Abstract
Objectives
To determine whether utilization of an alternative ankle-brachial index (ABI) calculation method improves mortality risk prediction compared to traditional methods.
Background
The ABI is used to diagnose peripheral arterial disease (PAD), and to identify those at risk for cardiovascular events. Traditionally, the ABI is calculated using the higher of the dorsalis pedis and posterior tibial ankle arteries. Studies directly comparing calculation methods are limited.
Methods
The ABI was calculated at baseline in 1,413 study participants undergoing non-emergent coronary angiography subsequently followed for all-cause and cardiovascular mortality. There were 224 individuals assigned to the traditional-PAD group (ABI < 0.90) using the traditional ABI method. Of those remaining, an alternative ABI method utilizing the lower of the two ankle pressures assigned 282 patients to the alternative-PAD group. The 862 individuals not assigned to PAD by either method were the no-PAD group.
Results
There were 163 mortalities during a median follow-up of 5.0 years. Adjusted Cox regression models showed that the alternative-PAD group had an increased risk for all-cause (HR=1.49; 95% CI, 1.01-2.19) and cardiovascular mortality (HR=3.21; 95% CI, 1.53-6.37) versus the no-PAD group. Additionally, in the no-PAD group, there was an 11% (HR=1.11; 95% CI, 1.05-1.17) increased risk of all-cause mortality per 1mm Hg increased difference between the left and right brachial systolic pressures.
Conclusion
The implementation of an alternative ABI method and use of the brachial difference identifies individuals at an increased risk for mortality who are currently missed using traditional ABI methods. Current ABI protocols may need to be evaluated.
Keywords: peripheral artery disease, mortality, diagnosis
Introduction
Peripheral arterial disease (PAD) results from atherosclerosis and narrowing of the arteries in the distal extremities. Estimates of PAD prevalence demonstrate a wide range from 4% in general populations to 30% among primary care practices(1,2). Individuals with PAD are at an increased risk for myocardial infarction, aortic aneurysm rupture, ischemia and amputation(3) with a 6-fold increased risk of death from cardiovascular causes(4,5). Concerningly, this cardiovascular morbidity and mortality has been demonstrated even in PAD patients without clinical symptoms(4). While aggressive medical treatment of PAD has been shown to reduce cardiovascular morbidity and mortality, many patients are not recognized and, as a result, are not adequately treated(2,6).
The ankle-brachial index (ABI) provides an objective clinical measure for the diagnosis of PAD with normal ABI defined as at least 1.00 to 1.40 and PAD classified as an ABI of less than 0.90. These criteria have been validated against peripheral angiography to calculate the sensitivity and specificity of the ABI as a diagnostic tool for PAD. While the specificity has been shown to be reproducibly high (>95%)(7-10), some validation studies have demonstrated low sensitivity(7,8). Therefore, methods to improve the sensitivity of the ABI, without a concomitant reduction in specificity, may improve risk prognostication.
According to current guidelines and a recent Scientific Statement from the American Heart Association(11), the preferred method to calculate the ABI requires measuring the systolic pressures of the left and right brachial arteries in addition to the dorsalis pedis and posterior tibial arteries in each leg. Then, for each leg, the higher of the dorsalis pedis and posterior tibial pressures is divided by the higher brachial pressure, and the lower overall ABI of the two legs is used to determine PAD status. Here we assess the implementation of an alternative measure of ABI, by instead utilizing the lower of the dorsalis pedis and posterior tibial pressures, and examine whether individuals reclassified into the PAD group using this alternative measure are at an increased risk of mortality. Additionally, we determine whether there is evidence to support the direct evaluation of the brachial difference in risk prediction among individuals undergoing ABI testing.
Methods
Study population
The Genetic Determinants of Peripheral Arterial Disease (GenePAD) study is comprised of individuals (n=1755) who underwent an elective, non-emergent coronary angiogram for angina, shortness of breath or an abnormal stress test at Stanford University and Mount Sinai Medical Centers between January 1, 2004 and March 1, 2008, as previously described (12-14).
Inclusion criteria
Participants were included in the study sample if complete data was available on all covariates (n=1,413). Individuals without both a dorsalis pedis and posterior tibial systolic pressure recorded in at least one leg were excluded (n=5), as calculation of both a traditional and alternative ABI value would not be possible. Remaining eligible patients with an ABI > 1.40 were excluded (n=40)(15). Using these criteria, 1,368 patients were included.
