Abstract
Over 51,000 individuals are diagnosed with a primary brain tumor in the United States each year, and for those with the most common type of malignant tumor, an astrocytoma, almost 75% will die within five years of diagnosis. While surgery, radiation, and chemotherapy have improved length of survival, mortality remains high, which underscores the need to understand how other factors affect the disease trajectory. Several recent studies have shown that depressive symptoms are independently associated with reduced quality of life and survival time after controlling for medial variables in patients with an astrocytoma. Thus, depressive symptoms represent a significant risk factor for adverse outcomes in this patient population.
A growing body of evidence indicates that depressive symptoms are linked to underlying biological phenomena, particularly inflammatory activation modulated through increased peripheral levels of proinflammatory cytokines. Recent research has shown that neoplastic astrocytes respond to elevated proinflammatory cytokine levels by secreting immune mediators within the central nervous system, including cytokines and glial fibrillary acidic protein (GFAP) that promote astrogliosis and angiogenesis, and may increase tumor growth and metastasis. However, because these biological factors have not as yet been measured in conjunction with depressive symptoms in these patients, little is known about the interactions that potentially influence the treatment trajectory.
In order to guide future research and provide a deeper understanding of the factors that may influence depressive symptoms and length of survival in patients with an astrocytoma, a review of the literature was undertaken. Publications over the past ten years were analyzed to examine the theoretical models and measures of depressive symptoms used in previous research. While numerous studies have documented the relationship between depression and reduced length of survival, there were several methodological concerns identified and there were no studies which included biological variables. Yet, research in the basic sciences provides compelling evidence of specific neuroendocrine-immune interactions orchestrated by astrocytes that can cause depressive symptoms and alter the tumor microenvironment so that standard treatments are not as effective. These findings support the need for clinically-based research so that we can begin to understand the potentially modifiable biobehavioral mechanisms underlying depressive symptoms in patients with an astrocytoma. Grounded in the biobehavioral research paradigm of psychoneuroimmunology, a novel research program is presented that may provide a new level of understanding regarding the high prevalence of depressive symptoms in patients with an astrocytoma and lead to new treatment strategies, with possible implications for improved symptom management and quality of life in patients with brain tumors.
Astrocytomas are a complex group of low- and high-grade tumors that comprise more than half of all primary brain tumors (Bondy et al., 2008). They are the most frequent cerebral tumor, with an incidence estimated at 1/12,500. Most patients diagnosed with an astrocytoma, regardless of the tumor grade, will not live beyond five years (Lin et al., 2002). Affecting primarily middle-age adults, astrocytomas disrupt the lives of thousands of individuals and families each year as neurocognitive and functional status often deteriorate rapidly over the ensuing months. Besides the grade of astrocytoma, patient age and functional status remain the known preoperative prognostic indicators of survival (McCarter et al., 2006; Lutterbach, Sauerbrei, & Guttenberg, 2003). However, several recent studies have found that preoperative depressive symptoms are predictive of shorter survival time specifically among patients with an astrocytoma as opposed to other types of brain tumors (Gathinji et al., 2008; Litofsky et al., 2004; Mainio et al., 2006). Depressive symptoms have long been regarded as part of the normal emotional response to cancer diagnosis, or a side-effect of treatment (Gross, Smith, & Stern, 2007). Intriguing recent evidence, however, suggests that a biological pathway involving immune mediators may not only cause or worsen depressive symptoms, but may increase tumor treatment resistance (Litofsky & Resnick, 2009; Miller & Raison, 2008; Samaras et al., 2007). While the grade of astrocytoma portends the disease trajectory and modalities of treatment, the proposed mechanisms underlying depressive symptoms may be present across grades due to the central role of astrocytes in regulating immune interactions within the central nervous system.
Epidemiological Overview of Astrocytoma
Astrocytomas represent 60% of all primary malignant brain tumors, and they are diagnosed in more than 24,000 people each year (American Cancer Society, 2007). Despite aggressive treatment, malignant astrocytomas cause more than 14,000 deaths annually. Astrocytomas are classified by tumor grade using the World Health Organization (WHO) system, which provides a prediction of their growth rate and ability to spread (Jemal et al., 2007). WHO grade I pilocytic astrocytomas and grade II diffuse astrocytomas are considered low-grade, while the highly-malignant forms, grade III anaplastic astrocytomas and grade IV glioblastoma multiforme (GBM), are high-grade. The annual incidence of astrocytoma in the United States is 4 out of every 100,000 people, with a male to female ratio of 3:2 (Central Brain Tumor Registry of the United States, 2008). Low-grade astrocytomas are more common in young adults, whereas high-grade astrocytomas primarily affect older adults. Caucasians are affected more often than any other race, and they occur more frequently in white non-Hispanics (American Cancer Society, 2007).
Pilocytic astrocytoma (WHO grade I) is a slow growing cystic tumor occurring most often in young adulthood, with only a small portion (10%) located in the cerebral hemispheres (Jemal et al., 2007). Diffuse astrocytomas (WHO grade II), which include fibrillary astrocytoma, gemistocytic astrocytoma, and protoplasmic astrocytoma, affect primarily young adults and have a tendency for malignant progression to anaplastic astrocytoma and, ultimately, GBM. These tumors develop most commonly in the cerebrum and represent 35% of all astrocytic brain tumors. Anaplastic astrocytomas (WHO grade III) are most commonly located in the cerebral hemispheres and have a tendency to progress to GBM. The mean age at biopsy is approximately 41 years. GBM (WHO grade IV) is the most frequent malignant brain tumor, accounting for 15% of all brain tumors, and 60% of all astrocytic tumors. The peak incidence occurs between the ages of 45 and 70 years, and these tumors are primarily found in the cerebral hemispheres (American Cancer Society, 2007).
Mean survival time varies by tumor grade, with the five-year survival of grade I and II being 38–45%, while grade III is 29% and grade IV is 3% (Bondy et al., 2008). The median survival of patients with low-grade astrocytomas is 5 years, and most patients die from progression of their disease to a high-grade astrocytoma. The median survival for anaplastic astrocytoma is three years from time of diagnosis. Grade IV astrocytoma, GBM, is the most common type of glioma and has a survival time varying from less than one year to three years after initial diagnosis. Patients with a GBM usually have tumor recurrence, often within a year after completion of first-line therapy (Lin et al., 2002).
