Abstract
Injury to the central nervous system (CNS) generally results in significant neuronal death and functional loss. In vitro experiments have demonstrated that neurotrophic factors such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and neurotrophin-4/5 (NT-4/5) can promote neuronal survival. However, delivery to the injured CNS is difficult as these large protein molecules do not efficiently cross the blood–brain barrier. Intranasal delivery of 70 μg [125I]-radiolabeled BDNF, CNTF, NT-4, or erythropoietin (EPO) resulted in 0.1–1.0 nM neurotrophin concentrations within 25 min in brain parenchyma. In addition, not only did these neurotrophic factors reach the CNS, they were present in sufficient concentrations to activate the prosurvival PI3Kinase/Akt pathway, even where lower levels of neurotrophic factors were measured. Currently traumatic, ischemic and compressive injuries to the CNS have no effective treatment. There is potential clinical relevancy of this method for rescuing injured CNS tissues in order to maintain CNS function in affected patients. The intranasal delivery method has great clinical potential due to (1) simplicity of administration, (2) noninvasive drug administration, (3) relatively rapid CNS delivery, (4) ability to repeat dosing easily, (5) no requirement for drug modification, and (6) minimal systemic exposure.
Keywords: Nasal drug delivery, protein delivery, central nervous system (CNS), blood–brain barrier, distribution, neurotrophic factor, ciliary neurotrophic factor, brain-derived neurotrophic factor, insulin growth factor-1, erythropoietin, neurotrophin-4/5, intranasal delivery, brain injury, Akt
Introduction
Injury to the central nervous system (CNS) of mammals generally results in rapid and permanent neuronal death. The primary wave of cell death after injury or disease is often followed by a second wave of neuronal death caused by the production of glutamate, aspartate, reactive oxygen species, inflammatory cytokines and other molecules released from the dying neurons (Ferrer & Planas, 2003). As the primary wave is too rapid to mitigate effectively, this second wave of cell death opens a therapeutic window of time to abate or reduce the extent of cell death, as it is slowly progressive over the course of weeks to months (Rami, Bechmann, & Stehle, 2008). Disorders such as Parkinson disease and amyotrophic lateral sclerosis also result from slow continuous degeneration of specific groups of neurons in the CNS. Currently, no clinically available treatments that are universally accepted exist to rescue these cells from degeneration.
Neurotrophic factors activate neuroprotective cell-survival and/or antiapoptotic pathways in the CNS, both in vitro and in vivo. Some in vivo strategies for delivering neurotrophic factors to the CNS include direct injection into the brain (Knusel et al., 1992), viral vector upregulation, (Mandel et al., 1999; Blits et al., 2003), or infusion pump-mediated delivery methods (Williams et al., 1986). Unfortunately, these methods presently lack practical clinical relevance for patient treatment. Part of the problem is that these large neurotrophic protein molecules to the CNS do not efficiently cross the blood–barrier into the CNS (Poduslo & Curran, 1996; Thorne & Frey, 2001). Clinical trials have demonstrated that systemic delivery at doses that are sufficiently high to result in therapeutic levels within the CNS parenchyma also result in significant systemic side-effects (Thoenen & Sendtner, 2002). These studies suggest the need for alternative methods of drug delivery to realize the clinical promise of these neuroprotective factors.
To bypass the blood–brain barrier and achieve potentially therapeutic levels of drugs in the CNS parenchyma compared to systemic treatment, efficient delivery can occur after an intranasal administration of nerve growth factor (NGF) and insulin-like growth factor-1 (IGF-1), proteins with well-characterized neuroprotective properties (Frey et al., 1997; Chen et al., 1998; Capsoni, Giannotta, & Cattaneo, 2002; Thorne et al., 2004; De Rosa et al., 2005) with elevated levels of some compounds as early as 5 min after nasal application (Zhang et al., 2006). These significantly elevated drug concentrations in the CNS occurred with reduced systemic exposure compared to intravenous and systemic administration techniques (Thorne et al., 2004; Dhanda et al., 2005). Although, the quantities that reach the brain via this mechanism may seem small, they appear to be in sufficient quantities to exert effects (Reger et al., 2006; 2008). Most of the early studies were performed in rats. Based in part on differences in nasal cavity size and structure between rats and man (Illum, 2004), several papers questioned the ability of drugs to access the brain in primates by the intranasal route (Merkus et al., 2003; Merkus & van den Berg, 2007). However, recent studies demonstrate intranasal delivery to the CNS occurs in nonhuman primates (Thorne et al, 2008; Yamada et al., 2008) and humans (Hallschmid et al., 2004; Benedict et al., 2008), supporting the potential clinical relevance of this approach. However, demonstration of drug transport does not necessarily indicate function, and efficacy in some cases must still be demonstrated (Hallschmid et al., 2008). Studies in humans have provided evidence for delivery of melanocortin (962.1 Da), vasopressin (1084.2 Da) (Born et al., 2002), angiotensin II (1046.18 Da) (Derad et al., 1998), and insulin (5808 Da) (Kern et al., 1999; Born et al., 2002) from the nasal mucosa to the cerebrospinal fluid (CSF). Intranasally delivered insulin improves memory, attention, and functional status in patients in the early stages of Alzheimer's disease without alteration in the blood levels of insulin or glucose (Reger et al., 2006; 2008). Intranasal insulin also improves memory in normal human adults (Benedict et al., 2004; 2007). Moreover, in a murine model of type I diabetic encephalopathy, long-term delivery of intranasal insulin reduces neurodegeneration and yields minimal systemic effects (Francis et al., 2008; 2009). In animal models, intranasal delivery of large protein neurotrophic factors in various forms of CNS disease and injury results in functional rescue. Intranasal application of either NGF or IGF-1 is neuroprotective after experimental induction of cerebral ischemia (Liu et al., 2004), and NGF also reduces degeneration (Capsoni, Giannotta, & Cattaneo, 2002) and rescues memory deficits in a mouse model of Alzheimer's disease (De Rosa et al., 2005). Thus, a great deal of evidence exists for the clinical potential of intranasal drug delivery, including neurotrophic factors.