Outcomes
The outcomes of interest in this analysis were death from any cause and death from cardiovascular causes including myocardial infarction, cardiac arrest, stroke, heart failure or aneurysm rupture. Ascertainment of mortality and cause of death was achieved through phone or postal communication, medical record review and the Social Security Death Index. New mortalities were identified through March 31, 2012.
Covariates
The use of lipid-lowering and anti-hypertensive medications was evaluated by direct medication inventory. Diabetes status was classified as self-reported use of insulin or oral hypoglycemic agents. Hypertension was defined as a SBP of ≥ 160 mm Hg or use of anti-hypertensive medications. Creatinine, total and HDL cholesterol levels were measured at the time of coronary angiography. An experienced cardiologist who was blinded to participant details evaluated coronary angiograms. Hemodynamically significant CAD was defined as > 60% stenosis(16,17).
ABI calculations
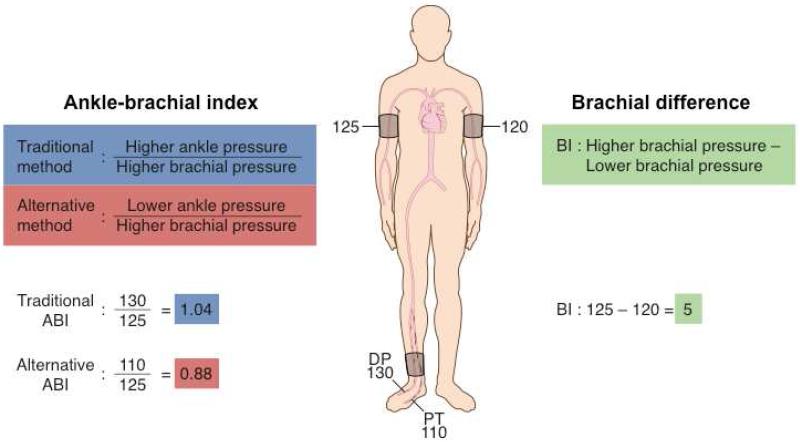
Prior to the coronary angiogram, posterior tibial, dorsalis pedis, and brachial artery systolic pressures were measured using a 5 MHz Doppler ultrasound. Calculation of the ABI using the traditional and alternative methods is illustrated in Figure 1. The traditional ABI was calculated for each leg by dividing the higher of the posterior tibial or dorsalis pedis pressures by the higher of the left or right brachial systolic pressures. Then, the leg with the lower ABI measurement was recorded as the traditional ABI value. The alternative ABI was calculated for each leg by dividing the lower of the posterior tibial or dorsalis pedis pressures by the higher of the left or right brachial systolic pressures. Then, the leg with the lower ABI measurement was recorded as the alternative ABI value. For each method the index leg was defined as the leg with the lower ABI and data from the contralateral leg was not included in the analysis.
Figure 1. Ankle-brachial index and brachial difference calculations.
ABI, ankle-brachial index; BD, brachial difference; DP, dorsalis pedis artery; PT, posterior tibial artery
PAD group classification
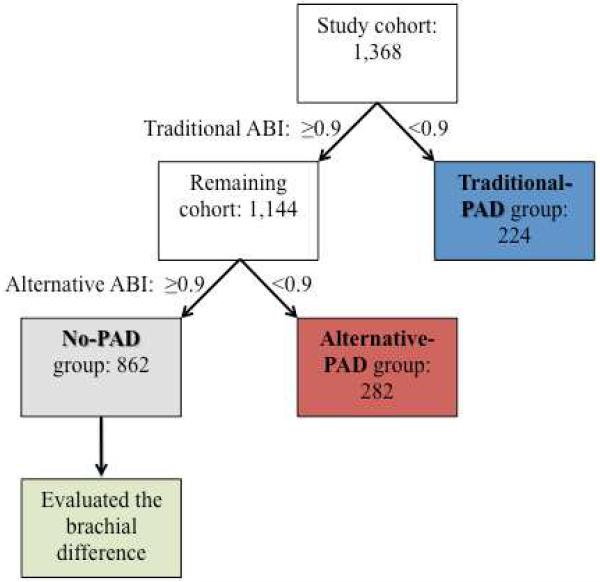
Study participant classification into mutually exclusive groups is represented in Figure 2. Patients with an ABI of < 0.90 in either leg using the traditional method were classified as having traditional-PAD. Of those remaining (who therefore had an ABI of ≥ 0.90 in both legs using the traditional method), individuals with an ABI of < 0.90 in either leg using the alternative method were classified as having alternative-PAD. Patients without PAD as classified by either method were used as the no-PAD reference group. Among individuals in the no-PAD group, we calculated the brachial difference as the difference between the left and right brachial systolic pressures.
