Abstract
Objective
Our goal is to determine if aging and Alzheimer’s disease (AD) have distinct effects on visual cortical motion processing for navigation and steering.
Methods
We recorded visual motion event related potentials (ERPs) in young (YNC) and older normal controls (ONC), and early AD patients (EADs) who viewed rapidly changing optic flow stimuli that simulate naturalistic changes in heading direction, like those that occur when following a path of self-movement through the environment. After a random series of optic flow stimuli, a vertical motion stimulus was presented to verify sustained visual attention by demanding a rapid push-button response.
Results
Optic flow evokes robust ERPs that are delayed in aging and diminished in AD. The interspersed vertical motion stimuli yielded shorter N200 latencies in EADs, matching those in ONCs, but the EADs’ N200 amplitudes remained small.
Conclusions
Aging and AD have distinct effects on visual sensory processing: aging delays evoked response, whereas AD diminishes responsiveness.
Keywords: aging, Alzheimer’s disease, vision, motion, event related potentials
INTRODUCTION
Visuospatial processing deficits contribute to the disabling navigational impairments seen in the earliest stages of AD [1, 2]. The disabling nature of these deficits [3, 4] justifies our considering visuospatial deficits with the same weight afforded to the verbal memory deficits that more commonly define AD.
The early stages of AD are often preceded by isolated verbal or visuospatial impairments [5]. This is recognized in the syndromic differentiation of amnestic and non-amnestic MCI [6, 7]. Such syndromic sub-types likely correspond to neuropathological variants with early signs of AD in either mesial temporal or extrastriate occipital cortices [8, 9].
We previously found a continuum of visuospatial perceptual deficits that extends across cognitive aging and AD [10, 11]. This continuum of deficits corresponds to gradations of impairment in the interpretation of navigational cues [12] related to real world navigation [13].
We have now used radial motion, optic flow to evoke scalp-recorded event related potentials (ERPs) synchronized on the onset of the motion [14, 15] (see Methods, Neurophysiological Recordings) to probe the cortical neurophysiological mechanisms of aging and AD related visuospatial processing deficits. These studies reveal evidence of distinct neural mechanisms underlying the visuospatial processing deficits in aging and AD that may be useful in distinguishing between these conditions.
METHODS
Subject Groups
We studied young (YNC) and older (ONC) normal control subjects and patients with early Alzheimer’s disease (EAD) with normal, or corrected to normal, vision: YNCs were undergraduate students, ONCs were either our patients’ spouses, or subjects recruited from healthy aging programs, and EADs were from the University of Rochester’s clinical programs with functionally significant memory impairment as documented by history and testing, and examination evidence of aphasia, agnosia, apraxia, inattention, or executive incapacity, and met NINCDS-ADRDA criteria for probable AD [16]. All subjects provided informed consent prior to enrollment, and all procedures were approved by the University’s Research Subjects Review Board.
Neurophysiological Experiments
All neurophysiological testing was controlled by the REX (real-time experimental) system [17] running on RTOS-QNX system for PCs. This allowed control of stimuli and displays, monitoring of eye position, and creation of data files. Subjects sat facing a rear-projection tangent screen’s 60° × 40° image while maintaining centered fixation (+/-10°) on a spot at the center of the screen. Eye and head position were monitored using infrared oculometry (ASL, Inc.). Subjects used their left and right index fingers to press an upper or lower button.
