structured leader RNA template it encounters secondary or tertiary structures that temporarily stall continued polymerization (7–9,41). Adding to the important mutual identity of the interacting RNA templates (1), such pause-inducing structures (8,9,11), points of RNA breakage, opening and interaction of stem–loops during reverse transcription (42) and acceptor RNA structures facilitating displacement of donor by acceptor RNA (10,43) have been proposed to induce template switching. Hairpin-dependent strand transfer has been proposed to involve an ‘interactive hairpin mechanism’ in which an increase in the local concentration of acceptor RNA is facilitated by the invasion of the displaced donor strand on the acceptor stem sequences (42). This proposed annealing of donor and acceptor hairpin stems closely resembles the annealing of donor and acceptor strands at the dimer linkage site, the latter generated independently of a prior invasion of the acceptor strand. According to this model, the donor–acceptor duplex is unwound by the growing cDNA and strand transfer is mediated by the annealing of cDNA and the displaced acceptor RNA. Recently, a so-called ‘dock and lock’ mechanism was suggested for pause-induced template switching (10). This model, supported by in vitro studies, suggests that enhanced RNaseH-mediated donor RNA degradation 3′ of the pause site facilitates annealing between acceptor RNA and cDNA. During this process, reverse transcription continues on the donor template until transfer of the nascent strand is induced by yet another nearby pause site leading to template switching upstream from the initial pause site. Although it remains unclear from the model how the 3′-end of the cDNA invades a stable hairpin on the acceptor RNA, this model could explain why an interaction between kissing hairpins leads to frequent template switching at both sides of the loop marker (LM14) with a preference for SIW14–15 (Fig. 2C). A similar model has been proposed from in vitro studies of recombination between HIV-1 templates that interact through kissing hairpins (44). In this case, KH-based template proximity was believed to allow the acceptor template to invade the donor–cDNA complex and promote subsequent strand transfer. This notion is supported by in vitro studies by Andersen and co-workers, suggesting that kissing hairpin-directed dimer formation and derived template proximity induce efficient HIV-1 template switching, although the site-distribution within the HIV-1 leader does not appear to be clustered around the kissing hairpin (45).
Our work provides indirect in vivo evidence that a specific loop–loop interaction between complementary loop motifs of KH2 is maintained within the mature RNA dimer. This conclusion adds to an increasing controversy about the biological importance of the kissing hairpins since disruption of the potential intermolecular loop interactions at KH2 abrogates neither MLV vector co-packaging (this study) nor full-length virus replication (30). Likewise, the lack of complementarity between dimer initiation sites in HIV-1 and -2 seems to explain the lack of in vitro heterodimerization of HIV-1 and -2 RNA (46), although alternative sites may support dimer formation (47). Alternative linkage sites have been defined within the MLV 5′ leader region, including an upstream kissing stem–loop containing a 10-nt palindrome (25) (located in Akv at positions 209–218 between LM3 and LM5, Fig. 1) and two GACG-loop-containing stems (48) (Akv positions 329–404, Fig. 1), one of which has been shown to possess intermolecular ‘kissing’ activity (28). However, in our experimental setting neither of these potential linkage sites induced template switching. Even when putative base pairing within the central kissing loop was disrupted, the junction sites were randomly distributed and no second-site ‘hotspots’ appeared. These results might indicate (i) that putative linkage sites flanking the central kissing loop are not maintained within the mature dimer, (ii) that these linkages are less stable and therefore fall apart more easily during reverse transcription, (iii) that the local structural context or sequence similarity does not allow frequent template switching or, alternatively, (iv) that Akv and MLEV 5′ leader RNAs do not heterodimerize through these signals. Partly in favor of the latter possibility, we cannot exclude that potential basepairing between the upper 10-nt palindromes of vector RNA is disturbed by an internal Akv–MLEV marker difference (LM4). Notably, in vitro RNA heterodimer formation (with RNAs that do not contain the upper kissing hairpin) depends exclusively on the association between loops, whereas co-packaging and possibly heterodimer formation occur in vivo even without matching kissing loop motifs. This discrepancy is most likely explained by the presence of alternative dimer-forming cis-elements located outside the core packaging and dimerization region.