Many neurotrophic factors have been implicated in diseases of the CNS and play a neuroprotective role after traumatic or ischemic brain injury. Four that have a good deal of supporting evidence for their neuroprotective properties in the widest number of CNS tissues are brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophin-4/5 (NT-4), and erythropoietin (EPO). The striatum of Huntington's disease patients shows a major loss of BDNF protein which likely plays a role in the etiology of the disease (Zuccato & Cattaneo, 2007), and exogenously administered BDNF decreases the onset and severity of motor dysfunction in a rat model of Huntington's disease (Canals et al., 2004). Lower than normal BDNF levels have been linked to neuronal loss in epilepsy (Azoulay, Urshansky, & Karni, 2008), and declines in BDNF, BDNF mRNA and/or proBDNF play a role in the pathologic changes in both aging and Alzheimer's diseased brains (Tapia-Arancibia et al., 2008). CNTF treatment of mouse models of both Alzheimer's and Huntington's disease results in rescue of learning and memory impairments (Emerich et al., 1997; Qu et al., 2008). After traumatic brain injury, treatment of the hippocampus with NT-4 reduces neuronal loss in pyramidal cells (Royo et al., 2006). The effect of EPO includes decreasing neuron loss associated with epilepsy (Chu et al., 2008), decreasing neuronal toxicity to cisplatin (Nowis et al., 2007), and decreasing neuronal loss after experimental traumatic brain injury (Grasso et al., 2007). A small amount of systemically administered EPO can cross the blood–brain barrier. However, administration of these other neurotrophic factors was performed by brain cannulation (NT-4, Royo et al., 2006; BDNF, Arancibia et al., 2008), direct implantation of cells expressing the factor of interest (CNTF, Emerich et al., 1997), or viral vector delivery of the genes (multiple factors in multiple laboratories). Although not currently clinically viable, these studies clearly demonstrate that diverse neurotrophic factors can protect CNS neurons from degeneration due to injury or disease.
The neuroprotective properties of neurotrophins stem from the binding of these proteins to their receptors and subsequent activation of intracellular signaling pathways that lead to activation of prosurvival pathways and/or inactivation of proapoptotic signaling. CNTF signals through the STAT3 and ERK pathways. Binding of CNTF to its receptor results in the formation of phosphorylated Stat3 (pSTAT3), which in turn activates survival pathways (Park et al., 2004). BDNF binds to the trkB receptor and activates the PI3K/Akt pathway, resulting in increases in cell survival and antiapoptosis programs. Upregulation of the appropriate signaling pathway demonstrates functionality of the neurotrophic factor within the tissue where it has distributed.
The goal in performing these studies was to determine if intranasal application of BDNF, CNTF, NT-4, or EPO would result in potentially therapeutic levels of the neurotrophic factors in widespread areas of the brain and upper spinal cord in adult rats and activate prosurvival pathways in brain tissues.
Materials and methods
Animals
Adult male Sprague–Dawley rats (180–250 g, Charles River Lab.) were housed at room temperature under a 12 h light/dark cycle. Food and water were provided ad libitum. Animals were cared for in accordance with the Regions Hospital, Health Partners Research Foundation Animal Care and Use Committee. A second strain of animals was used for the long-term functional analyses. Adult male Long Evans rats (225–500 g, Charles River Lab) were housed and cared for in similar conditions as described above. All procedures were approved by the Animal Care Committee of Regions Hospital and the University of Minnesota and conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Briefly, rats were treated intranasally with one of four radiolabeled neurotrophic factors. After either 25 or 60 min, the rats were euthanized and selected brain tissues were microdissected and analyzed to allow determination of the concentration of factor delivered based on radiolabel detection. In a second set of rats, the ability of these factors to activate prosurvival pathways was assessed for CNTF and BDNF. Two pathways associated with neuronal survival were assessed semi-quantitatively using western blot analysis, described below.
[125I]-labeled Neurotrophic Factors
Recombinant human (rh) BDNF (molecular weight 26,984 Da), NT-4 (22,428 Da), CNTF (22,706 Da), and EPO (glycosylated form 30,400 Da) were each purchased from Cell Sciences, Inc. (Canton, MA, USA). Lyophilized proteins were reconstituted in PBS and iodinated [125I] by GE Healthcare Bio-Sciences (Woburn, MA, USA) using the lactoperoxidase labeling method for EPO, BDNF and NT-4, while CNTF was labeled using chloramine-T labeling. The [125I]-BDNF solution contained less than 0.1% free iodide and had a radiochemical purity of greater than 98%, determined by high performance liquid chromatography (HPLC). Thin layer chromatography (TLC) determined the [125I]-NT-4 solution contained less than 1.0% free iodide, [125I]-erythropoietin less than 3.9% free iodide, and [125I]-CNTF less than 0.4% free iodide.
Intranasal administration
Intranasal administration of neuroprotective compounds was performed as previously described (Thorne et al., 1995; 2004). Briefly, rats were anesthetized with 40 mg/kg sodium pentobarbital i.p. (Nembutal, Ovation Pharmaceuticals, Deerfield, IL). The anesthetized rats were placed in a supine position, and the ventral surface of the head and neck were maintained horizontal using a small roll of gauze under the dorsal neck. Approximately 70 μg of [125I]-recombinant human neurotrophic factor (in 70 μL of sterile phosphate buffered saline) was administered by pipette in 7 μL drops, alternating between each naris every 2 min, over a total of 18 min. The contralateral naris was gently occluded during administration of each drop to facilitate snorting of the drops high into the nasal cavity. Animals survived for a total of 25 min (BDNF, CNTF, EPO, and NT-4, n = 7 for each factor for a total of 28, plus 3 unlabeled controls) or 1 h (BDNF, EPO, and NT-4, n = 3 for each factor for a total of 9). One hour postadministration time points were not performed with CNTF as there were solubility issues with one batch of this radioiodinated compound. The aorta was cannulated, and animals were perfused with 60 mL of saline followed by 360 mL of 4% paraformaldehyde using an infusion pump (15 mL/min: Harvard Apparatus, Inc., Holliston, MA, USA).
Collection and measurement of [125I]-labeled neurotrophic factor in tissue
Animals were anesthetized with pentobarbital sodium (Nembutal, 50 mg/kg i.p.; Abbot Laboratories, North Chicago, IL, USA) and remained in an anesthetized state until time of sacrifice. An abdominal aorta cannulation was performed to collect blood samples at 5 min intervals through the duration of the experiment. Animals were kept on a thermostatically controlled heating pad to maintain a 37°C body temperature (Fine Science tools, Inc., Foster City, CA, USA). A detailed necropsy was performed, removing cranial blood vessels and dura. Serial brain sections were made using a coronal precision rat brain matrix (Braintree Scientific). Discrete brain areas and spinal cord regions were microdissected. Trigeminal nerves and specific brain and spinal cord regions were carefully dissected and removed. Each sample was placed in a preweighed 5 mL tube using a microbalance (Sartorius MC210S, Goettingen, Germany). The wet weight of the samples was determined prior to gamma counting. The radioactivity levels in the tissues were measured using a gamma counter (Packard Cobra II Auto-Gamma counter). Tissue concentrations were calculated using the tissue weight, specific activity of the drug, and gamma counts minus the background radioactivity. Two data points from contaminated samples, with values 100-fold greater than other values of same tissue, were removed from the data analysis. Statistical outliers, those values greater than two standard deviations from the mean, were removed from the data analysis prior to the calculation of the mean and standard error. Very few data points fell in this range.