Simulated Optic Flow Stimulus Paradigm
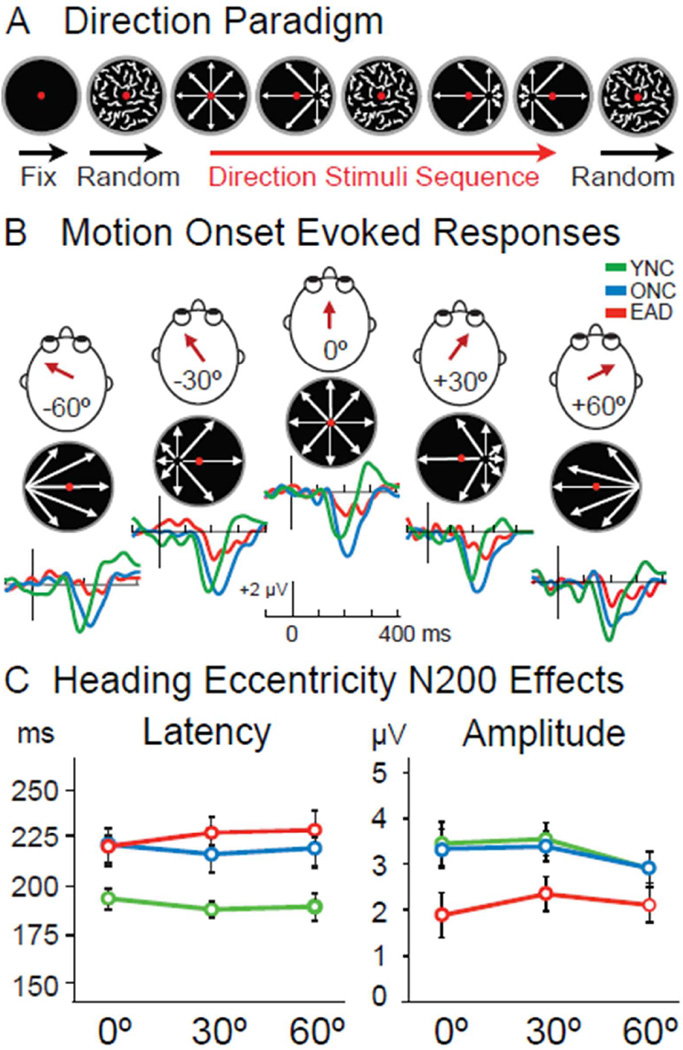
VMERPs were recorded in a direction change paradigm in which a series of 9 to 15 radial optic flow or random dot motion stimuli were presented for 500 ms per stimulus (Figure 1A). The radial optic flow included five different heading directions simulated by radial foci-of-expansion (FOEs) arranged along the horizontal meridian at eccentricities of: −60°, −30°, −60°, +30°, +60° (Figure 1B). All optic flow stimuli were presented at 100% motion coherence with an average dot speed of 31°/s. The interleaved random motion stimuli matched the radial optic flow but for the randomization of dot re-location yielding 0% motion coherence.
Figure 1.
Optic flow stimulus paradigm and ERPs of YNCs, ONCs, and EADs. A. Visual stimuli consisting of random dot kinematograms were projected on a screen, while central visual fixation was maintained. Each trial began with 500 ms of random dot motion followed by a randomized sequence of 9 to 15 stimuli. Each 500 ms stimulus consisted of random motion or radial optic flow patterns with either centered or laterally displaced focus of expansion (FOE) simulating different heading directions. B. We tested five possible heading directions as determined by the location of the radial motion FOE. YNC and ONC subjects showed robust N200 responses to OF stimuli that occurred first after random motion (OF onset), with EAD subjects showing only small N200s. C. Group averages of peak amplitude and latency of N200s evoked by OF onset reveal distinct aging and disease effects. While N200 latencies are prolonged in aging (p < .001), their amplitudes are only diminished in AD (p= .045). The location of the FOE at OF onset did not generate any significant difference in response amplitude or latency for any group.
Stimulus trials began with a black screen with a red dot for central fixation, followed by a random series of optic flow and random motion stimuli. All trials began with random motion and continued for a randomly selected 9 to 15 stimuli before ending with a vertical dot motion stimulus. The vertical motion cued the subjects to make a push button response to focus their attention on the stimulus stream. Subjects pressed the more distant of two buttons for upward dot motion and the closer of the two buttons for downward motion. These responses provided measures of the subject’s sustained attention during recording sessions. An audible tone was sounded if the subject failed to respond to the vertical motion within 5 s, to alert the subject and experimenter.
