Abstract
We tested whether visual processing impairments in aging and Alzheimer's disease (AD) reflect uniform posterior cortical decline, or independent disorders of visual processing for reading and navigation. Young and older normal controls were compared to early AD patients using psychophysical measures of visual word and motion processing. We find elevated perceptual thresholds for letters and word discrimination from young normal controls, to older normal controls, to early AD patients. Across subject groups, visual motion processing showed a similar pattern of increasing thresholds, with the greatest impact on radial pattern motion perception. Combined analyses show that letter, word, and motion processing impairments are independent of each other. Aging and AD may be accompanied by independent impairments of visual processing for reading and navigation. This suggests separate underlying disorders and highlights the need for comprehensive evaluations to detect early deficits.
Keywords: Aging, Alzheimer's disease, language, motion, vision
INTRODUCTION
Early Alzheimer's disease (AD) might be expected to have similar patterns of effects across neural systems; or might reflect the idiosyncratic strengths and weaknesses of each patient, creating distinct patterns of resilience and vulnerability to impairment. The dorsal and ventral visual cortical subsystems [1] present an opportunity to assess the homogeneity of AD pathophysiology in anatomically adjacent, functionally linked, brain areas processing visual cues for reading and navigation. Studying early AD patients enables the use of behavioral tasks that allow the characterization of their selective impairments.
Visual word processing impairments in early AD may be recognized as changes in reading habits, or may insidiously detract from the longevity and quality of life [2, 3]. Likewise, visual motion processing impairments may be seen as overt visuo-spatial disorientation, or more subtle declines in navigation that undermine driving safety and independent living [4].
The visual processing of written language requires the combined effects of visuo-spatial and lexical mechanisms. AD impairs the visuo-spatial processing of the shapes that define letters [5]. Perhaps more importantly, AD also impairs the processing of the orthographic shape cues that characterize words and promote word recognition [6].
Likewise, the visual processing of patterned motion combines movement direction and pattern integration mechanisms. AD impairs the processing of uniform directional arrays of planar motion [7, 8]. In addition, AD impairs the processing of the radial patterns of optic flow that inform the observer about the direction of self-movement [9, 10].
A critical question about early AD is whether visual cortical processing impairments reflect: a) aspects of a general decline in cortical information processing, or b) distinct syndromes in which AD first attacks different cortical subsystems in different patients. Here we compare the predictions of those hypotheses by focusing on whether visual cortical systems for reading and navigation are uniformly or independently impaired. We tested these alternatives by characterizing aging and AD related impairments in the visual processing of word and motion stimuli, recognizing that there is much more to language and navigation that is beyond the scope of this work.
METHODS
Subject groups
We studies 55 subjects in three groups: 18 young normal controls (YNCs), 18 older normal controls (ONCs), and 19 patients with probable AD (EAD) (Table 1). All subjects were free of other neurologic, ophthalmologic, or psychiatric illnesses with corrected binocular visual acuity of 20/40 or better (Snellen chart) and with contrast sensitivity profiles in the normal range (0.5–18 cycles/°, VisTech Consultants, Inc., Dayton, OH). All subjects were native speakers of American English. Two of the YNCs were bilingual.
Table 1.
Attributes of each subject group showing sample size, age, and the results of neuropsychological tests. Two-way ANOVA revealed significant group differences in all parameters (multivariate F18, 43 = 2354.29, p < 0.001; univariate p values in right column). Post-hoc tests of group differences for each test (THSD, p < 0.05) define the frames that enclose groups that were not different
| Test mn (se) | Comparisons of subject groups subject groups (n, % male) |
p value | ||
|---|---|---|---|---|
| YNC (18, 17%) | ONC (18, 44%) | EAD (19, 53%) | ||
| Age | 21.94 (1.04) | 74.28 (1.55) | 76.37 (1.65) | <0.001 |
| Mini-mental | 29.67 (0.14) | 28.72 (0.61) | 25.63 (0.72) | <0.001 |
| Road map | 30.11 (0.52) | 28.72 (0.94) | 25.37 (1.22) | =0.003 |
| Line orientation | 26.78 (0.56) | 25.94 (0.56) | 21.83 (1.49) | =0.002 |
| Figural memory | 8.44 (0.34) | 6.89 (0.42) | 4.89 (0.39) | <0.001 |
| Verbal recall | 21.06 (0.53) | 17.56 (0.68) | 8.53 (1.16) | <0.001 |
| Delayed recall | 7.72 (0.11) | 6.83 (0.25) | 2.84 (0.45) | <0.001 |
| Animal naming | 25.56 (1.65) | 20.72 (1.51) | 12.47 (0.99) | <0.001 |
AD patients were recruited from the clinical programs of the University of Rochester with the diagnosis made by a neurologist in accordance with the National Institutes of Neurological Diseases and Stroke criteria [11]. These patients were considered to have EAD by their having Mini-Mental State Examination (MMSE) score >18 and recognized impairment for less than three years. ONCs recruited were mainly the spouses of AD patients. YNCs were recruited from the student population at the University of Rochester. All subjects read and signed a consent form prior to participation. All methods were approved by the University of Rochester Medical Center's Research Subject Review Board.