Functional analyses
For the functional analyses, 84 adult male Long Evans rats (225–500 g, Charles River Lab.) were used. These rats were anesthetized with 1.2 mg/kg tribromoethanol (Sigma-Aldrich, St. Louis, MO), and were placed in a supine position. They received an intranasally delivered 70 μg dose of rhCNTF or BDNF (Peprotech) and were allowed to recover following the 18 min delivery time. Animals were sacrificed following a 25 min, 1 h, 24 h, 48 h, 72 h, or 96 h survival period (N = 5 for each time point and each neurotrophic factor, with an additional 12 controls). Discrete brain regions were dissected and flash frozen in liquid nitrogen. Whole cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, St. Louis, MO) containing protease (Pierce, Rockland, IL) and phosphatase inhibitor (Roche Applied Sci., Indianapolis, IN). Total protein concentrations were determined using bicinchoninic acid (BCA) protein assay (Pierce). Polyacrylamide gel electrophoresis was performed by loading 40 μg of protein onto 10% polyacrylamide Tris HCl gels (BioRad., Hercules, CA). The separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes for Western blot analysis, and immunoprobed overnight at 4°C for phosphorylated Stat3 (pStat3) (Cell Signaling Technol., Danvers, MA) or phosphorylated Akt (pAkt) (1:1000, Abcam, Cambridge, MA). Hypoxanthine guanine phosphoribosyltransferase (HPRT) (1:40,000, Abcam) was used as the loading control (van Wjngaarden et al., 2007). The following day, the membranes were washed, and incubated with a goat anti-rabbit IgG peroxidase-linked secondary antibody (1:1000, Cell Signaling). After washing, the membranes were reacted with the Lumi-light substrate (Roche, Basel, Switzerland). Densitometric analysis was performed using a G-box System (Syngene, Frederick, MD). Semi-quantization of molecular changes in pStat3 and pAkt were performed based on saline-treated controls. Data were analyzed using analysis of variance (ANOVA) Kruskal–Wallis test and Dunnett posttest for significance using the Prism and Statmate software (Graphpad, San Diego, CA). An F-test was used to verify that the significance of the variances were not significantly different. Data were considered significantly different if P ≤ 0.05.
Results
Following intranasal administration, high levels of the radiolabeled neurotrophic factors appeared in the olfactory bulb and trigeminal nerve of the treated rats. Additional distribution throughout the brain and cervical spinal cord was evident, with concentrations varying widely between brain regions. Levels of drug delivery for each will be discussed separately. In all cases, the presence of the neurotrophin was observed in the liver (Figures 1–4) and blood (not shown). However, levels are very low compared to those seen after the high levels needed to achieve similar CNS delivery using the intravenous route (Chen et al., 1998; Thoenen & Sendtner, 2002; Thorne et al., 2004).
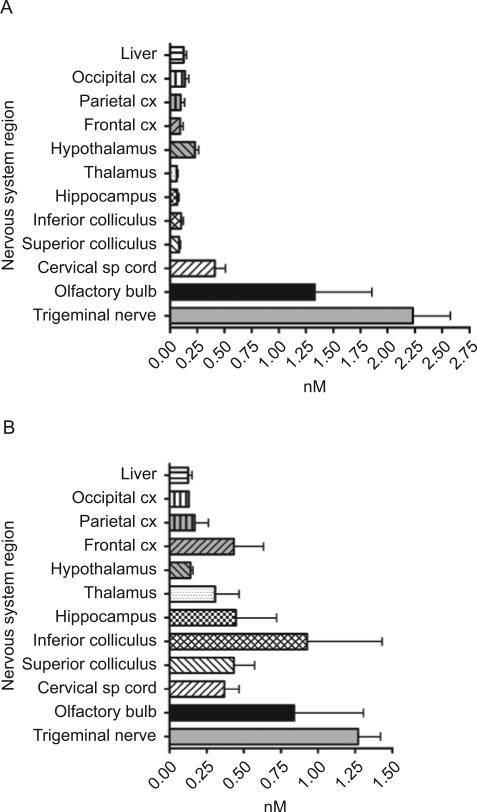
Figure 1.
(A) Intranasal delivery in nM of BDNF 25 min after intranasal delivery (N = 7). (B) Intranasal delivery in nM of BDNF 60 min after intranasal delivery (N = 3).
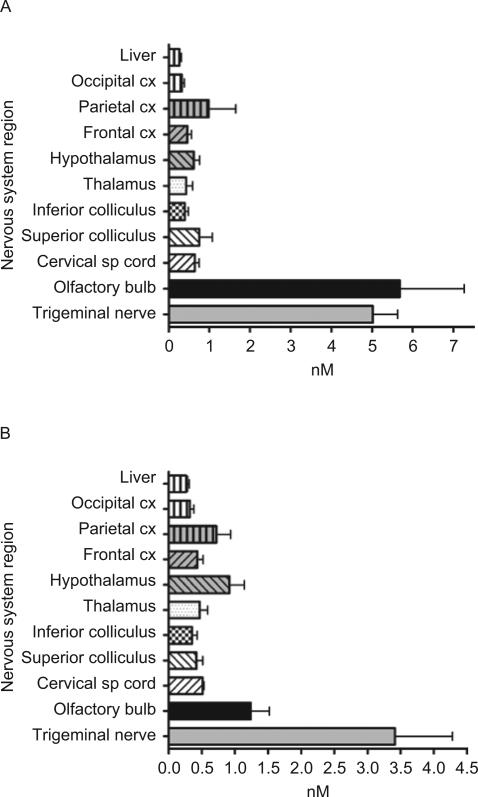
Figure 4.
(A) Intranasal delivery in nM of NT-4 25 min after intranasal delivery (N = 7). (B) Intranasal delivery in nM of NT-4 60 min after intranasal delivery (N = 3).
BDNF
Intranasal application of BDNF resulted in significant nM concentrations of BDNF throughout the brain and spinal cord by 25 min and generally increased further by 60 min (Figure 1A and 1B). Calculated as nanograms/mL of tissue at 25 min posttreatment, BDNF had spread throughout the brain and upper spinal cord, varying from 1.6 ± 0.3 ng/mL in thalamus to 11.1 ± 3.0 ng/mL in the upper cervical spinal cord (Table 1). In general, increases were seen after 60 min in the nervous tissues with a range of concentrations in these brain regions from 1.7 ± 0.6 ng/mL in hippocampus to 25.1 ± 1.36 ng/mL in inferior colliculus. It is thought that entry of the intranasally applied drugs is via both the olfactory nerve and trigeminal nerve paths. Both the olfactory bulbs and trigeminal nerves consistently displayed high neurotrophin concentrations at 25 min compared to any other CNS region, but these concentrations decreased significantly by 60 min (Tables 1 and 2). The concentration of BDNF decreased by 75.7% in the olfactory bulb from 25 to 60 min after intranasal administration.