Although magnetic resonance imaging (MRI) and positron emission tomography (PET) are used to provide a preliminary diagnosis, a biopsy must be performed to confirm diagnosis (Bondy et al., 2008). This typically occurs along with resection of the tumor. Tumor debulking, or resection, is the initial treatment for astrocytoma with the exception of tumors located in an area that would cause neurological devastation or when there is extensive metastasis (Claus & Black, 2006). Gross total resection has been associated with longer survival and improved neurological function (Brown et al., 2005). Surgery is followed by involved-field radiotherapy ranging from a total dose of 54 to 60 Gy, depending on tumor grade (Bondy et al., 2008). Adjuvant chemotherapy is not indicated for astrocytomas grade I–III, but may be used to treat recurrent grade III tumors. Chemotherapy is typically used for grade IV astrocytomas, with temozolomide being the currently preferred agent because it is administered orally, has a favorable side-effect profile, and is generally well tolerated by patients (Claus & Black, 2006). Unfortunately, despite advances in treatment modalities, little progress has been made in improving length of survival. Moreover, recent studies have shown that, regardless of the type and intensity of treatment, depressive symptoms are associated with shorter survival in patients with an astrocytoma (Gathinji et al., 2008; Litofsky et al., 2004; Mainio et al., 2005).
Literature Review
In order to guide future research and provide a deeper understanding of the factors that may influence depressive symptoms and length of survival in patients with an astrocytoma, a review of the literature was undertaken. The questions of interest were the following: Is there an optimal theoretical model or framework used in prior studies that may inform future biobehavioral-based research? What methods or instruments have been used to measure depressive symptoms in patients with an astrocytoma? What other factors have been associated with depressive symptoms in previous research?
The following databases were used to obtain relevant research studies that address the questions of interest: CINAHL Plus, PsychINFO, OVID, and PubMed Central. The databases were searched for publications ranging from 1999–2009 using a combination of the following keywords or MeSH terms: brain neoplasms depression, depressive disorder, cytokines, and biological factors, with the following restrictions: English and research articles.
Results of the Literature Review
From the initial search, 18 articles were identified that measured depression or depressive symptoms in patients with a brain tumor. These publications were initially perused to inspect the study design and measurement of depressive symptoms. A more thorough examination was then conducted to identify the theoretical model of the study, the methods used to measure depressive symptoms and other variables measured. The data from each article is listed in Table 1.
Table 1.
Literature Review Findings
| Authors and Year of Publication |
Study design and theoretical framework |
Factors measured | Measurement of depression |
Significant findings |
|---|---|---|---|---|
| Appleby et al. (2008) | Retrospective review of patients with primary or metastatic brain tumors. Theoretical framework not identified. | Age, gender, race, marital status, tumor type, location, and size, premorbid psychiatric diagnoses, treatment modalities | Treating physicians’ diagnosis of depression and anxiety or data in the chart that were suggestive of either disorder according to DSM-IV criteria | Depression and anxiety rates, as detected by physicians’ assessment, were similar to the 12-month prevalence rate in the general population. The most common correlate of reported depression and anxiety was a premorbid diagnosis of either disorder. |
| Armstrong et al. (2002) | Cross-sectional study of 57 patients with low-grade brain tumors following surgery. Biopsychosocial framework. | Age, gender, marital status, hand dominance, fatigue, tumor type and location, extent of surgery, cognitive function, vocational status, family support, psychological defenses, physical symptoms | Beck Depression Inventory (BDI), Multiphasic Personality Inventory-2 Depression (MPI-2D) | After controlling for fatigue, tumor location (posterior) and greater extent of surgery predicted a higher level of depression on MPI-2D. Higher BDI scores were associated with female gender, greater fatigue, more cognitive impairment and greater family support. |
| Arnold et al. (2008) | Cross-sectional study of 363 patients with a primary brain tumor. Biopsychosocial framework. | Age, gender, marital status, ethnicity, education, tumor type, grade, and site, previous psychiatric illness, anxiety, depression | Brief Patient Health Questionnaire (Brief PHQ) | Female gender, lower tumor grade, lower education level, and a history of psychiatric illness were predictors of symptoms consistent with anxiety and/or depression. |
| Brown et al. (2006) | Longitudinal study of 194 patients with high-grade gliomas. Biopsychosocial framework. | Age, gender, previous radiotherapy, tumor grade, location, laterality, extent of resection, medication, cognitive function, physical function, fatigue, depression, QOL | Profile of Mood States Short Form | QOL was positively associated with cognitive and physical function, but not demographic variables or tumor characteristics. |
| Brown et al. (2005) | Longitudinal study of 124 patients with high-grade gliomas before surgery and at 2-and 4-months post-surgery. Biopsychosocial framework. | Extent of surgery, medication, fatigue, depression, QOL | Profile of Mood States Short Form | Patients who received greater extent of surgery (gross total resection vs subtotal resection or biopsy) had higher QOL and were less likely to experience worsening depression over time. |
| D’Angelo et al. (2008) | Longitudinal study of 72 patients with a primary brain tumor before surgery and at 1, 3, 6, and 12-months post-surgery. Biopsychosocial framework. | Age, gender, tumor type, location, and laterality, anxiety, depression, cognitive impairment | Zung Self-rating Depression Scale, State-Trait Anxiety Inventory | A significant increase in the number of patients with depression was found at 1 and 3-months post-surgery. A positive relationship between trait anxiety at enrollment and depression after surgery was found. |
| Davies et al. (2003) | Cross-sectional study of 12 patients with a malignant brain tumor 24-months after surgery. Biopsychosocial framework. | Age, gender, functional status, work status, social activities, neuropsychological testing | Home-based interviews | Patients with a lower level of functioning were more likely to report symptoms of depression. |
| Gathinji et al. (2008) | Retrospective study of 1052 patients with malignant astrocytoma (WHO grade III or IV). Theoretical framework not identified. | Age, gender, marital status, tumor type, size, location, and laterality, treatment modalities | Clinical diagnosis and treatment by the primary provider or psychiatric physician | Preoperative depression was associated with reduced length of survival. The difference in percent survival between the depressed and nondepressed cohorts was particularly evident at 12 months (15% vs. 41%) and 20 months (0% vs. 21%). |