Subject testing
Neuropsychological testing
The MMSE was used in screening, inclusion requiring a score >18 [12]. The Money Road Map Test [13] assessed spatial orientation and sense of direction. The Judgment of Line Orientation Test, Form H [14] assessed basic visuo-spatial discrimination. The Figural Memory Test (Wechsler Memory Scale, Revised; WMS-R) [15] assessed visual recognition. Immediate and delayed verbal memory was assessed with the Verbal Paired Associates subtests of the WMS-R [15]. The Semantic Fluency Task (animal naming) [16] assessed semantic knowledge, memory, and executive function.
Psychophysical testing
Psychophysical testing was conducted with subjects seated in a dark enclosure facing a rear projection screen. A TV projector (Electrohome, Inc., Ontario, Canada) displayed animated sequences of white dots on a dark background created by the display computer. Letter and word test images spanned the central 20° × 10° of the visual field and motion test images spanned the central 60° × 40° of the visual field. An adjustable chin rest ensured consistent head alignment.
During stimulus presentation, subjects fixated a marker at the center of the screen. Fixation within the central 10° of the screen was continually monitored by infrared oculometry (ASL, Inc., Bedford, MA, USA). If eye position passed out of fixation window during stimulus presentation, the stimulus was extinguished, a tone sounded, and the next randomly selected trial was begun. A two button response box was placed in front of subjects to register task-related responses.
In these studies, static figures or moving patterns composed of white dots (0.1°, 2.69 cd/m2) were presented with a pseudo-random distribution of superimposed noise dots was used to obscure the stimuli; static noise dots with letter and word stimuli, randomly moving noise dots with visual motion stimuli. The number of noise dots in the entire stimulus array was specified by an adaptive staircase algorithm for determining psychophysical thresholds, the parameter estimation by sequential testing technique [17, 18]. After each test trial, a Weibull function [19] was fit to the data using a maximum likelihood method to determine the current best estimate of the stimulus coherence threshold that would yield 82.5% correct performance [20]. That estimate was used to specify the graded imposition of noise dots randomly positioned throughout the stimulus area to create the stimulus coherence in the next stimulus. Confidence interval measures determined when a reliable final threshold estimate was established to complete testing.
We presented all letter, word, and motion stimuli using comparable two alternative forced choice paradigms to focus on the elementary stages of ventral and dorsal stream cortical processing. Subjects who can discriminate between a letter versus a non-letter, word versus a non-word, or one type of motion versus another, in the presence of many noise dots will have a lower perceptual discrimination threshold than subjects who have a difficult time in the task. The density of noise dots across the stimulus area was kept uniform by spatial smoothing in every frame. All psychophysical experiments were controlled by the real-time experimental control system (REX) [21] running under a Unix operating system for PCs. REX controls stimulus presentation and monitors eye/head position as well as behavioral responses.
Letter discrimination task
The letter discrimination task assessed the subject's ability to distinguish normal English language letters from a block-wise random sequence of inverted letters, mirror-rotated letters, false fonts, or simple shapes (Fig. 1). Inverted and mirrored letters were used because they dissociate letter familiarity from their visuospatial orientation [22]. False fonts were used because they are unfamiliar but look like letters from a foreign language [23]. Simple shapes were used because they are readily recognized but have no linguistic meaning [24]. Each stimulus consisted of a left-right pair of two figures, one of which was a normal letter. During or after each stimulus presentation, subjects pressed a left or right-side button to indicate the side of the stimulus that contained the normal letter (Fig. 2A). Letters were chosen for the uniqueness with inversion (e.g., no Is) or mirror rotation (e.g., no As).
Fig. 1.