In summary, we propose that a stable intermolecular interaction directed by complementary loop motifs induces recombination through low RT processivity, or RT pausing, combined with increased template proximity and increased acceptor accessibility caused by ‘opening’ of the dimer linkage structure at the preferred transfer site.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Jane Hørlev for excellent technical assistance. This work was supported by the Danish Research Agency, the Bavarian Nordic Research Institute, the Danish Cancer Society and the Karen Elise Jensen Foundation.
REFERENCES
- 1.Hu W.-S. and Temin,H.M. (1990) Retroviral recombination and reverse transcription. Science, 250, 1227–1233. [DOI] [PubMed] [Google Scholar]
- 2.Fu W. and Rein,A. (1993) Maturation of dimeric viral RNA of Moloney murine leukemia virus. J. Virol., 67, 5443–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu W., Gorelick,R.J. and Rein,A. (1994) Characterization of human immunodeficiency virus type 1 dimeric RNA from wildtype and protease-defective virions. J. Virol., 68, 5013–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murti K.G., Bondurant,M. and Tereba,A. (1981) Secondary structural features in the 70S RNAs of Moloney murine leukemia virus and Rous sarcoma viruses as observed by electron microscopy. J. Virol., 37, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkelsen J.G. and Pedersen,F.S. (2000) Genetic reassortment and patch repair by recombination in retroviruses. J. Biomed. Sci., 7, 77–99. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan M., Fay,P.J. and Bambara,R.A. (2001) The kissing hairpin sequence promotes recombination within the HIV 5′ leader region. J. Biol. Chem., 276, 36482–36492. [DOI] [PubMed] [Google Scholar]
- 7.Buiser R.G., Bambara,R.A. and Fay,P.J. (1993) Pausing by retroviral DNA polymerases promotes strand transfer from internal regions of RNA donor templates to homopolymeric acceptor templates. Biochim. Biophys. Acta, 1216, 20–30. [DOI] [PubMed] [Google Scholar]
- 8.DeStefano J.J., Buiser,R.G., Mallaber,L.M., Fay,P.J. and Bambara,R.A. (1992) Parameters that influence synthesis and site-specific termination by human immunodeficiency virus reverse transcriptase on RNA and DNA templates. Biochim. Biophys. Acta, 1131, 270–280. [DOI] [PubMed] [Google Scholar]
- 9.Klasens B.I.F., Huthoff,H.T., Das,A.T., Jeeninga,R.E. and Berkhout,B. (1999) The effect of template RNA structure on elongation by HIV-1 reverse transcriptase. Biochim. Biophys. Acta, 1444, 355–370. [DOI] [PubMed] [Google Scholar]
- 10.Roda R.H, Balakrishnan,M., Kim,J.K., Roques,B.P., Fay,P.J. and Bambara,R.A. (2002) Strand transfer occurs in retroviruses by a pause-initiated two-step mechanism. J. Biol. Chem., 277, 46900–46911. [DOI] [PubMed] [Google Scholar]
- 11.Wu W., Blumberg,B.M., Fay,P.J. and Bambara,R.A. (1995). Strand transfer mediated by human immunodeficiency virus reverse transcriptase in vitro is promoted by pausing and results in misincorporation. J. Biol. Chem., 270, 325–332. [DOI] [PubMed] [Google Scholar]
- 12.Clever J.L., Wong,M.L. and Parslow,T.G. (1996) Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J. Virol., 70, 5902–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirac A.M., Huthoff,H., Kjems,J. and Berkhout,B. (2001). The dimer initiation site hairpin mediates dimerization of the human immunodeficiency virus type 2 RNA genome. J. Biol. Chem., 276, 32345–32352. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y.X., Fu,W., Winter,A.J., Levin,J.G. and Rein,A. (1995) Multiple regions of Harvey sarcoma virus RNA can dimerize in vitro. J. Virol., 69, 2486–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard P.-M., Bonnet-Mathonière,B., Muriaux,D. and Paoletti,J. (1995) A short autocomplementary sequence in the 5′ leader region is responsible for dimerization of MoMuLV genomic RNA. Biochemistry, 34, 9785–9794. [DOI] [PubMed] [Google Scholar]