Figure 2. Study participant peripheral arterial disease group classifications.
Traditional ABI refers to the method utilizing the higher of the dorsalis pedis and posterior tibial pressures while alternative ABI refers to the method utilizing the lower of the dorsalis pedis and posterior tibial pressures.
ABI, ankle-brachial index; PAD, peripheral arterial disease
Statistical methods
Baseline patient characteristics were calculated for the three PAD status groups and compared using a t-test or chi-squared test. For all survival analyses, the follow-up time was defined as the period between the enrollment interview and the last confirmed follow-up or date of death.
Hazard ratios (HR) were calculated using adjusted Cox proportional hazard models to compare all-cause and cardiovascular mortality risk in the traditional-PAD, alternative-PAD and no-PAD groups. Additionally, individuals with severely decreased ABI values of less than 0.40 have been shown to be at a further increased risk for PAD related sequelae (18). To examine the ability of the alternative ABI method to stratify mortality risk, we compared the risk of individuals with severely decreased ABIs using the traditional method to the risk of any individual in the full study cohort with an ABI < 0.40 only with the application of the alternative method. Similarly, we compared the risk of individuals with mild to moderately decreased ABIs (0.40 ≤ ABI< 0.90) using the traditional method to the risk of individuals with moderately decreased ABIs only when using the alternative method. All survival analyses were adjusted for age, sex, race, smoking history (ever or never), BMI, hypertension, use of lipid-lowering medications at enrollment, diabetes, total cholesterol, HDL cholesterol, creatinine and CAD. Proportional-hazards assumptions were evaluated by Schoenfeld’s residuals tests.
Next, we calculated the C-index to quantify the integrated sensitivity and specificity of the traditional and alternative PAD classifications in predicting all-cause and cardiovascular mortality. Therefore, the traditional and alternative ABI methods were separately applied to the full study cohort in order to ascertain the respective discriminatory capacity of each method in all comers. In survival analysis, the C-index interpretation is equivalent to the area under the ROC curve or c-statistic, while allowing for censored data(19).
We further examined whether the brachial difference provided additional prognostic information in predicting mortality risk amongst those with no detectable PAD by either the traditional or alternative method. This variable was calculated as the difference between the left and right brachial systolic pressures and examined as a continuous variable and as a binary variable of <10mm Hg or ≥10 mm Hg. The brachial difference was calculated in 200 patients in the no-PAD group after excluding individuals with only one brachial pressure due to IV placement or amputation.
Tests were considered significant if the two-sided P-value was < 0.05. All analyses were performed using Stata version 12.0 (StataCorp, College Station, Texas). Study data were collected and managed using REDCap electronic data capture tools hosted at Stanford University(20).
Results
Study population characteristics
There were 224 individuals in the traditional-PAD group, which consisted of those categorized as having PAD (ABI < 0.90) using the traditional ABI method. Among those remaining, the alternative ABI method placed 282 individuals into the alternative-PAD group. The no-PAD group consisted of the remaining 862 participants not classified as having PAD using the traditional or alternative ABI methods. The mean higher and lower ABI for the index leg in each group is presented in Table 1. The prevalence of PAD when applying each method to the full study cohort was 16.4% for the traditional ABI method and 37.0% for the alternative ABI method. There were 163 mortalities (12%), of which 47 were known to be from cardiovascular causes, during a median follow-up period of 5.0 years (interquartile range, 4.0-6.3).
Table 1.