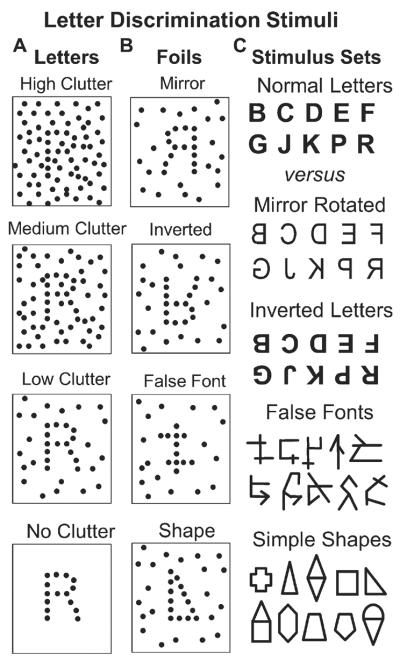
A fully interleaved sequence of letters and non-letters was presented to obtain static clutter discrimination thresholds for each type of non-letter. A) Letters formed by dots were presented with a continuously varied number of superimposed “clutter” dots that were randomly distributed across the area surrounding the letter; high to low clutter examples are at arbitrary levels of the continuously varying range of clutters presented in the stimuli. B) Four types of non-letter stimuli were presented with superimposed clutter dots: mirror image letters, inverted letters, false fonts, and simple shapes. C) Archetypes of the letter versus non-letter stimuli presented in the two-alternative forced choice letter discrimination task: Ten, graphically distinct, letters from the English alphabet, mirror rotated (horizontally inverted) versions of the normal letters, horizontally and then vertically inverted versions of the normal letters, false font figures that are graphically similar to letters, and simple shapes that are graphically similar to letters.
Fig. 2.
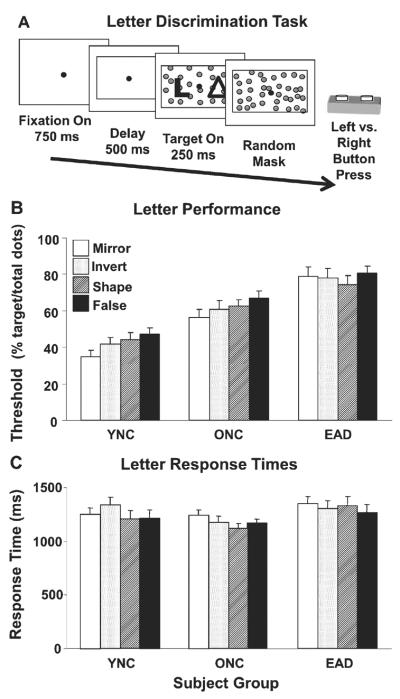
Letter discrimination assessed using stationary clutter coherence thresholds. A) The letter discrimination task consisted of a random sequence of paired figures, each pair consisting of a letter and one of four types of non-letter. Subjects pressed the left or right button corresponding to the side that contained the letter. The letters and non-letters were composed of dots, here shown as solid figures for clarity. All stimuli were presented with a varying number of superimposed randomly positioned noise dots to obtain letter discrimination thresholds. B) Letter discrimination thresholds for each subject group with each of the four types of non-letter stimuli. Group thresholds (mean±sem) are given as the percentage of all dots in the stimulus (target plus noise) that must form the target figures for the subjects to achieve 82.5% correct responses. The thresholds for YNCs are significantly lower than ONCs, which are significantly lower than EADs. C) Response time of subject groups (mean±se) for the four types of non-letter stimuli. Response times for YNCs and ONCs are not significantly different from each other, but both are significantly faster than EADs.
Word discrimination task
The word discrimination task assessed the subject's ability to distinguish common words from pseudo-words with varying amounts of visual noise (Fig. 3A). We varied the number of noise dots to adaptively determine the noise level at which the subject correctly identified which figure was the word in 82.5% of the trials. Test sessions began with up to three practice runs of ten trials. All subjects performed all 10 trials correctly by the third run. In this task, the push buttons were oriented nearer or further from the subject. In each trial, subjects pressed the nearer button if the real word was in the lower half of the display, the further button if the real word was in the upper half.
Fig. 3.
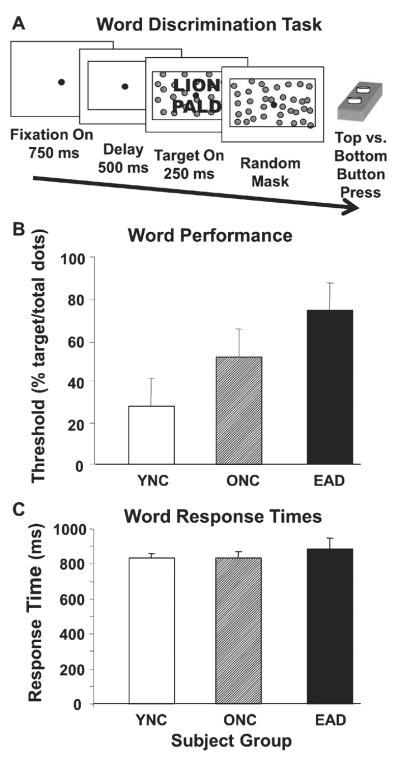
Word discrimination assessed using stationary clutter coherence thresholds. A) The word discrimination task consisted of a sequence of paired words and non-words. Subjects pressed the further or nearer of two buttons corresponding to the upper or lower position of the word. The words and non-words were composed of dots, here shown as solid figures for clarity. All stimuli were presented with a varying number of superimposed randomly positioned noise dots to obtain word discrimination thresholds. B) Word discrimination thresholds for each subject group are given as the percentage of the dots in the stimulus that are contained in the words and non-words. Thresholds for YNCs are significantly lower than ONCs, which are significantly lower than EADs. C) Response time of subject groups (mean±sem) for word versus non-word stimuli. Response times for YNCs, ONCs, and EADs are not significantly different. Axes labels are as in Fig. 2.