| Giovagnoli (1999) | Cross-sectional study of 57 patients with primary malignant brain tumor. Biopsychosocial framework. | Age, gender, marital status, education, cognitive functioning, emotional reactions, daily and work performance, QOL, tumor duration, type & location | Zung Self-rating Depression Scale, State-Trait Anxiety Scale | Significant predictors of QOL included level of depression and anxiety, and daily and work performance |
| Giovagnoli et al. (2005) | Cross-sectional study of 94 patients with Grades III to IV Anaplastic astrocytoma. Biopsychosocial framework. | Age, gender, marital status, education, tumor grade and location, cognition, physical performance, mood, QOL | Zung Self-rating Depression Scale, State-Trait Anxiety Inventory | QOL scores were significantly related to greater cognition, physical performance and mood. |
| Hahn et al. (2003) | Cross-sectional study of 68 patients with a primary brain tumor with no prior radiotherapy. Theoretical framework not identified. | Age, education, tumor type, lateralization, size, functional status, neuropsychological testing, depression | Beck Depression Inventory (BDI) | Patients with left hemisphere tumors reported significantly more memory problems and depressive symptoms. |
| Kilbride et al. (2007) | Longitudinal study of 51 patients with primary malignant brain tumor post-surgery, 3-weeks post-surgery, and pre-radiation treatment. Biopsychosocial framework. | Age, gender, tumor site, laterality, grade, functional status, depression, anxiety | Hospital Anxiety and Depression Scale (HADS) and unstructured interviews | HADS Underestimated the presence of depression. Patients who had suffered from depressive illness in the past were more likely to exhibit depression in the period between surgery and radiotherapy. |
| Litofsky et al. (2004) | Longitudinal study of 598 patients with high-grade glioma before surgery and 1, 3, and 6-months post-surgery. Theoretical framework not identified. | Age, gender, marital status, tumor grade, location, size, and laterality, treatment modalities | Primary physician diagnosis using DSM-IV criteria; patient report using the SF-36 Mental Health score; or a positive patient response to one of three questions related to depressive symptoms | Depressive symptoms were associated with shorter survival in patients with a glioblastoma (Grade IV) but not for those with Grade III astrocytoma. Mean survival time was significantly shorter among patients identified as depressed (34.0 weeks) compared with those who were identified as non-depressed by their physician (41.1 weeks). |
| Mainio et al. (2006) | Longitudinal study of 77 patients with Grades I–IV brain tumor before surgery, 3- and 12-months post-surgery. Biopsychosocial framework. | Age, gender, education, employment status, marital status, pre-morbid depression, tumor type, volume, and location, functional performance, premorbid depression, active depression | Pre-morbid depression measured with Crown-Crisp Experiential Index, active depression measured with Beck Depression Inventory (BDI) | After controlling for demographic variables, pre-injury depression, and tumor characteristics, level of depression predicted QOL at all treatment time intervals. |
| Mainio et al. (2005) | Longitudinal study of 77 patients with Grades I–IV brain tumor before surgery, 3- and 12-months post-surgery. Biopsychosocial framework. | Age, gender, education, employment status, marital status, pre-morbid depression, tumor type, volume, and location, functional performance, premorbid depression, active depression | Pre-morbid depression measured with Crown-Crisp Experiential Index, active, depression measured with Beck Depression Inventory (BDI) | A BDI score ≥10 was significantly related to decreased survival time in low-grade gliomas but not in high-grade gliomas. Patients with a preoperative BDI <10 or with a previous lifetime history of depression had a significantly longer survival time than those with higher preoperative depressive symptoms (60 vs. 51.7 months). |
| Pelletier et al. (2002) | Cross-sectional study among 73 patients with a primary brain tumor. Theoretical framework not identified. | Age, gender, marital status, education, tumor type, depression, fatigue, emotional distress, existential well-being, QOL | Beck Depression Inventory-II (BDI-II) | Of the sample, 38% had a BDI-II score in the clinically depressed range. The presence of depressive symptoms was the single most important independent predictor of QOL. |
| Pringle et al. (1999) | Cross-sectional study of 109 patients with an intracranial neoplasm. Theoretical framework not identified. | Age, gender, marital status, education, tumor type, location, and laterality | Hospital Anxiety and Depression Scale (HADS) | Anxiety was reported by 30% and depression by 16% of patients. Findings were not significantly different than other surgical groups. |
| Wellisch et al. (2002) | Cross-sectional study of 89 patients with PMBT post-surgery or radiation. Biopsychosocial framework. | Age, marital status, personal and family psychiatric history, tumor location, neuropsychological testing, self-reported symptoms | Major depressive disorder (MDD) diagnosed by structured psychiatric interview using DSM-IV criteria | Significant predictors of MDD included frontal location of tumor, sadness and lack of motivation symptoms, and family psychiatric history |
Theoretical models of previous research
The theoretical model most often identified in the literature review was the biopsychosocial framework (Armstrong et al., 2002; Arnold et al., 2008; Brown et al., 2006; Brown et al., 2005; D’Angelo et al., 2008; Davies et al., 2003; Giovagnoli, 1999; Giovagnoli et al., 2005; Mainio et al., 2006; Mainio et al., 2005; Wellisch et al., 2002). The remaining studies did not indicate or refer to a theoretical model when describing the study or in the discussion of the study results.
Methods used to measure depressive symptoms
Only one study included a diagnostic evaluation of depression by a neuropsychologist using MDD criteria of the Diagnostic and Statistical Manual of Mental Disorders version IV (DSM-IV). Wellisch, Kaleita, Freeman, Cloughesy, and Goldman (2002) evaluated depressive symptoms in 89 patients with a brain tumor, 70% of whom had an astrocytoma. Relevant symptoms of depression in these patients included energy loss, concentration problems, weight change, sadness, sleep disorder, motoric slowing, motivation loss/diminished interest in activities, guilt, and death ruminations. Six of the nine symptoms were reported by over 50% or more of the patients. Of the sample, 28% were diagnosed with MDD by the neuropsychologist, indicating that this patient population was more than 9 times more likely to have MDD than the general United States population.