Index leg ankle-brachial index values
| Index leg | ||
|---|---|---|
| Higher ABI ± SD (IQR) | Lower ABI ± SD (IQR) | |
| No-PAD | 1.10 ± 0.09 (1.02-1.16) | 1.02 ± 0.08 (0.95-1.07) |
| Traditional-PAD | 0.71 ± 0.16 (0.60-0.84) | 0.65 ± 0.19 (0.54-0.81) |
| Alternative-PAD | 1.02 ± 0.10 (0.94-1.07) | 0.81 ± 0.11 (0.77-0.88) |
ABI, ankle-brachial index; IQR; interquartile range; SD, standard deviation
Enrollment characteristics of the 1,368 participants in this study according to PAD status are displayed in Table 2. The alternative-PAD group significantly differed from the traditional-PAD group with a lower mean age, lower total cholesterol and creatinine levels and a significantly lower proportion of hypertensives, smokers, diabetics and individuals with CAD. Conversely, the only significant differences observed between the alternative-PAD group as compared to the no-PAD group were a higher mean age, higher HDL cholesterol and a greater proportion of females.
Table 2.
Baseline study population characteristics by peripheral arterial disease status (n=1,368)
| P-value for difference | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Traditiona | Alternativ | No | P, | P, trad | P, alt | |
| l-PAD | e-PAD | –PAD | trad | vs. no– | vs. no– | |
| Characteristic | (n=224) | (n=282) | (n=862) | vs. alt | PAD | PAD |
| Age, mean years (SD) | 71 (9) | 69 (10) | 65 (10) | 0.002 | < 0.001 | < 0.001 |
| Female, No. (%) | 107 (48) | 107 (38) | 254 (29) | 0.026 | < 0.001 | 0.008 |
| Ethnicity | ||||||
| Caucasian | 118 (53) | 151 (54) | 500 (58) | 0.846 | 0.152 | 0.189 |
| African | 43 (19) | 35 (12) | 81 (9) | 0.036 | < 0.001 | 0.145 |
| Hispanic | 30 (13) | 33 (12) | 88 (10) | 0.567 | 0.172 | 0.479 |
| Asian | 11 (5) | 26 (9) | 74 (9) | 0.064 | 0.068 | 0.743 |
| Other* | 22 (10) | 37 (13) | 119 (14) | 0.251 | 0.114 | 0.771 |
| Hypertensive, No. (%) | 197 (88) | 230 (82) | 666 (77) | 0.049 | < 0.001 | 0.128 |
| Body mass index, mean kg/m2 (SD) | 28 (6) | 30 (7) | 29 (6) | 0.018 | 0.108 | 0.109 |
| Lipids, mean mg/dL (SD) | ||||||
| Total cholesterol | 145 (40) | 137 (37) | 137 (36) | 0.028 | 0.004 | 0.910 |
| HDL cholesterol | 41 (13) | 42 (12) | 40 (12) | 0.447 | 0.160 | 0.008 |
| Ever smoker, No. (%) | 149 (67) | 160 (57) | 482 (56) | 0.025 | 0.004 | 0.809 |
| Cholesterol lowering medication, No. | ||||||
| (%) | 179 (81) | 223 (80) | 668 (78) | 0.766 | 0.285 | 0.428 |
| Clopidogrel, No. (%) | 91 (41) | 100 (36) | 263 (31) | 0.225 | 0.003 | 0.113 |
| Diabetic, No. (%) | 101 (45) | 88 (31) | 225 (26) | 0.001 | < 0.001 | 0.095 |
| Coronary artery disease, No. (%) | 197 (88) | 215 (76) | 621 (72) | 0.001 | < 0.001 | 0.168 |
| < | ||||||
| Creatinine, mean mg/dL (SD) | 1.4 (1.5) | 1.1 (0.3) | 1.2 (1.0) | 0.001 | 0.002 | 0.059 |
Includes Asian-Indian, Pakistani, Middle Eastern and Pacific Islander
Alt, alternative; HDL, high-density lipoprotein; No., number; PAD, peripheral arterial disease; SD; standard deviation; trad, traditional;
For all-cause and cardiovascular mortality, individuals in the traditional PAD group had significantly increased risk compared to those in the no-PAD group (Table 3). Individuals in the alternative-PAD group (who would have been considered ‘normal’ by the traditional ABI method) also had significantly elevated risk compared to the no-PAD group. Notably, the estimated risk in the traditional and alternative PAD groups were equally elevated, and did not significantly differ from each other for either all-cause (P=0.506) or cardiovascular mortality (P=0.653).
Table 3.