Visual motion discrimination task
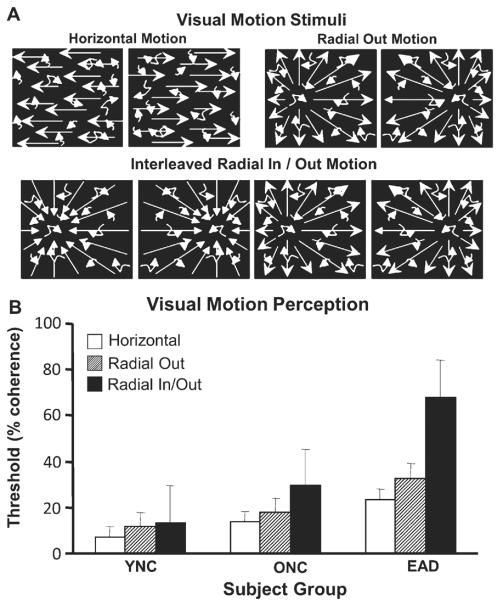
Three types of visual motion stimuli were used: horizontal, outward radial, and in/out radial. Subjects sat in the psychophysical enclosure while fixating the center of the screen ± 10° monitored by infrared oculometry during the rear projection of animated sequences of 500 moving white dots presented on a dark background at a 60 Hz frame rate. Subjects used a left/right oriented two-button response box to indicate choice responses in left/right two-alternative forced choice discrimination paradigm (Fig. 4A).
Fig. 4.
Motion discrimination assessed using random dot motion coherence thresholds. A) The motion discrimination task consisted of randomly positioned dots moving in a specified pattern: Horizontal motion to the left or right. Outward radial optic flow with a left or right sided focus of expansion. Interleaved inward or outward radial optic flow with left or right sided focus of contraction or expansion. All stimuli were presented with a varying number of superimposed randomly moving noise dots to obtain motion discrimination thresholds. Subjects pressed the left or right button corresponding to the side of the motion direction or focus of contraction or expansion. B) Visual motion discrimination thresholds for each subject group and each type of motion stimulus are given as the percentage of the dots that must be in the motion pattern to achieve 80% correct responses. Across all stimuli, thresholds for YNCs are significantly lower than ONCs, which are significantly lower than EADs. This is mainly attributable to the EADs having much larger thresholds for in/out radial optic flow.
Horizontal motion stimuli consisted of leftward or rightward moving dots requiring subjects to indicate the direction of motion. Outward radial motion stimuli consisted of dots moving in a radial pattern out from a focus of expansion on the horizontal meridian, 15° to the left or right of center. In/out radial motion consisted of dots moving in a radial pattern, outward from or inward toward a focus along the horizontal meridian, 15° to the left or right of center. These coherent motion patterns were intermixed with random dot motion, the percentage of coherently and randomly moving dots varied between trials for the determination of motion coherence thresholds. Individual dots were randomly assigned to coherent or random motion in each frame. All stimuli had the same dot density, luminance, contrast, and average dot speed.
RESULTS
Subject groups
The ONC and EAD groups were not different by age or gender composition; YNCs were younger and predominantly women. Neuropsychological testing confirmed subject group differences for all tests considered together (MANOVA F(18,43) = 2354.29, p < 0.001) or individually (follow-up ANOVAs' p's < 0.005). Post-hoc Tukey's Honestly Significant Differences (THSDs; p's < 0.05) revealed significant differences between the EADs and both the YNCs and ONCs for all tests, and with significant difference between all three groups for Figural Memory and Verbal Recall (Table 1). A series of psychophysical tests were conducted in the order presented, each test being conducted in a separate testing session to minimize fatigue effects in older subjects.
Letter discrimination
We used a letter discrimination task to measure our subjects' ability to distinguish between normal English language letters and letter-like figures (Fig. 1). Random dot clutter was superimposed on figures composed of ordered dots to determine the minimum percentage of dots in the target necessary for reliable letter discrimination, defined here as the percentage of the dots that must be in the figure for a subject to achieve 82.5% correct letter discrimination (Fig. 2A).