- 16.Laughrea M. and Jetté,L. (1996) Kissing-loop model of HIV-1 genome dimerization: HIV-1 RNAs can assume alternative dimeric forms and all sequences upstream and downstream of hairpin 248–271 are dispensable for dimer formation. Biochemistry, 35, 1589–1598. [DOI] [PubMed] [Google Scholar]
- 17.Mujeeb A., Clever,J.L., Billeci,T.M., James,T.L. and Parslow,T.G. (1998) Structure of the dimer initiation complex of HIV-1 genomic RNA. Nat. Struct. Biol., 5, 432–436. [DOI] [PubMed] [Google Scholar]
- 18.Paillart J.-C., Skripkin,E., Ehresmann,B., Ehresmann,C. and Marquet,R. (1996) A loop-loop ‘kissing’ complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc. Natl Acad. Sci. USA, 93, 5572–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen S.V., Mikkelsen,J.G. and Pedersen,F.S. (2002) Modulation of homo- and heterodimerization of Harvey sarcoma virus RNA by GACG tetraloops and point mutations in palindromic sequences. J. Mol. Biol., 323, 613–628. [DOI] [PubMed] [Google Scholar]
- 20.Skripkin E., Paillart,J.-C., Marquet,R., Ehresmann,B. and Ehresmann,C. (1994) Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc. Natl Acad. Sci. USA, 91, 4945–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laughrea M., Shen,N., Jetté,L. and Wainberg,M.A. (1999) Variant effects of non-native kissing-loop hairpin palindromes on HIV replication and HIV RNA dimerization: role of stem-loop B in HIV replication and HIV RNA dimerization. Biochemistry, 38, 226–234. [DOI] [PubMed] [Google Scholar]
- 22.Paillart J.-C., Westhof,E., Ehresmann,C., Ehresmann,B. and Marquet,R. (1997) Non-canonical interactions in a kissing-loop complex: the dimerization initiation site of HIV-1 genomic RNA. J. Mol. Biol., 270, 36–49. [DOI] [PubMed] [Google Scholar]
- 23.Girard P.-M., de Rocquigny,H., Roques,B.-P. and Paoletti,J. (1996) A model of PSI dimerization: destabilization of the C278-G303 stem–loop by the nucleocapsid protein (NCp10) of MoMuLV. Biochemistry, 35, 8705–8714. [DOI] [PubMed] [Google Scholar]
- 24.Ly H. and Parslow,T.G. (2002) Bipartite signal for genomic RNA dimerization in Moloney murine leukaemia virus. J. Virol., 76, 3135–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oroudjev E.M., Kang,P.C. and Kohlstaedt,L.A. (1999) An additional dimer linkage structure in Moloney murine leukemia virus RNA. J. Mol. Biol., 291, 603–613. [DOI] [PubMed] [Google Scholar]
- 26.Fisher J. and Goff,S.P. (1998) Mutational analysis of stem–loops in the RNA packaging signal of the Moloney murine leukemia virus. Virology, 244, 133–145. [DOI] [PubMed] [Google Scholar]
- 27.Mougel M., Zhang,Y. and Barklis,E. (1996) Cis-active structural motifs involved in specific encapsidation of Moloney murine leukemia virus RNA. J. Virol., 70, 5043–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim C.-H. and Tinoco,I. (2000) A retroviral RNA kissing complex containing only two G-C base pairs. Proc. Natl Acad. Sci. USA, 97, 9396–9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikkelsen J.G., Lund,A.H., Duch,M. and Pedersen,F.S. (2000) Mutations of the kissing-loop dimerization sequence influence the site specificity of murine leukemia virus recombination in vivo. J. Virol., 74, 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aagaard L., Rasmussen,S., Mikkelsen, J.G. and Pedersen, F.S. (2004) Efficient replication of full-length murine leukemia viruses modified at the dimer initiation site regions. Virology, in press. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen J.G., Lund,A.H., Kristensen,K.D., Duch,M., Sørensen,M.S., Jørgensen,P. and Pedersen,F.S. (1996) A preferred region for recombinational patch repair in the 5′ untranslated region of primer binding site-impaired murine leukemia virus vectors. J. Virol., 70, 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikkelsen J.G., Lund,A.H., Duch,M. and Pedersen,F.S. (1998) Recombination in the 5′ leader of murine leukemia virus is accurate and influenced by sequence identity with a strong bias toward the kissing-loop dimerization domain. J. Virol., 72, 6967–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund A.H., Mikkelsen,J.G., Schmidt,J., Duch,M. and Pedersen,F.S. (1999) The kissing-loop motif is a preferred site of 5′ leader recombination during replication of SL3-3 murine leukemia viruses in mice. J. Virol., 73, 9614–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Beveren C., Coffin,J.M. and Hughes,S. (1985) Nucleotide sequences complemented with functional and structural analysis. In Weiss,R., Teich,N., Varmus,H. and Coffin,J. (eds), RNA Tumor Viruses, Vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 790–805. [Google Scholar]
- 35.Mann R., Mulligan,R.C. and Baltimore,D. (1983) Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell, 33, 153–159. [DOI] [PubMed] [Google Scholar]
- 36.Pear W.S., Nolan,G.P., Scott,M.L. and Baltimore,D. (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA, 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Luna S., Soria,I., Pulido,D., Ortin,J. and Jimenez,A. (1988) Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene, 62, 121–126. [DOI] [PubMed] [Google Scholar]
- 38.Lund A.H., Duch,M., Lovmand,J., Jørgensen,P. and Pedersen,F.S. (1993) Mutated primer binding sites interacting with different tRNAs allow efficient murine leukemia virus replication. J. Virol., 67, 7125–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck E., Ludwig,G., Auerswald,E.A., Reiss,B. and Schaller,H. (1982) Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene, 19, 327–336. [DOI] [PubMed] [Google Scholar]
- 40.Paillart J.-C., Berthoux,L., Ottman,M., Darlix,J.-L., Marquet,R., Ehresmann,B. and Ehresmann,C. (1996) A dual role of the putative dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J. Virol., 70, 8348–8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison G.P., Mayo,M.S., Hunter,E. and Lever,A.M.L. (1998) Pausing of reverse transcriptase on retroviral RNA templates is influenced by secondary structures both 5′ and 3′ of the catalytic site. Nucleic Acids Res., 26, 3433–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.K., Palaniappan,C., Wu,W., Fay,P.J. and Bambara,R.A. (1997) Evidence for a unique mechanism of strand transfer from the transactivation response region of HIV-1. J. Biol. Chem., 272, 16769–16777. [DOI] [PubMed] [Google Scholar]
- 43.Negroni M. and Buc,H. (2000) Copy-choice recombination by reverse transcriptases: reshuffling of genetic markers mediated by RNA chaperones. Proc. Natl Acad. Sci. USA, 97, 6385–6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balakrishnan M., Roques,B.P., Fay,P.J. and Bambara,R.A. (2003) Template dimerization promotes an acceptor invasion-induced transfer mechanism during human immunodeficiency virus type 1 minus-strand synthesis. J. Virol., 77, 4710–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen E.S., Jeeninga,R.E., Damgaard,C.K., Berkhout,B. and Kjems,J. (2003) Dimerization and template switching in the 5′ untranslated region between various subtypes of human immunodeficiency virus type 1. J. Virol., 77, 3020–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dirac A., Huthoff,H., Kjems,J. and Berkhout,B. (2002) Requirements for RNA heterodimerization of the human immunodeficiency virus type 1 (HIV-1) and HIV-2 genomes. J. Gen. Virol., 83, 2533–2542. [DOI] [PubMed] [Google Scholar]
- 47.Berkhout B. and van Wamel,J.L. (1996) Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J. Virol., 70, 6723–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Tapia M., Metzler,V., Mougel,M., Ehresmann,B. and Ehresmann,C. (1998) Dimerization of MoMuLV genomic RNA: redefinition of the role of the palindromic stem-loop H1 (278–303) and new roles for stem-loops H2 (310–352) and H3 (355–374). Biochemistry, 37, 6077–6085. [DOI] [PubMed] [Google Scholar]