Hazard ratios for the association of peripheral arterial disease status with mortality outcomes
| All-cause mortality | HR (95% CI) | P- value |
P-value for difference |
|---|---|---|---|
| No-PAD | ref (1.0) | ref | |
| Traditional PAD | 1.72 (1.17- 2.54) |
0.006 | 0.506 |
| Alternative PAD | 1.49 (1.01- 2.19) |
0.046 |
| Cardiovascular mortality |
HR (95% CI) | P- value |
P-value for difference |
|---|---|---|---|
| No-PAD | ref (1.0) | ref | |
| Traditional PAD | 2.64 (1.25- 5.56) |
0.011 | 0.653 |
| Alternative PAD | 3.21 (1.53- 6.37) |
0.002 |
Adjusted for age, gender, race, smoking history, body mass index, hypertension, use of lipid-lowering medication, diabetes, total cholesterol, high-density lipoprotein cholesterol, coronary artery disease and creatinine
CI, confidence interval; HR, hazard ratio; PAD, peripheral arterial disease; ref, reference
Patients with a severely decreased ABI (< 0.40) using the traditional ABI method, as well as those with an ABI < 0.40 only when using the alternative method, had a greater than three-fold increased risk for all-cause mortality (Table 4). This risk did not significantly differ between ABI calculation methods (P=0.712). Similarly, patients placed in the mild/moderate ABI category (0.40 ≤ ABI < 0.90) using the traditional method and individuals moved into this group only with the application of the alternative method were at a significantly increased risk for death from any cause with a HR that did not statistically differ between these two groups (P=0.618).
Table 4.
Adjusted hazard ratios for the association of ankle-brachial index severity status with all-cause mortality
| Severe (ABI < 0.4) | HR (95% CI) | P-value | P-value for difference |
|---|---|---|---|
| No-PAD (n=862) | ref (1.0) | ref | |
| Using the traditional ABI method (n=11) | 4.13 (1.57- 10.90) |
0.004 | 0.618 |
| Only when using the alternative ABI method (n=30) |
3.13 (1.59-6.16) | 0.001 |
| Mild/moderate (0.4 ≤ ABI < 0.9) | HR (95% CI) | P-value | P-value for difference |
|---|---|---|---|
| No-PAD (n=862) | ref (1.0) | ref | |
| Using the traditional ABI method (n=213) | 1.61 (1.08-2.41) | 0.020 | 0.712 |
| Only when using the alternative ABI method (n=280) |
1.48 (1.00-2.19) | 0.049 |
Adjusted for age, gender, race, smoking history, body mass index, hypertension, use of lipid-lowering medication, diabetes, total cholesterol, high-density lipoprotein cholesterol, coronary artery disease and creatinine
CI, confidence interval; HR, hazard ratio; PAD, peripheral arterial disease; ref, reference
We then directly compared the discriminatory capacity of the traditional and alternative ABI methods amongst the entire cohort. The C-index demonstrated that there was no loss of integrated sensitivity and specificity in predicting all-cause or cardiovascular mortality with the implementation of the alternative ABI method to designate PAD status as compared to the traditional method amongst all comers (Table 5). Because the cardiovascular mortality event rate was so high amongst those in the alternative-PAD group (Table 3), and they would have been inappropriately classified as low-risk using the traditional method, the classification of PAD using the alternative ABI method resulted in a significantly greater C-index for cardiovascular mortality (P=0.013) that was maintained even with adjustment for CAD status (P=0.021).
Table 5.
C-index for peripheral arterial disease status classified using the traditional and alternative ankle-brachial index methods
| All-cause mortality | C-index (95% CI) |
P-value | P-value for difference |
|---|---|---|---|
| Traditional-PAD | 0.570 (0.534- 0.606) |
< 0.001 | 0.150 |
| Alternative-PAD | 0.596 (0.555- 0.636) |
< 0.001 |
| Cardiovascular mortality |
C-index (95% CI) |
P-value | P-value for difference |
|---|---|---|---|
| Traditional-PAD | 0.575 (0.508- 0.641) |
< 0.001 | 0.013 |
| Alternative-PAD | 0.665 (0.595- 0.734) |
< 0.001 |
ABI, ankle -brachial index, CI, confidence interval; PAD, peripheral arterial disease
PAD indicates an ABI < 0.9 by the stated method
Among the no-PAD group, who would be classified as lowest risk using both ABI methods, we found that the brachial difference predicted an 11% (95% confidence interval [CI], 1.05-1.17; P < 0.001) increase in all-cause mortality risk per 1mm Hg difference between the left and right brachial systolic pressures (n=200). Furthermore, individuals with a brachial difference of at least 10mm Hg (n=16) demonstrated a nearly a four-fold increased risk of all-cause mortality compared to those with a brachial difference of less than 10mm Hg (HR=3.96; 95% CI, 1.21-13.02; P=0.023).