Letter discrimination thresholds were determined for each of the four non-letter stimulus types (Fig. 2B). Two-way ANOVA revealed a group effect (F(2,216) = 63.33, p < 0.001) with the thresholds of the YNCs being lower than ONCs, and ONCs lower than EADs (THSDs, p < 0.05). There was no significant effect of stimulus type (F(3,216) = 1.658, p = 0.177) and no significant group-by-stimulus interaction (F(6,216) = 0.522, p = 0.791).
Mean response times were determined for each of the four stimulus types in each subject group (Fig. 2C). As with thresholds, two-way ANOVA of response times revealed a significant effect of group (F(2,216) = 4.001, p = 0.020) without a significant difference between YNCs and ONCs, but both YNCs and ONCs are faster than EADs (THSDs, p < 0.05). There was no significant effect of which stimulus type was used (p = 0.481), and no significant group-by-stimulus interaction (p = 0.841). Using response time as a co-variate in ANCOVAs of letter discrimination thresholds did not alter the group comparisons (F(2,216) = 63.42, p < 0.001).
Word discrimination
We used a word discrimination task to measure our subjects' ability to distinguish between English language words and word-like groups of letters. As with letter discrimination, random dot clutter was superimposed on words composed of ordered dots to determine the minimum percentage of dots in the target necessary for 82.5% correct word discrimination (Fig. 3A).
Despite the greater complexity of word stimuli, our subjects are faster with words than with letters, possibly reflecting the greater linguistic content of the word stimuli. Mean word discrimination thresholds (Fig. 3B) were entered in to a one-way ANOVA that revealed a significant group effect (F(2,51) = 20.83, p < 0.001) with the thresholds of the YNCs being lower than ONCs, and ONCs being lower than EADs (THSDs, p < 0.05). Average response times (Fig. 3C) did not show a significant group effect (F(2,51) = 0.004, p = 0.996) and using response times as a covariate did not substantially alter group effects on thresholds (F(3,50) = 12.37, p < 0.001).
We used multiple linear regression to assess whether impaired letter discrimination accounts for impairments in word discrimination. Combining all four letter thresholds, and all three subject groups, reveals a modest relationship (R2 = 0.43, F(4,51) = 10.77, p < 0.001). This effect is driven by group differences; when the groups are considered separately, the relationship is marginal in EADs (R2 = 0.41, F(4,15) = 3.6, p = 0.041), and non-significant in YNCs (p = 0.11) or ONCs (p = 0.16). Thus, all groups show comparable thresholds for letters and words, with faster responses to words than letters.
Motion discrimination
We used motion coherence to determine visual discrimination thresholds for three types of motion stimuli (Fig. 4A): Left/right horizontal dot motion thresholds reflect the ability to discriminate the direction of dot motion. Left/right centered outward radial motion thresholds reflect pattern motion discrimination simulating left- or right- forward self-movement headings in optic flow. Left/right centered inward or outward radial motion thresholds reflect global pattern motion discrimination simulating left- or right, back- or forward self-movement headings in optic flow.
Mean motion coherence thresholds were determined for each of the three motion stimulus conditions in each subject group (Fig. 4B). We found significant effects of group (F(2,149) = 26.26, p < 0.001) and motion stimulus (F(2,149) = 13.88, p < 0.001). These effects can be considered secondary to a significant group-by-stimulus interaction (F(4,149) = 4.23, p = 0.003) from elevated radial in/out motion coherence thresholds in EADs: the EADs are different from the other two groups, and their radial in/out scores are different from those of the other two tests (THSDs, p < 0.05).
Linking words and motion
We combined letter, word, and motion processing thresholds to test for relationships between impairments in these domains. Bivariate correlations were not significant in YNCs. In contrast, ONCs and EADs showed similar patterns whether they were analyzed separately or as a combined older subjects group. These patterns are clearest in partial correlations controlling for age and MMSE: Letter thresholds are all positively correlated with each other (r2 = 0.69 to 0.75, dfs = 35, p's < 0.001) and negatively correlated with word thresholds (r2 = −0.54 to 0.63, dfs = 35, p's ≤ 0.002). In addition, there are several significant correlations between letter thresholds and language related neuropsychological test scores: immediate verbal recall with false font (r2 = −0.55, df = 35, p = 0.019) and inverted letter (r2 = −0.51, df = 35, p = 0.032) thresholds, delayed verbal recall with false font (r2 = −0.64, df = 35, p = 0.004), shape (r2 = −0.56, df = 35, p = 0.016), inverted letter (r2 = −0.57, df = 35, p = 0.014), and mirrored letter (r2 = −0.58, df = 35, p = 0.012) thresholds. In the visuospatial domain, there are significant correlations between outward radial and in/out radial thresholds (r2 = 0.37, df = 35, p = 0.037) and between horizontal motion thresholds and line orientation scores (r2 = 0.74, df = 35, p = 0.001) but motion thresholds are not correlated with letter or word thresholds. Animal naming scores are correlated with figural memory (r2 = 0.53, df = 35, p = 0.02) and road map (r2 = 0.54, df = 35, p = 0.018) scores. Finally, from the basic visual tests, better eye acuity was correlated with face recognition (r2 = −0.43, df = 35, p = 0.009) but not with other neuropsychological or any psychophysical measures.