Other studies used well established screening instruments for depression, such as the Beck Depression Inventory (Armstrong et al., 2002; Hahn et al., 2003; Mainio et al., 2006; Mainio et al., 2005; Pelletier et al., 2002), Multiphasic Personality Inventory-2 Depression (Armstrong et al., 2002), Brief Patient Health Questionnaire (Arnold et al., 2008), Hospital Anxiety and Depression Scale (Kilbride et al., 2007; Pringle et al., 1999), Zung Self-rating Depression Scale (D’Angelo et al., 2008; Giovagnoli 1999; Giovagnoli et al., 2005). The remaining studies used mood assessment instruments, such as the Profile of Mood States - Short Form (Brown et al., 2006; 2005), patient interviews (Davies et al., 2003) or documentation of clinical depression in the patient’s medical record by the physician or data suggestive of depression (Appleby et al., 2008; Gathinji et al., 2008; Litofsky et al., 2004).
Other factors that influence depressive symptoms
Of the demographic variables measured, female gender (Armstrong et al., 2002; Arnold et al., 2008) and those with a lower educational level (Arnold et al., 2008) reported more symptoms of depression. A premorbid diagnosis of depression or diagnosis of active depression at the time of surgery were associated with more depressive symptoms in four studies (Appleby et al., 2008; Arnold et al., 2008; Gathinji et al., 2008; Kilbride et al., 2007). Tumor characteristics, such as lower grade (Arnold et al., 2008; Manio et al., 2005) or left hemisphere location (Armstrong et al., 2002; Hahn et al., 2003; Wellisch et al., 2002), were related to increased severity of depressive symptoms. The severity of other symptoms, including fatigue (Armstrong et al., 2002), cognitive impairment (Armstrong et al., 2002; Brown et al., 2006), and physical impairment (Brown et al., 2006; Davies et al., 2003; Giovagnoli, 1999; Giovagnoli et al., 2005) were found to increase depressive symptoms. Greater family support (Armstrong et al., 2002) and trait anxiety (D’Angelo, 2008; Giovagnoli, 1999) were found to be significantly related to depressive symptoms. And finally, a greater extent of surgery was related to decreased depressive symptoms in two studies (Brown et al., 2005; Gathinji et al., 2008) and increased depressive symptom in one study (Armstrong et al., 2002).
Discussion
Theoretical models of previous research
The literature review revealed that the biopsychosocial framework of depression was the most common theoretical model used in exploring the relationships among depressive symptoms and outcomes in patients with a cerebral tumor. This framework emphasizes the dynamic interactions among neuro-cognitive factors, psychological processes and the social environment and is particularly useful for exploring the factors associated with depression. However, the biopsychosocial model does not incorporate the biological interactions occurring within the individual, which may modulate neuro-cognitive factors and psychological processes.
In addition, while most studies that used the biopsychosocial framework documented the tumor type, or histology, the analyses did not appear to control for this variable, which may dilute the significance of tumor biology on depressive symptoms. For example, gliomas (eg. astrocytomas, oligodendrogliomas, and ependymomas) may be associated with depressive symptoms due to the involvement of immunocompetent cells, whereas other types of primary brain tumors (eg. meningiomas or acoustic neuromas) may not (Litofsky & Resnick, 2009). Two studies reported that the association between depressive symptoms and decreased length of survival was more evident in low-grade tumors (Arnold et al., 2008; Mainio et al., 2005) while two studies controlled for tumor type and grade in the inclusion criteria and analyses (Gathinji et al., 2008; Litofsky et al., 2004). Since tumor histology and grade may affect the production and release of biological factors that cause depressive symptoms, these variables should be included in the theoretical framework.
None of the research studies reviewed measured biological factors in conjunction with depressive symptoms in patients with a brain tumor. Yet, extensive research in psychiatry and other areas of health science have shown that biological factors can trigger the onset of depressive symptoms (Dantzer & Kelley, 2007; Kelley et al., 2003; Muller & Schwartz, 2007). Proinflammatory cytokines (particularly interleukin [IL]-1, IL-6, and tumor necrosis factor-alpha [TNF-α]), molecules involved in the initiation of the inflammatory response, cause or exacerbate depressive symptoms and activate astrocytes to release cytokines within the central nervous system (CNS; Raghavendra, Tanga, & DeLeo, 2004; Vollmer-Conna et al., 2004; Wichers & Maes, 2002).
Astrocytes are the major glial cell population within the CNS and act as immunocompetent cells; that is, they regulate local immune responses through the production and release of cytokines, such as interleukin(IL)-1, IL-6, and tumor necrosis factor (TNF)-α (Dong & Benveniste, 2001). When the integrity of astrocytes is compromised, such as occurs with neoplasia, astrocytes also release glial acidic fibrillary protein (GFAP) into the CNS in addition to increased cytokines. GFAP is a Type III intermediate filament protein, the major protein component of the astrocyte cytoskeleton (Laping, Teter, Nichols, Rozorski, & Finch, 2008). Thus, GFAP serves as a biomarker of astrogliosis, and serum levels have been found to be highly correlated with astrocytoma volume (Brommeland, Rosengran, Fridland, Hennig, & Isaksen, 2007). Local levels of GFAP, along with elevated cytokines have been implicated as driving factors in astrocytic differentiation and growth, angiogenesis and astrogliosis, and may alter the tumor microenvironment so that immune cells do not attack the tumor cells (Korzhevskii, Otellin, & Grigor’ev, 2005; Radeff Huang, Seasholtz, & Brown, 2005; Schneider, Sailer, Ansorge, Firsching, & Reinhold, 2006). Altered GFAP levels have also been described in post-mortem samples of brain tissue from physically healthy individuals with major depressive disorder (Davis et al., 2002; Muguel-Hidalso, 2000). Because GFAP levels have been found to rise with tumor progression (Brommeland et al., 2007), the relationship to depressive symptoms warrants further exploration.
