Van Beest et al. [1] performed a post hoc analysis of 53 patients with severe sepsis or septic shock to investigate the interchangeability of mixed and central venous-to-arterial carbon dioxide (CO2) differences (mvaCO2gap and cvaCO2gap, respectively) and the relation between the cvaCO2gap (“pCO2gap” or the “gap”), cardiac index (CI), and outcome. The authors observed a strong agreement between pCO2 measured from either mixed venous or central venous sites with relatively small limits of agreement. The authors claim that combining ScvO2 values, as easily obtained from a central venous catheter, as a surrogate for global tissue hypoxia, and pCO2gap as a surrogate for CI, obtained from the same central venous catheter, may be useful in assessing cardiovascular state during resuscitation in critically ill patients. We cannot agree more and propose thereafter a tentative “ScvO2-cvaCO2gap-guided protocol” (Fig. 1). Cuschieri et al. [2] previously demonstrated in a mixed population of critically ill patients that the relationships between the mvaCO2gap or the cvaCO2gap and the CI were equivalent. Since central venous blood is readily available from a central venous catheter, whereas mixed venous blood requires a pulmonary artery catheter, the cvaCO2gap, as an easily available clinical monitoring tool, is attractive. At ICU admission, 24 patients in the van Beest et al. study had a pCO2gap greater than 0.8 kPa (or 6 mmHg). Persistence of such a large pCO2gap after 24 h of treatment was predictive of higher mortality.
Fig. 1.
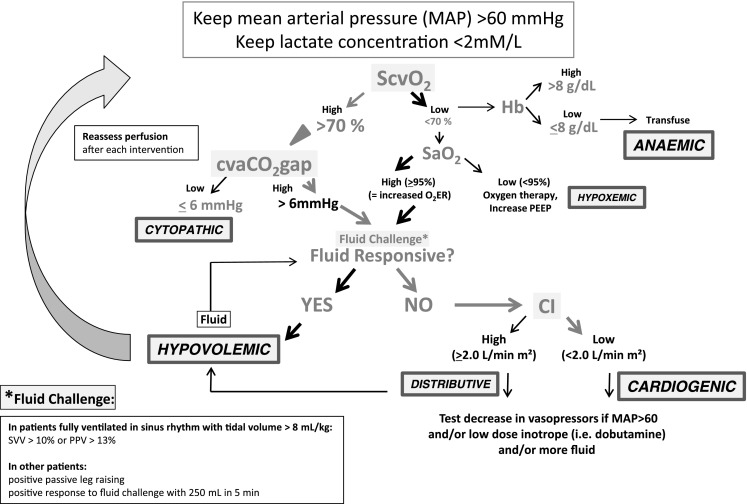
The ScvO2-cvaCO2gap-guided protocol. ScvO 2 venous central O2 saturation, cvCO 2 gap central venous-to-arterial pCO2 difference, SaO 2 arterial O2 saturation, CI cardiac index, SVV stroke volume variability, PPV pulse pressure variability
These data are in line with results of Vallée et al. [3] who prospectively tested this hypothesis in 56 septic shock patients resuscitated to an ScvO2 greater than 70 % (according to the Rivers’ study results [4]). They found that patients who still had altered tissue perfusion (assessed by serum lactate levels greater than 2 mmol L−1) in spite of a normalized ScvO2 displayed a large cvaCO2gap (greater than 6 mmHg). CO2 is the end product of aerobic metabolism and its concentration in the venous blood reflects the global tissue blood flow relative to metabolic demand. Since CO2 is about 20 times more soluble than O2 the likelihood of it diffusing out of ischemic tissues and into the venous effluent is great, making it a very sensitive marker of hypoperfusion. Thus, in situations where an O2 diffusion barrier exists (resulting from non-functional and obliterated capillaries), “masking” poor O2 extraction (O2ER) and increased tissue O2 debt, CO2 still diffuses to the venous effluent, “unmasking” the low perfusion state for the clinician when venous-to-arterial CO2 difference is evaluated. Consistently Vallée et al. [3] evidenced that patients with high cvaCO2gap values had lower lactate clearance and CI values, and presented a significant lower decrease in Sepsis-related Organ Failure Assessment score than patients with a low cvaCO2gap. Thus, the cvaCO2gap represents a useful complementary tool to identify patients who remain inadequately resuscitated when the 70 % ScvO2 threshold value has been reached.