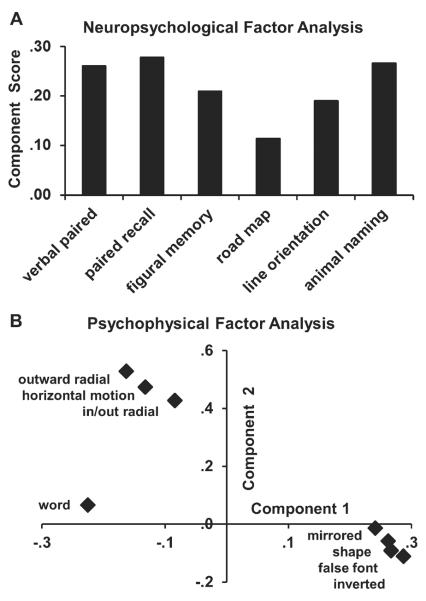
We focused on the relationships between test scores using factor analyses, recognizing the limits imposed by our small sample size. Guided by the bivariate correlations, we compared young and all older adult subjects (ONCs plus EADs). Parallel analyses were conducted for the neuropsychological and psychophysical tests, because of the very different structure of these tests and the need to limit the number of parameters relative to the number of subjects. Factor identification was based-on principal component analysis, selecting variables yielding eigenvalues >1, after varimax rotation with Kaiser normalization.
Neuropsychological tests yield a single component model that is dominated by verbal mediation: verbal memory and fluency (Fig. 5A). Psychophysical tests yield a two component model to explain 70% of the variance: Factor 1 loads all four letter thresholds with negative scores for word thresholds. Factor 2 loads all three motion thresholds (Fig. 5B). Thus, in this small sample, the impairments of aging and early AD suggest the lack of correlations between letter or word impairments and motion impairments that are seen as two independent factors in the psychophysical decline of these subjects.
Fig. 5.
Factor analyses of neuropsychological and psychophysical tests scores in older adult subjects. A) The six neuropsychological tests yielded a single component model that was dominated by the verbally mediated tests: verbal paired associates, delayed verbal recall, and animal naming. B) The eight psychophysical tests yielded a two component model with component 1 (abscissa) loading on the four letter discrimination test scores, with negative loading on word discrimination test scores, and component 2 loading on the three visual motion discrimination test scores.
DISCUSSION
Letter and word deficits
These studies extend previous findings of slower letter discrimination in AD [25] (Fig. 2). The loss of accuracy in our study might reflect post-stimulus masking by the random dots in EADs [26]. We expected that the high contrast of our white-on-black letters would benefit letter discrimination in EAD [27]. Considering both of these effects, we speculate that AD confers vulnerability to the disruption of perceptual signals regardless of signal strength. However, these analyses are not comprehensive as they do not consider non-visual influences on language and navigation or the corresponding roles of non-extrastriate cortical areas in those behaviors.
Word discrimination showed successive worsening in ONCs and EADs (Fig. 3) consistent with previous studies [28–30]. The increasing impairment of letter and word discrimination across these groups suggests that a cumulative effect of age and AD related impairments. However, we find that individual subjects' threshold for letters and words are negatively correlated: subjects with higher word discrimination thresholds have lower letter discrimination thresholds, impaired word processing with preserved letter processing. Thus, word processing impairments include effects that are not evident in similarly designed measures of letter processing impairments.
Our separation of letter and word processing capacities may have been facilitated by our using the same block letters in both stimuli, minimizing orthographic word cues. This may force our subjects to rely on phonological word processing that is disrupted in AD, as seen with diminished left temporal activation by phonological tasks, with apparently compensatory right temporal activation [30].
Motion processing impairments
These studies confirm elevated visual motion thresholds in aging and early AD [7, 9] with similar increases in horizontal and outward radial motion thresholds across subject groups (Fig. 4). ONCs and EADs show correlations between the three motion processing measures, possibly because they rely on local motion processing, having lost global motion processing capacities [10, 31]. The independence of local and global processing strategies, and their selective engagement with specific stimulus sets, is supported by our related studies of single neuron mechanisms of visual motion processing in behaving monkeys neurophysiology [32, 33].