While increased secretion of several specific cytokines from astrocytoma cells have been described, there is mounting evidence that cytokine patterns, or the balance between pro- and anti-inflammatory cytokines, may be more important than isolated, individual cytokine levels in understanding astrocytoma growth and resistance to treatment (Hao et al., 2002). Indeed, Samaras et al. (2007) recently compared levels of IL-6 and IL-10 in astrocytic neoplasms and in peripheral blood. They found that levels of both cytokines were higher in peripheral blood compared to healthy controls, and that tumor and peripheral levels were highly correlated. The median IL-10 expression level by the neoplastic cells was significantly lower than that of IL-6, suggesting a localized cellular mechanism of immune suppression. Moreover, the principal IL-6 positive cell type was the neoplastic astrocyte; whereas microglial cells and macrophages appeared to be the major source of IL-10. Similarly, Kumar et al. (2006) documented significantly lower levels of serum IL-12, and higher levels of IL-10 among newly diagnosed patients with anaplastic astrocytoma and glioblastoma multiforme. The authors concluded that astrocytomas cause a Th1/Th2 cytokine imbalance. Although these particular cytokines are implicated in regulating cell growth, resistance to chemotherapy, and angiogenesis (Liebrich et al., 2007), the possible involvement of other cytokines has yet to be determined.
Within the past few years, advances in technology have enabled a broad spectrum of cytokine measurements from a single specimen, which could be used to study pro- and anti-inflammatory cytokine interactions in the blood and tissue. Immunohistochemistry or genetic biomarkers may be used to identify the specific cytokine-releasing cells, providing a new level of understanding about the biological interactions that affect depressive symptoms, quality of life, and the disease trajectory.
In order to incorporate the possible multiple interactions among depressive symptoms, biological factors, and outcomes, the integrative paradigm of psychoneuroimmunology (PNI) could be used to guide a program of research in this area (Fox, Shepherd & McCain, 1999; Starkweather, Witek-Janusek & Mathews, 2005). PNI is a biobehavioral framework focused on the mechanisms of multidimensional psychobehavioral-neuroendocrine-immune system interactions that influence disease-specific outcomes (McCain, Gray, Walter, & Robins, 2005).
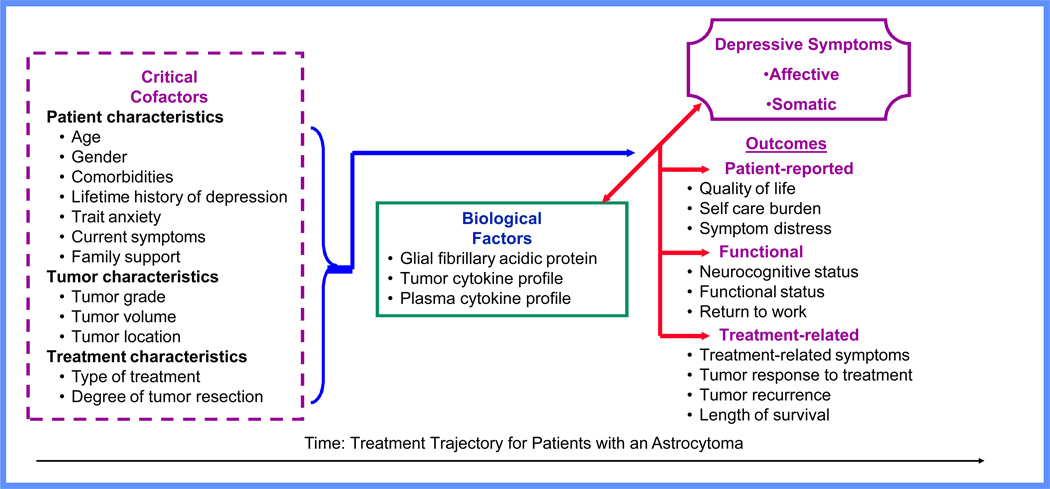
Critical cofactors are viewed as moderators of neuroendocrine-immune (biological) interactions that potentially predispose the individual to depressive symptoms (see Fig. 1). These may include personal characteristics (demographics, comorbidities, current symptoms, or social support), disease characteristics (tumor type and location) and treatment characteristics (extent of surgery, radiation, chemotherapy). Neuroendocrine-immune interactions are central to the framework, denoting their importance in mediating states of health and disease. As noted above, for patients with an astrocytoma, specific biological factors have been implicated: glial fibrillary acidic protein, a biomarker of astrocytic activation, and local and circulating cytokines (Brommeland et al., 2007; Kumar et al., 2006; Liebrich et al., 2007; Samaras et al., 2007). Use of the PNI framework allows an examination of the psychosocial and biological factors affecting symptoms of depression, and a method to develop hypotheses regarding the interactions that affect clinical outcomes.
Figure 1.
Psychoneuroimmunology Framework: Depressive Symptoms in Patients with an Astrocytoma
Methods used to measure depressive symptoms
Although most, if not all, studies purported to be measuring depression, only one study included a diagnostic neuropsychological examination, the gold standard method of diagnosing clinical depression (Wellisch et al., 2002). In addition, there was a wide range of instruments used, with most relying on self-report questionnaires or chart review, making comparisons among studies impossible. Due to these discrepancies in the definition of depression and depressive symptoms, it remains unclear whether patients with clinical depression are at risk of decreased survival time or whether those who have a high severity of depressive symptoms but do not qualify as clinically depressed are also at risk.
Depressive symptoms include emotional, cognitive, and physical effects, and many symptoms overlap with those engendered by the disease process or as side-effects of treatment in patients with an astrocytoma. Although the detrimental effects of clinical depression on health is well recognized, there remains controversy over the significance of depressive symptoms in this population because symptoms caused by the presence of an astrocytoma, such as fatigue and insomnia, may be part of their disease process (somatic symptoms) as opposed to an emotional aspect of depression (affective symptoms) (Pasquini & Biondi, 2007). Thus, the measurement of depressive symptoms in patients with an astrocytoma may grossly overestimate the presence of clinical depression.