The risk of all-cause and cardiovascular mortality did not significantly differ between participants excluded (n=387) and included (n=1,368) in the study-cohort and tests for interaction showed that the reported results were not significantly different according to gender or racial group. Sensitivity analyses adjusting for clopidogrel and, separately, removing heart failure and aneurysm rupture as causes of cardiovascular mortality, yielded results consistent with our primary data. Schoenfeld’s residuals tests demonstrated that the proportional hazards assumption was met for all models.
Discussion
The ABI is a validated and widely used measure to detect the presence of PAD. Currently, the ABI is recommended as the first line test for the diagnosis of PAD among individuals with symptoms and clinical findings suggestive of PAD(11). Because PAD is considered a coronary heart disease risk equivalent(21), the ABI is also a simple tool that can identify those at risk of major adverse cardiovascular events (MACE). The current methods used to identify the presence or absence of PAD rely on criteria that may be insensitive(7,8) and have been suggested to falsely classify some at-risk individuals as ‘normal’. In this study, we compared participants classified as having PAD (ABI < 0.90) using the traditional ABI calculation method with patients that were classified as having PAD only when using an alternative ABI calculation method, to see if we could enhance the prognostic utility of this widely used test.
Individuals who were reclassified as having PAD only when implementing the alternative ABI calculation had a mortality risk that was significantly greater than those without PAD by either method. This is important because these patients would normally be classified as ‘free-of-disease’ and would not meet criteria to have risk reducing therapies initiated. Additionally, the risk in this alternative group was as high as the risk for those individuals classified as having PAD using the traditional ABI method. These findings were consistent for both all-cause and cardiovascular mortality as well as when examining categories of ABI severity. Finally, among those without PAD detected by either ABI method, the brachial difference was a significant predictor of all-cause mortality risk, which is consistent with previous studies demonstrating an association between increased brachial difference and markers of subclinical atherosclerosis(22). This finding emphasizes that vascular disease in any anatomic distribution may provide important prognostic information and should not be ignored.
Whereas a similarly increased risk of mortality was demonstrated for the traditional and alternative PAD groups compared to the no-PAD group, examination of baseline characteristics revealed that the alternative-PAD group had far fewer established cardiovascular risk factors, such as significantly lower rates of hypertension, smoking, diabetes and CAD, than the traditional-PAD group. This is an important finding because their pre-test probability of disease is low, and it is probable that a ‘normal’ traditional ABI would lead many practitioners to ‘rule-out’ disease, according to Bayesian principles. It is unclear why individuals in this group resemble lower risk individuals and harbor disease in an alternative anatomic pattern, but it is clear that their risk is significantly elevated and that they are often clinically ignored. Future studies are required to understand if these individuals possess other unmeasured risk factors (e.g. premature endothelial dysfunction) which accelerate their risk of death, or if isolated/focal tibial disease may reflect a more aggressive variant of PAD.
When applied to all-comers, the implementation of the alternative ABI calculation method will often result in a lower ABI value compared to the traditional method. Therefore, a greater number of individuals will be classified as having PAD using the alternative method, making it a more sensitive predictor of risk. While this study shows that the use of the alternative method detects a group of individuals at increased risk for mortality that was not previously identified, we wished to determine whether the implementation of this more sensitive measure in the full study cohort resulted in a net loss of integrated sensitivity and specificity for predicting MACE. We evaluated this using the C-index, which provided evidence against any loss of integrated sensitivity and specificity for all-cause mortality. Indeed, because the risk of death was so high in the alternative-PAD group, and this group was falsely classified as normal by the traditional ABI method, we found that the ability to predict future cardiovascular death was significantly increased when using the alternative ABI method to define PAD status in the entire cohort. Thus, our concern that re-defining the interpretation of this test to capture more at risk individuals would enhance sensitivity at an unacceptable cost in specificity (for MACE) appears unfounded.