We have seen that impaired optic flow heading discrimination is independent of memory loss in AD [34], but linked to navigational deficits [4] and posterior cortical neurophysiological unresponsiveness to visual motion [35]. Heading discrimination from optic flow and from object motion are also independent in AD [31] with conflicts between superimposed optic flow and object motion resulting in heading misperceptions that are unique to AD [36]. Together, these studies illustrate both the perceptual and neural mechanism of impaired motion processing, and the potentially grave naturalistic implications of such impairments.
Independence of words and motion
We find that visual word and motion processing show independent decline in aging and early AD (Fig. 5). This is supported by the presences of significant correlations between letter thresholds and verbal memory scores, and between motion thresholds and line orientation scores, but not across the language and motion/spatial domains. The independence of word and motion processing impairments may reflect pathology in extrastriate visual association cortical areas that separately process object oriented and visuo-spatial signals [37, 38].
Our limited sample size warrants caution in interpreting these results and we emphasize that these findings do not detract from the importance of the disorders of primary visual centers in aging and AD [39]. Rather, these findings demand a fuller exploration of interactions between retino-geniculate deficits and extrastriate cortical dysfunction. In particular, the selective vulnerability of retinal X and Y ganglion cells, and of parvocellular and magnocellular geniculate neurons, might play roles in both the functional manifestations of ventral and dorsal extrastriate dysfunction, as well as in the pathophysiology of extrastriate cortical subsystem dysfunction.
The continuum of independent impairments across aging and early AD suggests that we might recognize groups of older adults as being more or less impaired depending on which neural subsystem are assessed. This is consistent with the clinical and pathological identification of separate sites of early pathological changes in AD patients with primarily verbal versus visuospatial symptoms [40, 41].
In addition, the independent decline of visual word and motion processing in AD implies selective effects on neural substrates that are anatomically adjacent, functionally linked, and sharing the same genetic and molecular context. This may reflect unrecognized genetic or molecular differences between these groups, or idiosyncratic profiles of functional strength and weakness that might lead to distinct patterns of disease resistance and vulnerability. In either case, the independence of impairments should be an important consideration in the early detection of AD and the monitoring of therapeutic efficacy for established and newly introduced treatments.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Teresa Steffenella in data collection, and the expertise of William Vaughn in developing the stimulus systems. Drs. Garrett Riggs and Michael Rossen contributed to the design of these studies. This work was supported by NIA grants AG17596 and AG20647, and NEI grant EY10287.
Footnotes
Authors' disclosures available online (http://www.jalz.com/disclosures/view.php?id=1296).
REFERENCES
- [1].Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. MIT Press; Cambridge: 1982. pp. 549–586. [Google Scholar]
- [2].Bowen JD, Malter AD, Sheppard L, Kukull WA, McCormick WC, Teri L, Larson EB. Predictors of mortality in patients diagnosed with probable Alzheimer's disease. Neurology. 1996;47:433–439. doi: 10.1212/wnl.47.2.433. [DOI] [PubMed] [Google Scholar]
- [3].Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- [4].Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71:888–895. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perfetti CA. Comprehending written language: A blueprint of the reader. In: Brown CM, Hagoort P, editors. The Neurocognition of Language. Oxford University Press; New York: 1999. pp. 167–208. [Google Scholar]
- [6].Glosser G, Friedman RB, Kohn SE, Sands L, Grugan P. Cognitive mechanisms for processing nonwords: Evidence from Alzheimer's disease. Brain Lang. 1998;63:32–49. doi: 10.1006/brln.1997.1924. [DOI] [PubMed] [Google Scholar]
- [7].Trick GL, Silverman SE. Visual sensitivity to motion: Age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991;41:1437–1440. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- [8].Gilmore GC, Wenk HE, Naylor LA, Stuve TA. Motion perception and aging. Psychology Aging. 1992;7:654–660. doi: 10.1037//0882-7974.7.4.654. [DOI] [PubMed] [Google Scholar]
- [9].Tetewsky SJ, Duffy CJ. Visual loss and getting lost in Alzheimer's disease. Neurology. 1999;52:958–965. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- [10].O'Brien HL, Tetewsky SJ, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in Alzheimer's disease. Cereb Cortex. 2001;11:1083–1092. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- [11].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS- ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [12].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [13].Money J, Alexander D, Walker HT. A Standardized Road Map Test of Direction Sense. Johns Hopkins Press; Baltimore, MD: 1965. [Google Scholar]