Table 1.
Concentration of drug 25 min after intranasal delivery in nanograms/mL.
| Nervous system region | BDNF(N = 7) | CNTF(N = 7) | EPO(N = 7) | NT-4(N = 7) |
|---|---|---|---|---|
| Upper cervical spinal cord | 11.1 ± 3.0 | 28.2 ± 5.0 | 19.5 ± 3.8 | 34.1 ± 13.0 |
| Superior colliculus | 2.2 ± 0.5 | 10.5 ± 1.3 | 11.9 ± 4.9 | 11.1 ± 4.6 |
| Inferior colliculus | 2.8 ± 0.6 | 7.3 ± 1.7 | 12.0 ± 3.0 | 12.2 ± 4.9 |
| Thalamus | 1.6 ± 0.3 | 7.2 ± 1.6 | 13.0 ± 5.8 | 7.0 ± 2.6 |
| Hypothalamus | 6.1 ± 1.2 | 20.2 ± 1.8 | 18.8 ± 5.2 | 35.4 ± 13.1 |
| Hippocampus | 1.7 ± 0.5 | 6.8 ± 1.4 | - | 20.1 ± 7.0 |
| Frontal cortex | 2.5 ± 0.9 | 9.7 ± 2.0 | 13.9 ± 3.7 | 25.4 ± 12.3 |
| Parietal cortex | 2.6 ± 1.1 | 8.3 ± 2.1 | 6.5 ± 1.5 | 17.2 ± 4.5 |
| Occipital cortex | 3.6 ± 1.1 | 12.4 ± 2.1 | 9.8 ± 2.2 | 10.3 ± 4.8 |
| Olfactory bulbs | 20.3 ± 8.0 | 75.3 ± 13.9 | 160.3 ± 53.1 | 25.3± 4.5 |
| Trigeminal nerve | 60.2 ± 10.7 | 129.4 ± 18.6 | 152.4 ± 21.6 | 62.7 ± 8.4 |
| Liver | 4.5 ± 1.3 | 20.3 ± 3.9 | 8.0 ± 1.4 | 8.4 ± 1.4 |
Table 2.
Concentration of drug 60 min after intranasal delivery in nanograms/mL.
| Nervous system region | BDNF(N = 3) | EPO(N = 3) | NT-4(N = 3) |
|---|---|---|---|
| Upper cervical spinal cord | 10.0 ± 4.6 | 15.5 ± 1.3 | 11.4 ± 5.4 |
| Superior colliculus | 11.2 ± 5.0 | 12.7 ± 4.0 | 5.9 ± 2.1 |
| Inferior colliculus | 25.1 ± 1.36* | 10.8 ± 3.2 | 9.0 ± 0.8 |
| Thalamus | 14.3 ± 5.2 | 14.3 ± 5.2 | 4.9 ± 2.6 |
| Hypothalamus | 19.7 ± 0.8 | 19.7 ± 8.7 | 14.4 ± 0.7 |
| Hippocampus | 1.7 ± 0.6 | - | 5.8 ± 3.2 |
| Frontal cortex | 4.0 ± 0.2 | 13.1 ± 3.7 | 7.0 ± 1.7 |
| Parietal cortex | 4.4 ± 2.3 | 13.6 ± 7.1 | 4.7 ± 1.7 |
| Occipital cortex | 3.5 ± 0.003 | 9.7 ± 2.6 | 5.3 ± 2.3 |
| Olfactory bulbs | 4.93 ± 0.00 | 37.6 ± 12.0 | 65.1 ± 24.4 |
| Trigeminal nerve | 34.3 ± 7.1 | 103.7 ± 37.5 | 126.3 ± 30.7 |
| Liver | 2.7 ± 0.5 | 7.0 ± 2.5 | 13.7 ± 4.0 |
indicates only two data points.
CNTF
Intranasal CNTF was able to access the brain and spinal cord at higher concentrations (Figure 2) than seen with BDNF administration (Figure 1A). Every CNS structure analyzed had 3- to almost 7-fold greater nM concentration of CNTF at 25 min compared to BDNF at 25 min. For CNTF, concentrations varied from 0.36 ± 0.06 nM in hippocampus to 1.3 ± 0.2 nM in spinal cord. When calculated in nanogram/mL, the concentration of CNTF varied throughout the CNS tissues, ranging from 6.8 ± 1.4 in hippocampus to 28.2 ± 5.0 in the upper cervical spinal cord (Table 1). Olfactory bulb and trigeminal nerves exhibited high concentrations of CNTF after intranasal application: 75.3 ± 13.9 ng/mL CNTF in the olfactory bulb and 129.4 ± 18.6 ng/mL CNTF in the trigeminal nerve at 25 min.
Figure 2.
Intranasal delivery in nM of CNTF 25 min after intranasal delivery.
EPO
At 25 min after intranasal EPO administration, concentrations in CNS varied from 0.4 ± 0.8 nM in inferior colliculus to 0.99 ± 0.6 nM in parietal cortex (Figure 3A). The levels of EPO in the majority of CNS tissues were relatively unchanged by 60 min after drug delivery. Concentrations of EPO in nanograms/mL levels varied at 25 min from 6.5 ± 1.5 in parietal cortex to 19.5 ± 3.8 in upper cervical spinal cord and at 60 min ranged from 9.7 ± 2.6 in occipital cortex to 19.7 ± 8.7 in hypothalamus (Tables 1 and 2). Hippocampus was not sampled in EPO animals. The olfactory bulbs and trigeminal nerve had extremely elevated levels of EPO compared to other nervous system structures. Olfactory bulbs contained 160.3 ± 53.1 ng/mL at 25 min, and this level was reduced 76.5% at 60 min. The trigeminal nerve contained 152.4 ± 21.6 ng/mL at 25 min and was reduced 32% by 60 min after EPO delivery.
Figure 3.
(A) Intranasal delivery in nM of EPO 25 min after intranasal delivery (N = 7). (B) Intranasal delivery in nM of EPO 60 min after intranasal delivery (N = 3).