The current study differs from previous reports that attempted to identify more sensitive methods to diagnose PAD(23,24) or prognosticate the risk of future events(25). Utilization of the lower ankle pressure in calculating the ABI has been shown to predict a higher prevalence of PAD while having greater sensitivity for atherosclerotic disease(23,24). Although a recent AHA Scientific Statement does acknowledge that the lower ankle pressure can be used as a prognostic marker for cardiovascular events, it gives this approach a Level C recommendation and raises concerns about “overdiagnosis” and “unnecessary tests” that might be associated with less specific ABI methods(11). To our knowledge, only one previous study has examined the implementation of this alternative ABI calculation in predicting clinical outcomes(25). Similar to the findings reported here, their study reported an increased risk of cardiovascular events amongst those subjects assigned to the ‘Suspected PAD’ group by an alternative ABI method. In contrast to the current report, however, their study evaluated a significantly younger patient population with fewer co-morbidities, and did not observe a statistically significant difference in risk for cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) in fully adjusted Cox-regression models. These findings may be explained in part by the fact that both the presence and systemic sequelae of PAD increase significantly with age, and thus may not have been fully manifest in the younger cohort from the prior study(26). In the current report, the alternative method correctly prognosticated risk after controlling for all comorbidities, and did so without a reduction in net sensitivity and specificity, which has not been previously shown.
Recently published guidelines(11) for calculating the ABI utilize the higher brachial pressure and higher of the dorsalis pedis and posterior tibial pressures in each leg. The higher brachial pressure is uniformly used in order to limit inaccurate ABI calculations among individuals with lower brachial pressures in one arm due to subclavian or axillary artery stenosis. However, the rationale for using the higher ankle pressure is less clear and direct comparisons of different methods are limited. It is obvious that single vessel coronary disease is associated with cardiovascular events and mortality in CAD(27) and that the severity of a stenosis does not always predict the likelihood of future plaque rupture(28). It could be argued that single vessel disease in the lower vasculature should not be overlooked, as when utilizing the higher ankle pressure and dismissing the lower pressure recorded in a potentially diseased vessel. While concerns about overdiagnosis, false positivity and resource utilization with more sensitive ABI methods are valid(11), consideration should be given to the potential value of identifying at-risk individuals earlier in the disease process The classical ABI is currently recommended as the first-line non-invasive test for the diagnosis of suspected PAD, as well as a screening test for those considered to be at high-risk for cardiovascular disease(11). When taken in the context of prior studies(25), the current findings suggest that the ABI interpreted with the alternative method might have an additional role in the risk stratification of subjects with an indeterminate risk profile, such as patients with few comorbidities, but symptoms severe enough to warrant cardiac catheterization.
Limitations
The current study was undertaken in a cohort of individuals undergoing coronary angiography and may not be generalizable to other populations. Additionally, we were unable to evaluate the accuracy of PAD diagnosis using the traditional and alternative ABI methods against a gold-standard such as peripheral angiography. Larger cohorts will be needed to confirm the utility of the alternative ABI method by ABI severity as this analysis had limited events within these subgroups. Additionally, future studies are needed to examine the additive value of the alternative ABI method compared to established risk models.
Conclusion
Peripheral arterial disease is associated with an increased risk of MACE. The ABI is widely used to diagnose PAD and to inform the medical management of patients. Here we show that the implementation of an alternative ABI calculation method identifies a large proportion of individuals at an increased risk for all-cause and cardiovascular mortality. These individuals would not be identified using traditional ABI methods and otherwise might be ignored due to their clinical risk profile. Additionally, we provide evidence that the evaluation of the brachial difference among patients not classified as having PAD may provide useful data for risk prediction that is not directly evaluated under current guidelines. These methods improved the prognostic utility of the ABI without a net impairment in sensitivity and specificity. These findings should be replicated in lower risk general populations, and randomized controlled trials will be needed to determine whether the use of the lower ankle pressure in ABI calculations improves clinical outcomes. The results of this study suggest that protocols commonly employed for this widely used diagnostic test may need to be re-evaluated.
Acknowledgments
Grant support: This work was funded by grants from the National Institutes of Health (K12HL087746 to JPC) and the American Heart Association (10BGIA3290011 to NJL).