- [14].Benton A, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment: A clinical manual. Oxford University Press; New York: 1983. [Google Scholar]
- [15].Wechsler D. Wechsler Memory Scale, Revised Manual. The Psychological Corp.; San Antonio, TX: 1987. [Google Scholar]
- [16].Rosen WG. Verbal fluency in aging and dementia. J Clin Neuropsychol. 1980;2:135–146. [Google Scholar]
- [17].Pentland A. Maximum likelihood estimation: The best PEST. Percept Psychophys. 1980;28:377–379. doi: 10.3758/bf03204398. [DOI] [PubMed] [Google Scholar]
- [18].Harvey LO. Efficient estimation of sensory thresholds with ML-PEST. Spatial Vis. 1997;11:121–128. doi: 10.1163/156856897x00159. [DOI] [PubMed] [Google Scholar]
- [19].Weibull W. A statistical distribution function of wide applicability. J Appl Mech Trans ASME. 1951;18:293–297. [Google Scholar]
- [20].Harvey LO. Efficient estimation of sensory thresholds. Behav Res Meth Instrum Comput. 1986;18:623–632. [Google Scholar]
- [21].Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc. 1982;2:1–10. [Google Scholar]
- [22].Tetewsky S. Familiarity effects in visual comparison tasks and their implications for studying human intelligence. J Exp Psychol Learn Mem Cogn. 1992;18:577–594. doi: 10.1037//0278-7393.18.3.577. [DOI] [PubMed] [Google Scholar]
- [23].Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- [24].Lezak MD. Neuropsychological Assessment. Oxford University Press, Inc.; New York: 1995. [Google Scholar]
- [25].Cummings JL, Houlihan JP, Hill MA. The pattern of reading deterioration in dementia of the Alzheimer type: Observations and implications. Brain Lang. 1986;29:315–323. doi: 10.1016/0093-934x(86)90051-9. [DOI] [PubMed] [Google Scholar]
- [26].Schlotterer G, Moscovitch M, Crapper-McLachlan D. Visual processing deficits as assessed by spatial frequency contrast sensitivity and backward masking in normal ageing and Alzheimer's disease. Brain. 1984;107:309–325. doi: 10.1093/brain/107.1.309. [DOI] [PubMed] [Google Scholar]
- [27].Gilmore GC, Cronin-Golomb A, Neargarder SA, Morrison SR. Enhanced stimulus contrast normalizes visual processing of rapidly presented letters in Alzheimer's disease. Vision Res. 2005;45:1013–1020. doi: 10.1016/j.visres.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [28].Glosser G, Gallo J, Duda N, de V, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:198–206. [PubMed] [Google Scholar]
- [29].Patterson K, Hodges JR. Deterioration of word meaning: Implications for reading. Neuropsychologia. 1992;30:1025–1040. doi: 10.1016/0028-3932(92)90096-5. [DOI] [PubMed] [Google Scholar]
- [30].Peters F, Majerus S, Collette F, Degueldre C, Del Fiore G, Laureys S, Moonen G, Salmon E. Neural substrates of phonological and lexicosemantic representations in Alzheimer's disease. Hum Brain Mapp. 2009;30:185–199. doi: 10.1002/hbm.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mapstone M, Logan D, Duffy CJ. Cue integration for the perception and control of self-movement in ageing and Alzheimer's disease. Brain. 2006;129:2931–2944. doi: 10.1093/brain/awl201. [DOI] [PubMed] [Google Scholar]
- [32].Logan DJ, Duffy CJ, Logan DJ, Duffy CJ. Cortical area MSTd combines visual cues to represent 3-D self-movement. Cerebral Cortex. 2006;16:1494–1507. doi: 10.1093/cercor/bhj082. [DOI] [PubMed] [Google Scholar]
- [33].Page WK, Duffy CJ. Active steering by global motion enhances MST's optic flow responses. Soc Neurosci Abstr. 2003 Program No. 179.3. [Google Scholar]
- [34].Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: Getting lost between aging and AD. Neurology. 2003;60:802–808. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- [35].Kavcic V, Ni H, Zhu T, Zhong J, Duffy CJ. White matter integrity linked to functional impairments in aging and early Alzheimer's disease. Alzheimers Dement. 2008;4:381–389. doi: 10.1016/j.jalz.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mapstone M, Duffy CJ. Approaching objects cause confusion in patients with Alzheimer's disease regarding their direction of self-movement. Brain. 2010;133:2690–2701. doi: 10.1093/brain/awq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kleist K. Uber form und orstsblindheit bei verletzungen des hinterhautlappens. Deutsch Z Nervenheilk. 1935;138:206–214. [Google Scholar]
- [38].Ungerleider LG, Haxby JV. `What' and `where' in the human brain. Curr Biol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- [39].Gilmore GC, Wenk HE, Naylor LA, Koss E. Motion perception and Alzheimer's disease. J Gerontol. 1994;49:P52–P57. doi: 10.1093/geronj/49.2.p52. [DOI] [PubMed] [Google Scholar]
- [40].Renner JA, Burns JM, Hou CE, McKeel DW, Storandt M, Morris JC. Progressive posterior cortical dysfunction: A clinicopathologic series. Neurology. 2004;63:1175–1180. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- [41].Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathological characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]