NT-4
NT-4 also displayed excellent entry into the CNS after intranasal delivery at concentrations similar to that seen for BDNF (Figures 1 and 4). Interestingly, every CNS tissue examined 25 min after intranasal delivery, except the olfactory bulbs, contained higher levels of NT-4 than at 60 min. The concentration varied from 0.28 ± 0.09 nM in thalamus to 1.8 ± 0.5 nM in hypothalamus at the 25 min postadministration interval and a range in CNS structures from 0.12 ± 0.02 nM in hippocampus to 0.43 ± 0.2 nM in parietal cortex at the 60 min posttreatment time point. At 25 min, the concentration of NT-4 in the CNS tissues spanned from 7.0 ± 2.6 ng/mL in thalamus to 35.4 ± 13.1 ng/mL in hypothalamus (Table 1). By 60 min after intranasal NT-4 application, the concentrations decreased in almost all of the CNS tissues examined, from a low of 4.7 ± 1.7 ng/mL in parietal cortex to 14.4 ± 0.7 ng/mL in hypothalamus (Table 2). Olfactory bulb NT-4 concentration was 25.3 ± 4.5 ng/mL at 25 min, and increased to 65.1 ± 24.4 ng/mL at 60 min. The trigeminal nerve had the highest concentrations of NT-4, with 62.7 ± 8.4 ng/mL and 126.3 ± 30.7 ng/mL at 25 and 60 min, respectively.
Activation of pAkt following CNTF or BDNF intranasal delivery
Following intranasal treatment with either 70 μg CNTF or BDNF, activation of the prosurvival signaling protein Akt was examined using western blots and densitometric analysis (Figure 5). Frontal cortex (BDNF) and occipital cortex (CNTF) were examined for upregulation of pAkt, whose results from treatment with either of activation by these two growth factors. A single intranasal treatment with either BDNF or CNTF resulted in a significant upregulation of pAkt, the activated form of Akt, from 24 h up to 96 h after a single intranasal drug administration. Similar results were seen for pStat3 (data not shown).
Figure 5.
Western blot examination of activation of the pAkt pathway caused by intranasal delivery of either CNTF or BDNF. (A) Representative western blot of control and CNTF-treated occipital cortex 24 and 48 h after a single intranasal application. Control pAkt protein is on the far right. HPRT was used as a loading control. (B) Densitometric examination of relative amounts of pAkt in CNTF-treated occipital cortex 24, 48, 72, and 96 h after a single intranasal application compared to saline-only control cortex (N=6 for each time point). * indicates significantly different from control. (C) Densitometric examination of relative amounts of pAkt in BDNF-treated frontal cortex 24, 48, 72, and 96 h after a single intranasal application compared to saline-only control cortex (N = 6 for each time point). * indicates significantly different from control.
Discussion
The present study demonstrates that intranasal delivery of BDNF, CNTF, EPO, and NT-4 in adult rats resulted in substantial concentrations of these neurotrophic factors widely throughout the brain and cervical spinal cord. Comparable delivery was seen for CNTF, EPO, and NT-4. Intranasal BDNF resulted in the lowest neurotrophin concentration, although concentrations were still 1–25 ng/mL in all CNS tissues examined. The olfactory epithelium expresses the trkB receptor for BDNF (Deckner et al., 1993), and it may be that receptor binding slows movement of drug into the brain tissues. Other large proteins can also reach the CNS after intranasal delivery. Using similar techniques, significant amounts of intact NGF reach the brain after intranasal application (Frey et al., 1997; Chen et al., 1998). Molecular weight does not seem to be the most important factor relative to the amount of intranasal transport into the brain parenchyma. Depending on the brain region examined, EPO, the largest of the factors at 30.4 kDa, had almost 5-fold more protein in ng/mL than that achieved following BDNF administration. Delivery of intact neurotrophic factor is substantiated by the demonstration that activated pAkt was significantly upregulated in the CNS, indicative that the protein bound to its receptor and resulted in downstream alterations in prosurvival pathways.
Studies have demonstrated CNS neurotrophic effects after intranasal administration of IGF-1, EPO, and NGF. IGF-1 activates prosurvival signaling pathways (Thorne et al., 2004), and intranasal IGF-1 administration after experimentally induced middle cerebral artery occlusion (MCAO) in rats results in a significant reduction in infarct volume and improved somatosensory function (Liu et al., 2004). Intranasal EPO also reduces infarct volume and cell damage after a similar experimental focal ischemia (Yu et al., 2005). Intranasal administration of NGF reaches the brain, where it prevents neurodegeneration and loss of cholinergic markers as well as rescues recognition memory deficits in the anti-NGF transgenic mouse model of Alzheimer's disease (Capsoni, Giannotta, & Cattaneo, 2002; De Rosa et al., 2005). Our study suggests that BDNF, CNTF, and NT-4, whose levels in brain and spinal cord after intranasal delivery are the same or greater than seen with IGF-1 and NGF (Chen et al., 1998; Thorne et al., 2004) have the potential to show neuroprotective effects when used in models of CNS injury and disease.
The blood–brain barrier is a well-known barrier to entry of drugs into the brain parenchyma (Poduslo & Curran, 1996), and intranasal delivery circumvents this barrier. By targeting drugs directly to the CNS, their potential systemic toxicity is decreased and their potential tissue-specific concentrations are increased (Misra et al., 2003). The two principle routes of drug delivery directly from the nasal mucosa to the CNS are along the olfactory and trigeminal neural pathways (Illum, 2004; Thorne et al., 2004; 2008; Dhanda et al., 2005). Although transport of some molecules may occur via the relatively slow intracellular axonal transport mechanism from the olfactory epithelium to the olfactory bulb (Thorne et al., 1995), most interest has focused on the nonsaturable extracellular or paracellular transport that carries a wide variety of molecules from the nasal mucosa to the CSF, brain, and spinal cord. With this mode of delivery, uptake into the CNS can occur within minutes (Born et al., 2002; Thorne et al., 2004; Zhang et al., 2006). Despite the obvious anatomical differences between rodents and man, both nonhuman primate and human studies support the potential of this method for drug delivery of a variety of neuropeptides and protein therapeutics (Pietrowsky et al., 1996; Kern et al., 1999; Reger et al., 2006; 2008, Benedict et al., 2007, Thorne et al., 2008). Neuropeptide transport to the CNS after intranasal delivery was seen in human volunteers by direct sampling of CSF (Born et al., 2002). The direct intranasal pathway from the nasal mucosa to the CNS holds promise for clinical applications.
The intranasal dose of a neurotrophic factor needed to rescue neurons from injury-induced death is very difficult to determine. One can estimate a potential dose range that might prevent neuronal death in a given species based on a variety of concentrations of other molecules having a similar receptor Kd in that species or the Kd of the neurotrophin for its receptor and the known pharmacokinetic profile for the neurotrophin in that species. For example, 0.48 nmol (12.72 μg) NGF (administered once every 2 days as 48 μL of 10 μM NGF solution) was demonstrated to reduce brain neurodegeneration in the transgenic mouse model of Alzheimer's disease (Capsoni, Giannotta, & Cattaneo, 2002), while 9.86 nmol (75 μg) IGF-1 administered 10 min, 24 h, and 48 h after MCAO reduced infarct size in a rat MCAO stroke model (Liu et al., 2001). As little as 157 nmol (910 μg or 20 IU) of intranasal insulin improved memory, attention, and functioning in patients in early stages of Alzheimer's disease when administered twice a day for 21 days (Reger et al., 2008). Because delivery is believed to occur from the nasal mucosa to the CNS and does not require absorption into the general circulation with subsequent delivery across the blood–brain barrier, it should not be assumed that the effective dose range is a direct function of body weight.