Recent opinions within the oncology literature, however, encourage the use of an inclusive approach toward identifying depressed individuals by counting the number of depressive symptoms, even if they may be disease or treatment related (Guo et al., 2006; Pasquini & Biondi, 2007; Reich, 2008). Much data demonstrate that subsyndromal depression is associated with significant subsequent morbidity in individuals both with and without cancer (Goodwin, Zhang, & Ostir, 2004; Myers, 2008). Likewise, depressive symptoms, even when not qualifying as clinical depression or major depressive disorder, are predictive of increased morbidity and mortality across a range of medical conditions (Hjerl et al., 2003; Lima et al., 2007).
The findings of Litofsky et al. (2004) underscore the need to understand the symptom manifestations of depression versus depressive symptoms, the effect of depression and/or depressive symptoms on the treatment trajectory, and the type of interventions that may be effective. In their prospective study of 598 patients with high-grade glioma they found that while physicians reported depression in 15% of patients during the early postoperative period, 93% of patients reported symptoms consistent with depression. Symptoms of depression increased across the 6-month period after surgery and were associated with reduced quality of life scores. Length of survival was significantly shorter among patients with depression, even among those who received anti-depressant therapy, compared to patients who were not depressed.
These findings support the need to measure depressive symptoms and include serial diagnostic neuropsychological evaluations over time so that we can begin to understand the differences between patients who experience clinical depression versus depressive symptoms. The measurement of biological factors may also provide insight on the associated mechanisms so that new treatment strategies can begin to be developed. For instance, proinflammatory cytokines can cause different behavioral features, including both a depressive syndrome, distinguished primarily by affective symptoms, and a neurovegetative syndrome characterized by somatic symptoms such as fatigue and loss of appetite (Anisman & Merali, 2003; Dantzer, 2006; Maletic et al., 2007; Miller & O’Callaghan, 2005; Reich, 2008). While the depressive syndrome is typically responsive to antidepressants, the neurovegetative syndrome appears to be resistant to antidepressant treatment (Miller & Raison, 2008; Reich, 2008). Thus, antidepressants may provide only part of the solution to cytokine-associated behavioral disturbances.
Novel approaches to symptom management may include pharmacologic agents that directly inhibit proinflammatory cytokines, or enhance glucocorticoid signaling and inhibit inflammatory signaling (Chen et al., 2002). Biobehavioral nursing interventions may complement medical therapies. Mindfulness-based meditation has been shown to be an effective strategy for reducing depressive symptoms in patients with cancer and immune-based disease (McCain et al., 2003; McCain et al., 2008).
Other factors that influence depressive symptoms
Additional factors identified in the literature review that may influence depressive symptoms can be broadly categorized into patient characteristics [gender, educational level, premorbid diagnosis of depression, trait anxiety, current symptoms (fatigue, cognitive impairment, physical functioning), and family support], tumor characteristics (tumor grade and location), and treatment characteristics (extent of tumor resection). These factors were added into the proposed theoretical model developed by the authors which will be used to guide future research in this area (Fig. 1).
Summary
The literature review revealed several methodological concerns that should be addressed in future research. Most studies mixed tumor types into one sample, which could dilute the significance of the study findings for patients with an astrocytoma. The measurement of depression or depressive symptoms was inconsistent between studies, creating variability in how patients are categorized (as depressed or non-depressed). It would be helpful if researchers could agree to use one method or instrument to measure depression and/or depressive symptoms in future studies so that results can be compared and the effect of various treatment modalities between sites can be more fully examined.
The pervasive nature of depressive symptoms over the disease trajectory; recent evidence indicating a relationship between depressive symptoms and reduced length of survival; and, research showing that astrocytes orchestrate local cytokine concentrations, suggest a common biobehavioral mechanism underlying the presence of depressive symptoms in patients with an astrocytoma. The literature review supports the need to gain a better understanding of how neuroendocrine-immune interactions influence outcomes with the goal of facilitating the development of effective treatment strategies. An initial step of a possible program of research would be to determine the relationships among depressive symptoms, the selected biological factors, and patient-reported, functional, and treatment-related outcomes over time in patients with a cerebral astrocytoma. The relationship between cytokine and GFAP levels in tumor tissue and serum could be evaluated to determine whether systemic concentrations correlate with concentrations within the CNS. Although previous studies have shown that tumor tissue and serum levels of some individual cytokines are highly correlated, a PNI based program of research would examine a broader range of both pro- and anti-inflammatory cytokines. Without a clear understanding of how inflammatory mediators affect somatic versus affective symptoms and clinical outcomes over time there is a great risk that these patients will continue to suffer from a potentially treatable aspect of the disease process.
Characterization of the cytokine profile and the balance between pro- and anti-inflammatory cytokines over time should be established to determine how cytokine interactions affect depressive symptoms, astrocytoma growth and recurrence, and length of survival. Application of the PNI framework will provide an integrated perspective, enhancing the exploration of the multiple interactions among biological factors, depressive symptoms and critical cofactors so that the effect on outcomes can be better understood. The results of such a program of research might potentially provide empirical evidence regarding the biological mechanisms underlying depressive symptoms which may ultimately lead to better symptom management and more speculatively to reduced morbidity and mortality in patients with an astrocytoma.
Contributor Information
Angela Starkweather, Assistant Professor, Virginia Commonwealth University School of Nursing.
Paula Sherwood, Assistant Professor, University of Pittsburgh School of Nursing.
Debra E. Lyon, Associate Professor, Interim Chair, Department of Family and Community Health Nursing, Virginia Commonwealth University School of Nursing.
Nancy L. McCain, Professor, Virginia Commonwealth University School of Nursing.