Abbreviations
- ABI
Ankle-brachial index
- BMI
Body mass index
- CI
Confidence interval
- CAD
Coronary artery disease
- HRs
Hazard ratios
- HDL
High-density lipoprotein
- MACE
Major adverse cardiovascular events
- PAD
Peripheral arterial disease
- SBP
Systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationships with industry: None
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Shemanski L, Manolio TA. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.Nead KT, Zhou M, Caceres RD, Olin JW, Cooke JP, Leeper NJ. The Walking Impairment Questionnaire predicts mortality and improves risk discrimination and reclassification in a high-risk cohort. Circulation: Cardiovascular Quality and Outcomes. 2013 doi: 10.1161/CIRCOUTCOMES.111.000070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leeper NJ, Myers J, Zhou M, et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–8. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 8.Nassoura ZE, Ivatury RR, Simon RJ, Jabbour N, Vinzons A, Stahl W. A reassessment of Doppler pressure indices in the detection of arterial lesions in proximity penetrating injuries of extremities: a prospective study. Am J Emerg Med. 1996;14:151–6. doi: 10.1016/S0735-6757(96)90122-9. [DOI] [PubMed] [Google Scholar]
- 9.Fowkes FG. The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol. 1988;17:248–54. doi: 10.1093/ije/17.2.248. [DOI] [PubMed] [Google Scholar]
- 10.Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA. Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol. 1994;140:526–34. doi: 10.1093/oxfordjournals.aje.a117279. [DOI] [PubMed] [Google Scholar]
- 11.Aboyans V, Criqui MH, Abraham P, et al. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement From the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 12.Wilson AM, Sadrzadeh-Rafie AH, Myers J, et al. Low lifetime recreational activity is a risk factor for peripheral arterial disease. Journal of vascular surgery: official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2011;54:427–32. 432 e1–4. doi: 10.1016/j.jvs.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadrzadeh Rafie AH, Stefanick ML, Sims ST, et al. Sex differences in the prevalence of peripheral artery disease in patients undergoing coronary catheterization. Vasc Med. 2010;15:443–50. doi: 10.1177/1358863X10388345. [DOI] [PubMed] [Google Scholar]
- 14.Nead KT, Zhou MJ, Caceres RD, et al. Usefulness of the Addition of Beta-2-Microglobulin, Cystatin C and C-Reactive Protein to an Established Risk Factors Model to Improve Mortality Risk Prediction in Patients Undergoing Coronary Angiography. The American journal of cardiology. 2013 doi: 10.1016/j.amjcard.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creager MA, Belkin M, Bluth EI, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS Key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to develop Clinical Data Standards for peripheral atherosclerotic vascular disease) J Am Coll Cardiol. 2012;59:294–357. doi: 10.1016/j.jacc.2011.10.860. [DOI] [PubMed] [Google Scholar]
- 16.Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–21. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 17.Atar D, Ramanujam PS, Saunamaki K, Haunso S. Assessment of coronary artery stenosis pressure gradient by quantitative coronary arteriography in patients with coronary artery disease. Clin Physiol. 1994;14:23–35. doi: 10.1111/j.1475-097x.1994.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Aboyans V, Kamineni A, Allison MA, et al. The epidemiology of subclavian stenosis and its association with markers of subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;211:266–70. doi: 10.1016/j.atherosclerosis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison MA, Aboyans V, Granston T, et al. The relevance of different methods of calculating the ankle-brachial index: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010;171:368–76. doi: 10.1093/aje/kwp382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oksala NK, Viljamaa J, Saimanen E, Venermo M. Modified ankle-brachial index detects more patients at risk in a Finnish primary health care. Eur J Vasc Endovasc Surg. 2010;39:227–33. doi: 10.1016/j.ejvs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118:961–7. doi: 10.1161/CIRCULATIONAHA.107.763227. [DOI] [PubMed] [Google Scholar]
- 26.Criqui MH. Peripheral arterial disease--epidemiological aspects. Vascular medicine. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 27.Henderson RA, Pocock SJ, Sharp SJ, et al. Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting. Randomised Intervention Treatment of Angina. Lancet. 1998;352:1419–25. doi: 10.1016/s0140-6736(98)03358-3. [DOI] [PubMed] [Google Scholar]
- 28.Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. Journal of the American College of Cardiology. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]