The cellular machinery for action of these neurotrophic factors is present, as many neurons express receptors for these neuroprotective compounds, and the amount of factor reaching the CNS should be sufficient to rescue neurons based on the literature. BDNF receptors are expressed on many neurons, including cerebellum, spinal cord, hippocampus and cerebral cortex (Kokaia et al., 1993), and in animal models BDNF is neuroprotective for a large number of neuronal types (e.g., Hyman et al., 1991; Yan, Elliott, & Snider, 1992; Widmer, Knusel, & Hefti, 1993). In vitro, concentrations of BDNF between 10 and 50 ng/mL enhances neuronal survival (Lindholm et al., 1993; Lefebvre et al., 1994); direct injections of 0.6–4.5μg BDNF into the brain also has a beneficial effect on neuronal survival (Widmer, Knusel, & Hefti, 1993; Tsukahara et al., 1994). Intranasal delivery of 70 μg BDNF results in concentrations within the frontal cortex, hippocampus, and cervical spinal cord at 60 min of 10.7 ng/mL, 12.1 ng/mL, and 18.1 ng/mL, respectively (Table 2). These concentrations are within the range consistent with those used in vitro to promote neuronal survival. The CNTF receptor also is expressed on many CNS neurons (Lee, Hofmann, & Kirsch, 1997), and it supports neuronal survival and function in a number of diseases and types of injury (Clatterbuck, Price, & Koliatsos, 1993; Anderson et al., 1996). Based on in vitro studies, concentrations of CNTF needed to promote neuronal survival are in the 30–40 ng/mL range (Skaper et al., 1992; Magal et al., 1993). Although the average nanogram level of intranasal CNTF delivered to the brain and cervical spinal cord was 13.31 ng/mL of tissue, the elevation of prosurvival pathways (Figure 5) demonstrates that CNTF at these levels binds to its receptor and is functional. CNTF is particularly attractive as its effects on the CNS can be particularly long-lasting (Fischer et al., 2004). Ongoing studies are assessing its neuroprotective effects in injury models.
Increased concentrations of CNTF compared to BDNF in the CNS tissues were seen. Several factors may play a role in this difference. As the BDNF receptor, trkB, is highly expressed in the olfactory epithelium, less BDNF may be available for transport (Deckner et al., 1993). In addition, the method used for radio-iodination of CNTF differed from that used for other factors. Lactoperoxidase-catalyzed iodination of proteins results in milder labeling than the harsh oxidizing agent employed in the chloramine-T iodination method used for CNTF. This can result in lower specific activity of the labeled protein (Samuelson, 2001; Wajchenberg et al., 1978). However, the chloramine-T method also results in greater iodination, which may have contributed to the larger distribution values detected in CNTF-treated tissue.
EPO is a hormone involved in regulation of erythropoiesis, but recent laboratory studies have demonstrated that it has potent neuroprotective functions. The EPO receptor is expressed on many neurons throughout the CNS, but it is particularly enriched in cortex and hippocampus (Brines et al., 2000). Ischemia results in EPO receptor upregulation in the brain, suggesting that exogenously added EPO might have therapeutic potential for this type of brain injury (Siren et al., 2001). EPO can reduce neuronal death after experimentally induced ischemic injuries (Sakanaka et al., 1998), and also exerts an anti-inflammatory effect in the CNS (Agnello et al., 2002; Diem et al., 2005). Low levels of EPO, in contrast to CNTF and BDNF, are able to cross the blood–brain barrier (Brines et al., 2000). Systemic doses in animal experiments range up to 5000 units/kg, which translates into an approximate blood level of 40 ng/mL and CSF EPO levels of 1000 mUnits/mL (which is ~8.4 ng/mL) (Brines et al., 2000). However, these systemic doses are quite large and result in significant increases in blood volume (Agnello et al., 2002). In vitro studies show increased neuronal survival at EPO concentrations of 10–84 ng/mL (Digicaylioglu et al., 2004; Danielyan et al., 2005). Intranasal administration of EPO in rats protected neurons against focal cerebral ischemia, but brain levels in this study were not determined (Yu et al., 2005). We demonstrate that between 6.5 and 160.3 ng/mL EPO reaches the CNS of adult rats after intranasal delivery. Thus, intranasal EPO appears promising as a neuroprotective agent. EPO is already approved for use in humans by the Food and Drug Administration for treatment of various forms of anemia, and the transition to a human trial may be easier to accomplish as toxicity and other needed data are already known for this factor.
NT-4 has been shown to increase neuronal survival after injury (Ip et al., 1993); it also protects the brain from neuronal death due to stroke (Endres et al., 2003). In vitro studies show increased neuronal survival at drug levels of 10–50 ng/mL (Ip et al., 1993; Rabacchi et al., 1999), and we show that similar levels are seen in the CNS after intranasal delivery. NT-4, like BDNF, binds primarily to the trkB receptor, although NT-4 binds with lower affinity (Sadick et al., 1997). This may explain why NT-4 transport was higher than BDNF.
Not only do nanogram levels of the four neurotrophic factors reach the CNS, but they cause a functional response in prosurvival pathways. Numerous in vitro studies show that BDNF protects neurons after injury by activating intracellular signaling cascades. Binding of BDNF to its receptor activates the phosphatidylinositol 3-kinase/Akt (PI3-K/Akt) pathway; neuroprotective effects of BDNF are eliminated in the presence of inhibitors of this pathway (Sun et al., 2008). CNTF also increases neuronal survival after injury or in disease by specific activation of pAkt, causing downstream effects on pro-survival and antiapoptotic signaling molecules such as Bcl-xl (Ikeda et al., 2004; Azadi et al., 2007). Elevation by BDNF and CNTF of the pAkt prosurvival pathway in both frontal and occipital cortex demonstrates that sufficient drug concentrations for a functional effect were obtained.
The potential therapeutic use of these factors to treat CNS and spinal cord injury and/or disease is supported by many studies. Unfortunately, the adult CNS generally expresses only low levels of these neuroprotective factors. While they upregulate them after injury alone, the injured tissue cannot maintain sufficient levels to prevent neuronal death (Ikeda et al., 2004; Azadi et al., 2007). Viral vector gene transfer or direct injection of these factors increase neuronal survival after various forms of injury (Weise et al., 2000). However, the clinical use of brain infusion, direct brain injection, or viral vector upregulation of these neurotrophic factors is not attractive as a treatment alternative for patients.