Dana Howard Bovbjerg, Director - Biobehavioral Medicine in Oncology Program, University of Pittsburgh Cancer Institute, Visiting Professor - Departments of Psychiatry, Psychology and Behavioral & Community Health Sciences, Hillman Cancer Center
William C. Broaddus, Professor, Department of Neurosurgery, Virginia Commonwealth University
References
- American Cancer Society. Cancer facts and figures, 2007. Atlanta: Author; 2007. Retrieved August 30, 2007, from: www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf. [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress and depressive illness: Brain-immune interactions. Annals of Medicine. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Appleby BS, Appleby KK, Rabin PV. Predictors of depression and anxiety in patient with intracranial neoplasms. Journal of Neuropsychiatry and Clinical Neuroscience. 2008;20:447–449. doi: 10.1176/jnp.2008.20.4.447. [DOI] [PubMed] [Google Scholar]
- Armstrong CL, Goldstein B, Cohen B, Jo MY, Tallent EM. Clinical predictors of depression in patients with low-grade brain tumours: Consideration of a neurological versus a psychogenic model. Journal of Clinical Psychology in Medical Settings. 2002;9:97–107. [Google Scholar]
- Arnold SD, Forman LM, Brigidi BD, Carter KE, Schweitzer HA, Quinn HE, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-Oncology. 2008;10:171–181. doi: 10.1215/15228517-2007-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy ML, Scheurer ME, Malmer B, Barnholtz Sloan JS, Davis FG, Il’yasova D. Brain tumor epidemiology: Consensus from the brain tumor epidemiology consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommeland T, Rosengran L, Fridland S, Hennig R, Isaksen V. Serum levels of glial fibrillary acidic protein correlate to tumour volume of high-grade gliomas. Acta Neurologica Scandinavica. 2007;116:380–384. doi: 10.1111/j.1600-0404.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. Journal of Neuro-oncology. 2006;76:283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: The impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57:495–504. doi: 10.1227/01.neu.0000170562.25335.c7. [DOI] [PubMed] [Google Scholar]
- Central Brain Tumor Registry of the United States (CBTRUS) Primary brain tumors in the United States: Statistical report 2005–2007 years data collected. Hinsdale, IL: CBTRUS; 2008. [Google Scholar]
- Chen TC, Wasten P, Su S, Rawlinson N, Hofman FM, Hill CK, et al. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21 (Cip 1) and p27 (Kip 1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biological Therapeutics. 2002;1:268–276. doi: 10.4161/cbt.80. [DOI] [PubMed] [Google Scholar]
- Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: Data from SEER program, 1973–2001. Cancer. 2006;15:1358–1363. doi: 10.1002/cncr.21733. [DOI] [PubMed] [Google Scholar]
- D’Angelo C, Mirijello A, Leggio L, Ferrulli A, Carotenuto V, Icolaro N, et al. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-year longitudinal study. Journal of Neurosurgery. 2008;108:281–286. doi: 10.3171/JNS/2008/108/2/0281. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokines, sickness behavior and depression. Neurology Clinic. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E, Hall S, Clarke C. Two year survival after malignant cerebral glioma: Patients and relative reports of handicap, psychiatric symptoms and rehabilitation. Disability and Rehabilitation. 2003;25:259–266. doi: 10.1080/0963828021000024915. [DOI] [PubMed] [Google Scholar]
- Davis S, Thomas A, Perry R, Oakley A, Kalaria RN, O’Brien JT. Glial fibrillary acidic protein in late life major depressive disorder: An immunocytochemical study. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:556–560. doi: 10.1136/jnnp.73.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Fox S, Shephard TJ, McCain N. Neurological mechanisms in psychoneuroimmunology. Journal of Neuroscience Nursing. 1999;31:87–96. doi: 10.1097/01376517-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Gathinji M, McGirt MJ, Attenello FJ, Chaichana KL, Than K, Olivi A, et al. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surgical Neurology. 2008 doi: 10.1016/j.surneu.2008.07.016. epub Sept 10, 1–7. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR. Quality of life in patients with stable disease after surgery, radiotherapy, and chemotherapy for malignant brain tumour. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;67:358–363. doi: 10.1136/jnnp.67.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade gliomas. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:562–568. doi: 10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment and survival of older women with breast cancer. Journal of the American Geriatric Society. 2004;52:106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AF, Smith FA, Stern TA. Is depression an appropriate response to having cancer? A discussion of diagnostic criteria and treatment decisions. Journal of Clinical Psychology. 2007;9:382–387. doi: 10.4088/pcc.v09n0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Musselman DL, Manatunga AK, Gilles N, Lawson KC, Porter MR, et al. The diagnosis of major depression in patients with cancer: a comparative approach. Psychosomatics. 2006;47:376–384. doi: 10.1176/appi.psy.47.5.376. [DOI] [PubMed] [Google Scholar]
- Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. International Journal of Radiation Oncology, Biology and Physics. 2003;55:992–999. doi: 10.1016/s0360-3016(02)04205-0. [DOI] [PubMed] [Google Scholar]
- Hao C, Parney IF, Roa WH, Turner J, Petruk KC, Ramsay DA. Cytokines and cytokine receptor mRNA expression in human glioblasomas: Evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathologica. 2002;103:171–178. doi: 10.1007/s004010100448. [DOI] [PubMed] [Google Scholar]
- Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortenson PB, Jorgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44:24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: A Cancer Journal for Clinicians. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behaviors. Brain, Behavior and Immunity. 2003;S1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kilbride L, Smith G, Grant R. The frequency and cause of anxiety and depression amongst patients with malignant brain tumours between surgery and radiotherapy. Journal of Neurooncology. 2007;84:297–304. doi: 10.1007/s11060-007-9374-7. [DOI] [PubMed] [Google Scholar]
- Korzhevskii DE, Otellin VA, Grigor’ev IP. Glial fibrillary acidic protein in astrocytes in the human neocortex. Neuroscience and Behavioral Physiology. 2005;35:789–792. doi: 10.1007/s11055-005-0125-y. [DOI] [PubMed] [Google Scholar]