The obvious limitation of ScvO2 is therefore that normal/high values cannot discriminate whether delivery is adequate or in excess to demand. High ScvO2 profiles have even been shown to be related to elevated blood lactate concentration and poor survival rates [5]. Although ScvO2 may thus not miss any global oxygen delivery (DO2) disorder, it may remain “blind” to local perfusion disorders, which abound in sepsis because of impaired microcirculation. Under conditions where O2 uptake (VO2) does not meet O2 demand, tissue dysoxia occurs, leading to organ failure and death. The crucial point here is that these tissues might remain however accessible to conventional therapeutic hemodynamic management (inotropes and fluid infusion).
Whether the resultant effect on pCO2 gap depends in principle on the flow state or anaerobic CO2 production was tested by Vallet et al. [6] in an experimental model of isolated limb in which ischemic hypoxia (IH) and hypoxic hypoxia (HH) were compared. The authors demonstrated that when DO2 was reduced beyond its critical threshold in IH (dysoxia), this was associated with an increased limb venous-to-arterial pCO2gap [6]. Conversely, in HH, pCO2gap did not increase in spite of a marked VO2 and VCO2 reduction, clearly evidencing the gap as a marker of adequacy of venous blood flow to remove CO2 produced rather than a marker of tissue hypoxia or dysoxia.
In conclusion, determining the gap during resuscitation of critically ill patients is useful when deciding when to stop resuscitation despite persistent evidence of organ ischemia and an ScvO2 of greater than 70 % (see Fig. 1, Table 1). All forms of circulatory stress are potentially associated with hyperlactatemia, but hyperlactatemia is not a discriminatory factor in defining the cause of that stress. A goal of a gap lower than 6 could be a useful complementary tool to evaluate the adequacy of blood flow to global metabolic demand. In this regard it can help to titrate inotropes in order to adapt DO2 to VCO2, or to choose between hemoglobin correction or fluid/inotrope infusion. Whatever way you use it or like it, from mixed or central venous, in patients with septic shock, please “mind the gap”!
Table 1.
Lactate-ScvO2-cvaCO2gap as shock diagnostic tools
| Shock type | Lactate | O2ER | ScvO2 | cvaCO2gap |
|---|---|---|---|---|
| Cardiogenic hypovolemic | HI | HI | LO | HI |
| Anemic hypoxemic | HI | HI | LO | LO |
| Distributive | HI | LO | HI | HI |
| Cytopathic | HI | LO | HI | LO |
ScvO 2 venous central O2 saturation, cvCO 2 gap central venous-to-arterial pCO2 difference, O 2 ER O2 extraction ratio, HI high, LO low
Conflicts of interest
None.
References
- 1.Van Beest PA, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial pCO2 difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013;39:1034–1039. doi: 10.1007/s00134-013-2888-x. [DOI] [PubMed] [Google Scholar]
- 2.Cuschieri J, Rivers EP, Donnino MW, Katilius M, Jacobsen G, Nguyen HB, Pamukov N, Horst HM. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med. 2005;31:818–822. doi: 10.1007/s00134-005-2602-8. [DOI] [PubMed] [Google Scholar]
- 3.Vallée F, Vallet B, Mathe O, Parraguette J, Mari A, Silva S, Samii K, Fourcade O, Genestal M. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med. 2008;34:2218–2225. doi: 10.1007/s00134-008-1199-0. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Heffner AC, Kline JA, Jones AE, Emergency Medicine Shock Research Network (EMSHOCKNET) Prognostic value and agreement of achieving lactate clearance or central venous oxygen saturation goals during early sepsis resuscitation. Acad Emerg Med. 2012;19:252–258. doi: 10.1111/j.1553-2712.2012.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallet B, Teboul JL, Cain S, Curtis S. Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia. J Appl Physiol. 2000;89:1317–1321. doi: 10.1152/jappl.2000.89.4.1317. [DOI] [PubMed] [Google Scholar]