Despite differences in anatomy between small rodents and man, there is increasing evidence that intranasal drug delivery, of even large proteins, results in significant delivery to the CNS and with appropriate dosing, may result in therapeutic effects (Reger et al., 2006; 2008). We demonstrated high concentrations of delivered neurotrophic factors in the hippocampus, cerebral cortex, and cervical spinal cord. Most forms of traumatic brain and spinal cord injury have limited therapies to enhance functional recovery. One of the main problems with traumatic and ischemic brain injury is the secondary wave of neuronal death caused by the death of the neurons directly injured, compounding the functional loss from the initial injury (Ferrer & Planas, 2003). This opens up a window of opportunity where efficient delivery of neuroprotective agents could potentially prevent this large and prolonged secondary wave of neuronal death and the associated loss of function.
The advantages of intranasal drug delivery, administered as nose drops or nasal spray, are (1) administration is easy and does not require significant medical training, (2) administration of drug is noninvasive, (3) delivery to the CNS is relatively rapid, (4) dosages are repeatable, (5) drugs can be packaged in a portable form, (6) no modification of the drug is required, and (7) systemic exposure is minimized. Currently, there are no viable treatments available for patients with traumatic and ischemic injury to the CNS; thus the clinical potential of the intranasal drug delivery method is quite significant.
Acknowledgments
Declaration of interest
This work was supported by EY11374 from the National Eye Institute, Prevent Blindness America, the Glaucoma Foundation, the Minnesota Lions and Lionesses, the Lew Wasserman Mid-Career Merit Award from Research to Prevent Blindness (RPB), and an unrestricted grant to the Department of Ophthalmology from RPB Inc.
Financial Disclosures: None: ARAB, MSL, LRH, AAM, WHF, LKM.
References
- Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–134. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Panayotatos N, Corcoran TL, Lindsay RM, Wiegand SJ. Ciliary neurotrophic factor protects striatal output neurons in an animal model of Huntington disease. Proc Natl Acad Sci USA. 1996;93:7346–7351. doi: 10.1073/pnas.93.14.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Azadi S, Johnson LE, Paquet-Durand F, Perez MTR, Zhang Y, Ekstrom PAR, van Veen T. CNTF + BDNF treatment and neuroprotective pathways in the rd1 mouse retina. Brain Res. 2007;1129:116–129. doi: 10.1016/j.brainres.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Urshansky N, Karni A. Low and dysregulated BDNF-secretion from immune cells of MS patients is related to reduced neuroprotection. J Neuroimmunol. 2008;195:186–193. doi: 10.1016/j.jneuroim.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- Blits B, Oudega M, Boer GJ, Barlett Bunge M, Verhaagen J. Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience. 2003;118:271–281. doi: 10.1016/s0306-4522(02)00970-3. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan SS, Angello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Giannotta S, Cattaneo A. Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc Natl Acad Sci USA. 2002;99:12432–12437. doi: 10.1073/pnas.192442999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey WH., II Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis. 1998;1:35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- Chu K, Jung KH, Lee ST, Kim JH, Kang KM, Kim HK, Lim JS, Park HK, Kim M, Lee SK, Roh JK. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia. 2008;49:1723–1732. doi: 10.1111/j.1528-1167.2008.01644.x. [DOI] [PubMed] [Google Scholar]
- Clatterbuck RE, Price DL, Koliatsos VE. Ciliary neurotrophic factor prevents retrograde neuronal death in the adult central nervous system. Proc Natl Acad Sci USA. 1993;90:2222–2226. doi: 10.1073/pnas.90.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielyan L, Mueller L, Proksch B, Kabisch D, Weller M, Wiesinger H, Buniatian GH, Gleiter CH. Similar protective effects of BX-123 and erythropoietin on survival of neural cells and generation of neurons upon hypoxic injury. Eur J Cell Biol. 2005;84:907–913. doi: 10.1016/j.ejcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Deckner ML, Frisen J, Verge VM, Hokfelt T, Risling M. Localization of neurotrophin receptors in olfactory epithelium and bulb. Neuroreport. 1993;13:301–304. doi: 10.1097/00001756-199312000-00030. [DOI] [PubMed] [Google Scholar]
- De Rosa R, Garcia A, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A. Intranasal administration of NGF rescues recognition memory deficits in anti-NGF transgenic mice. Proc Natl Acad Sci USA. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanda DS, Frey WH, II, Leopold D, Kompella UB. Nose-to-brain delivery: Approaches for drug deposition in the human olfactory epithelium. Drug Delivery Technol. 2005;5:64–72. [Google Scholar]
- Diem R, Sattler MB, Merkler D, Demmer I, Maier K, Stadelmann C, Ehrenreich H, Bahr M. Combined therapy with methylprednisolone and erythropoietin in a model of multiple sclerosis. Brain. 2005;128:375–385. doi: 10.1093/brain/awh365. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor 1. Proc Natl Acad Sci USA. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Cain CK, Greco C, Saydoff JA, Hu ZY, Liu H, Lindner MD. Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington's disease. Cell Transplant. 1997;6:249–266. doi: 10.1177/096368979700600308. [DOI] [PubMed] [Google Scholar]
- Endres M, Fan G, Hirt L, Jaenisch R. Stroke damage in mice after knocking the neurotrophin-4 gene into the brain-derived neurotrophic factor locus. J Cereb Blood Flow Metab. 2003;23:150–153. doi: 10.1097/01.WCB.0000043949.67811.C6. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, Tuor UI, Glazner G, Hanson LR, Frey WH, II, Toth C. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131:3311–3334. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH, II, Toth C. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes. 2009;58:934–945. doi: 10.2337/db08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Frey WH, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA, Rahman YE. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Born J, Fehm HL, Kern W. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol Behav. 2004;30:55–64. doi: 10.1016/j.physbeh.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolis brain insulin signaling. Int J Obes (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Tatsuno T, Noguchi H, Nakayama C. Ciliary neurotrophic factor protects rat retina cells in vitro and in vivo via PI3 kinase. Curr Eye Res. 2004;29:349–355. doi: 10.1080/02713680490516279. [DOI] [PubMed] [Google Scholar]
- Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci USA. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Hofmann HD, Kirsch M. Expression of ciliary neurotrophic factor receptor-alpha messenger RNA in neonatal and adult rat brain: an in situ hybridization study. Neuroscience. 1997;68:979–990. doi: 10.1016/s0306-4522(96)00476-9. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moghadass M, Van de Water TR, Moonen G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. NeuroReport. 1994;5:865–868. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Dechant G, Heisenberg CP, Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993;5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Hanson LR, Frey WH., II The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13:16–23. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, DeFor T, Frey WH., II Noninvasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett. 2001;308:91–94. doi: 10.1016/s0304-3940(01)01982-6. [DOI] [PubMed] [Google Scholar]
- Magal E, Louis JC, Oudega M, Varon S. CNTF promotes the survival of neonatal rat corticospinal neurons in vitro. NeuroReport. 1993;4:779–782. doi: 10.1097/00001756-199306000-00046. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Gage FH, Clevenger DG, Spratt SK, Snyder RO, Leff SE. Nerve growth factor expressed in the medial septum following in vivo gene delivery using a recombinant adeno-associated vector protects cholinergic neurons from fimbria-fornix lesion-induced degeneration. Exp Neurol. 1999;155:59–64. doi: 10.1006/exnr.1998.6961. [DOI] [PubMed] [Google Scholar]
- Merkus P, Guchelaar HJ, Bosch A, Merkus FWHM. Direct access of drugs to the human brain after intranasal drug administration? Neurology. 2003;60:1669–1671. doi: 10.1212/01.wnl.0000067993.60735.77. [DOI] [PubMed] [Google Scholar]
- Merkus FWHM, van den Berg MP. Can nasal drug delivery by-pass the blood-brain barrier? Drugs RD. 2007;8:133–144. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharmaceut Sci. 2003;6:252–273. [PubMed] [Google Scholar]
- Nowis D, Legat M, Bil J, Kurzaj Z, Issat T, Stoklosa T, Mioduszewska B, Kaczmarek L, Jakobisiak M, Golab J. Erythropoietin reduces cisplatin-induced neurotoxicity without impairment of cytotoxic effects against tumor cells. Int J Oncol. 2007;31:1547–1552. doi: 10.3892/ijo.31.6.1547. [DOI] [PubMed] [Google Scholar]
- Park K, Luo JM, Hisheh S, Harvey AR, Cui Q. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci. 2004;24:10806–10815. doi: 10.1523/JNEUROSCI.3532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrowsky R, Struben C, Molle M, Fehm HL, Born J. Brain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose-to-brain pathway for peptide effects in human. Biol Psychiat. 1996;39:332–340. doi: 10.1016/0006-3223(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Qu HY, Zhang T, Li XL, Zhou JP, Zhao BQ, Li Q, Sun MJ. Transducible P11-CNTF rescues the learning and memory impairments induced by amyloid-beta peptide in mice. Eur J Pharmacol. 2008;594:93–100. doi: 10.1016/j.ejphar.2008.06.109. [DOI] [PubMed] [Google Scholar]
- Rabbacchi SA, Kruk B, Hamilton J, Carney C, Hoffman JR, Meyer SL, Springer JE, Baird DH. BDNF and NT4/5 promote survival and neurite outgrowth of pontocerebellar mossy fiber neurons. J Neurobiol. 1999;40:254–269. [PubMed] [Google Scholar]
- Rami A, Bechmann I, Stehle JH. Exploiting endogenous anti-apoptotic proteins for novel therapeutic strategies in cerebral ischemia. Prog Neurobiol. 2008;85:273–296. doi: 10.1016/j.pneurobio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH, II, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Royo NC, Conte V, Saatman KE, Shimizu S, Belfield CM, Soltesz KM, Davis JE, Fujimoto ST, McIntosh TK. Hippocampal vulnerability following traumatic brain injury: a potential role for neurotrophin-4/5 in pyramidal cell neuroprotection. Eur J Neurosci. 2006;23:1089–1102. doi: 10.1111/j.1460-9568.2006.04642.x. [DOI] [PubMed] [Google Scholar]
- Sadick MD, Galloway A, Shelton D, Hale V, Weck S, Anicetti V, Wong WL. Analysis of neurotrophin/receptor interactions with a gD-flat-modified quantitative kinase receptor activation (gD.KIRA) enzyme-linked immunosorbent assay. Exp Cell Res. 1997;234:354–361. doi: 10.1006/excr.1997.3614. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson LE. Iodination of soluble and membrane-bound proteins. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0811s11. Chapter 8: Unit 8.11. [DOI] [PubMed] [Google Scholar]
- Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Negro A, Dal Toso R, Facci L. Recombinant human ciliary neurotrophic factor alters the threshold of hippocampal pyramidal neuron sensitivity to excitotoxin damage: synergistic effects of monosialogangliosides. J Neurosci Res. 1992;33:330–337. doi: 10.1002/jnr.490330217. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhou H, Luo X, Li S, Yu D, Hua J, Mu D, Mao M. Neuroprotection of brain-derived neurotrophic factor against hypoxic injury in vitro requires activation of extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Int J Dev Neurosci. 2008;26:363–370. doi: 10.1016/j.ijdevneu.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaqa E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer's disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nature Neurosci. 2002;5:1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Emory CR, Ala TA, Frey WH. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Frey WH. Delivery of neurotrophic factors to the brain. Clin Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH. Delivery of interferon-b to the monkey nervous system following intranasal administration. Neuroscience. 2008;162:785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of IGF-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Yonekawa Y, Tanaka K, Ohara O, Watanabe S, Kimura T, Nishijima T, Taniguchi T. The role of brain-derived neurotrophic factor in transient forebrain ischemia in the rat brain. Neurosurgery. 1994;34:323–331. doi: 10.1227/00006123-199402000-00016. [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden P, Brereton H, Coster DJ, Williams KA. Stability of housekeeping gene expression in the rat retina during exposure to cyclic hyperoxia. Mol Vis. 2007;13:1508–1515. [PubMed] [Google Scholar]
- Wajchenberg B, Pinto H, Torres de Toledo e Souza I, Lerario AC, Pieroni RR. Preparation of iodine-125-labeled insulin for radioimmunoassay: Comparison of lactoperoxidase and chloramine-T iodination. J Nucl Med. 1978;19:900–905. [PubMed] [Google Scholar]
- Weise J, Isenmann S, Klocker N, Kugler S, Hirsch S, Gravel C, Bahr M. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- Widmer HR, Knusel B, Hefti F. BDNF protection of basal forebrain cholinergic neurons after axotomy: complete protection of p75NGFR-positive cells. NeuroReport. 1993;4:363–366. doi: 10.1097/00001756-199304000-00005. [DOI] [PubMed] [Google Scholar]
- Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, Gage FH. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci USA. 1986;83:9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Hasegawa M, Kametani S, Ito S. Nose-to-brain delivery of TS-002, prostaglandin D2 analogue. J Drug Target. 2007;15:59–66. doi: 10.1080/10611860601029496. [DOI] [PubMed] [Google Scholar]
- Yan Q, Elliott J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360:753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387:5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Zhang QZ, Zha LS, Zhang Y, Jiang WM, Lu W, Shi ZQ, Jiang XG, Fu SK. The brain targeting efficiency following nasally applied MPED-PLA nanoparticles in rats. J Drug Target. 2006;14:281–290. doi: 10.1080/10611860600721051. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]