- Kumar R, Kamdar D, Madden L, Hills C, Crooks D, O’Brien D, et al. Th1/Th2 cytokine imbalance in meningioma, anaplastic astrocytoma and glioblastoma multiforme patients. Oncology Reports. 2006;15:1513–1516. doi: 10.3892/or.15.6.1513. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Teter B, Nichols NR, Rozorski I, Finch LE. Glial fibrillary acidic protein: Regulation by hormones, cytokines, and growth factors. Brain Pathology. 2008;4:259–275. doi: 10.1111/j.1750-3639.1994.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Liebrich M, Guo LH, Schluesener HJ, Schwab JM, Dietz K, Will BE. Expression of interleukin-16 by tumor-associated macrophages/activated microglia in high-grade astrocytic brain tumors. Archivum Immunologiae et Therapiae Experimentalis. 2007;55:41–47. doi: 10.1007/s00005-007-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VP, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- Lin CL, Lieu AS, Lee KS, Yang YHC, Kuo TH, Hung MH, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surgical Neurology. 2002;60:402–406. doi: 10.1016/s0090-3019(03)00322-7. [DOI] [PubMed] [Google Scholar]
- Litofsky NS, Farace E, Anderson F, Jr, Meyers CA, Huang W, Laws ER, Jr, et al. Depression in patients with high-grade glioma: Results of the Glioma Outcomes Project. Neurosurgery. 2004;54:358–366. doi: 10.1227/01.neu.0000103450.94724.a2. [DOI] [PubMed] [Google Scholar]
- Litofsky NS, Resnick AG. The relationships between depression and brain tumors. Journal of Neurooncology. 2009 doi: 10.1007/s11060-009-9825-4. published online March 5, 2009. [DOI] [PubMed] [Google Scholar]
- Lutterbach J, Sauerbrei W, Guttenberg R. Multivariate analysis of prognostic factors in patient with glioblastoma. Strahlentherapie und Onkologie. 2003;1:8–15. doi: 10.1007/s00066-003-1004-5. [DOI] [PubMed] [Google Scholar]
- Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Gender difference in relation to depression and quality of life among patients with a primary brain tumor. European Psychiatry. 2006;21:194–199. doi: 10.1016/j.eurpsy.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Mainio A, Tuunanen S, Hakko H, Timonen M, Niemela A, Koivukangas J, Rasanen P. Depression in relation to survival among neurosurgical patients with a primary brain tumor: A 5-year follow-up study. Neurosurgery. 2005;56:1234–1241. doi: 10.1227/01.neu.0000159648.44507.7f. [DOI] [PubMed] [Google Scholar]
- Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J. Neurobiology of depression: An integrated view of key findings. International Journal of Clinical Practice. 2007;61:2030–2040. doi: 10.1111/j.1742-1241.2007.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain NL, Gray DP, Elswick RK, Robins JW, Tuck I, Walter JM, Rausch SM, Ketchum JM. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. Journal of Consulting and Clinical Psychology. 2008;76:431–441. doi: 10.1037/0022-006X.76.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain NL, Gray DP, Walter JM, Robins J. Implementing a comprehensive approach to the study of health dynamics using the psychoneuroimmunology paradigm. Advances in Nursing Science. 2005;28:320–332. doi: 10.1097/00012272-200510000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain NL, Munjas BA, Munro CL, Elswick RK, Robins JW, Ferreira-Gonzales A, Baliko B, Kaplowitz LG, Fisher EJ, Garrett CT, Brigle KE, Kendall LC, Lucas V, Cochran KL. Effects of stress management on PNI-based outcomes in persons with HIV disease. Research in Nursing & Health. 2003;26:102–117. doi: 10.1002/nur.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter H, Furlong W, Whitton AC, Feeny D, DePauw S, Willan AR, et al. Health status measurements at diagnosis as predictors of survival among adults with brain tumors. Journal of Clinical Oncology. 2006;24:3636–3643. doi: 10.1200/JCO.2006.06.0137. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. Immune system contributions to the pathophysiology of depression. Focus. 2008;6:36–45. [Google Scholar]
- Miller DB, O’Callaghan JP. Depression, cytokines and glial function. Metabolism Clinical and Experimental. 2005;54:S33–S38. doi: 10.1016/j.metabol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Muguel-Hidalso JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biological Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Molecular Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Myers S. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncology Nursing Forum. 2008;35:802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- Osaba D, Brada M, Prados MD, Yung WKA. Effects of disease burden on health-related quality of life in patients with malignant gliomas. Neurooncology. 2000;2:221–228. doi: 10.1093/neuonc/2.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumour patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. Journal of Neurooncology. 2002;57:41–49. doi: 10.1023/a:1015728825642. [DOI] [PubMed] [Google Scholar]
- Pasquini M, Biondi M. Depression in cancer patients: A critical review. Clinical Practice and Epidemiology in Mental Health. 2007;3:1–9. doi: 10.1186/1745-0179-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. British Journal of Neurosurgery. 1999;13:45–51. doi: 10.1080/02688699944177. [DOI] [PubMed] [Google Scholar]
- Radeff Huang J, Seasholtz TM, Brown JH. Role of S1P signaling in TNF-alpha mediated astrocytoma cell proliferation. FASEB Journal. 2005;19:A526–A527. [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. European Journal of neuroscience. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Reich M. Depression and cancer: Recent data on clinical issues, research challenges and treatment approaches. Current Opinion in Oncology. 2008;20:353–359. doi: 10.1097/CCO.0b013e3282fc734b. [DOI] [PubMed] [Google Scholar]
- Samaras V, Piperi C, Korkolopoulou P, Zisakis A, Levidou G, Themistocleous M, et al. Application of the ELISPOT method for comparative analysis of interleukin (IL)-6 and IL-10 secretion in peripheral blood of patients with astroglial tumors. Molecular and cellular Biochemistry. 2007;304:343–351. doi: 10.1007/s11010-007-9517-3. [DOI] [PubMed] [Google Scholar]
- Schneider T, Sailer M, Ansorge S, Firsching R, Reinhold D. Increased concentrations of transforming growth factor β1 and β2 in the plasma of patients with glioblastoma. Journal of Neurooncology. 2006;79:61–65. doi: 10.1007/s11060-005-9116-7. [DOI] [PubMed] [Google Scholar]
- Starkweather AR, Witek-Janusek L, Mathews HL. Psychoneuroimmunology: An integrated research framework for neuroscience nurses. Journal of Neuroscience Nursing. 2005;37:56–61. [PubMed] [Google Scholar]
- Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, et al. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behavior in humans. Psychological Medicine. 2004;34:1289–1297. doi: 10.1017/s0033291704001953. [DOI] [PubMed] [Google Scholar]
- Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11:230–238. doi: 10.1002/pon.562. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]