Optimization of serial stimulation was accomplished by using Kautz random sequences [18, 19] with a repetition span of s*(s−1)(q−1), where s is the number of test stimuli (6 = 5 optic flows plus random motion) and q is the sequence’s memory span (3 in this experiment).
Neurophysiological Recordings
We used NeuroScan Labs (Neurosoft, Inc.) equipment, and electrode caps (Quik-Cap; Compumedics Neuroscan) to record scalp EEG at a sample rate of 500 Hz with 32 bit resolution. The electrode montage conformed to the International 10–20 System with an additional 13 electrodes. Impedances were maintained below 5 kΩ per channel, balanced within 2 kΩ across channels, and low and high pass filters were set at 100 and 0.1 Hz, respectively, with a 3 dB cutoff and a roll-off of 12 dB per octave at both sides.
Scan 4.3 (Neurosoft, Inc.) was used for off-line analysis, including ocular artifact reduction (ARTCOR) and band-pass filtering (0.1 and 50 Hz) with visual artifact rejection. The averaged response were used to identified and measured waveform components for each subject, electrode, and stimulus condition. Resulting peak amplitudes and latencies of each component from all recording sites were entered in to mixed measures ANOVA designs with group (YNC, ONC, and EAD) as a between subjects factor, and levels of each condition as a within subjects factor, followed by post-hoc Tukey’s Honestly Significant Differences (THSDs) analyses. [SPSS 2010]
RESULTS
We recorded visual motion ERPs to heading direction changes simulated by radial optic flow from 15 YNCs, 15 ONCs, and 12 EADs. These subject groups show differences on neuropsychological tests consistent with EADs’ impairments on all but the line orientation and face processing tests. YNCs and ONCs differ only by age. (Table 1)
Table 1.
Demographic and neuropsychological characteristics of the three subject groups. Two-way ANOVA revealed significant group differences in all parameters (multivariate F18, 43 = 2354.29, P < .001; univariate P values in right column). Post-hoc tests of group differences for each test (Tukey’s Honestly Significant Differences, THSD, p < .05) define the frames that enclose groups that were not different.
| Nauropsychological Profiles | ||||
|---|---|---|---|---|
| Subject Group (n, % male | ||||
| Test mn (se) |
YNC (15,47%) |
ONC (15,33%) |
EAD (12,58%) |
p value |
| Age | 26.00 (1.34) | 75.07 (2.37) | 78.42 (2.51) | < .001 |
| MMSE | 29.60 (024) | 29.00 (0.31) | 25.50 (0.67) | < .001 |
| Road Map | 30.00 (0.89) | 29.27 (0.81) | 25.33 (1.12) | =.003 |
| Line Orient | 26.60 (0.78) | 23.67 (1.06) | 22.00 (2.21) | =.068 |
| Facial Matching | 47.60 (1.01) | 46.47 (0.93) | 44.75 (2.03) | =.334 |
| Figural Memory | 8.53 (0.35) | 7.20 (0.37) | 5.17 (0.46) | < .001 |
| Verbal Recall | 20.67 (0.50) | 17.60 (0.91) | 11.42 (1.59) | < .001 |
| Delayed Recall | 7.80 (0.11) | 6.87 (0.32) | 4.08 (0.54) | < .001 |
| Animal Naming | 24.13 (1.62) | 20.40 (1.63) | 12.75 (0.91) | < .001 |
Optic flow ERPs were most consistent at CPZ and we focused on that lead. Group effects on latency (F2,36 = 26.812, p < .001) reflect earlier responses in YNCs than ONCs or EADs. Group differences in optic flow onset N200 amplitudes (F2,36 = 3.392, p = .045) reflect larger responses in YNCs and ONCs than EADs. (Figure 1)
Successive optic flow stimuli evoke smaller N200s (F2,72 = 23.07, p < .001), without stimulus order by subject group interactions. Successive stimuli also delay N200s (F2,36 = 17.46, p < .001), with ONCs and EADs being more affected than YNCs. There are no heading eccentricity, or eccentricity by group interactions, on N200 amplitude or latency. Thus, all heading directions yield similar responses that are delayed in ONCs and diminished in EADs.
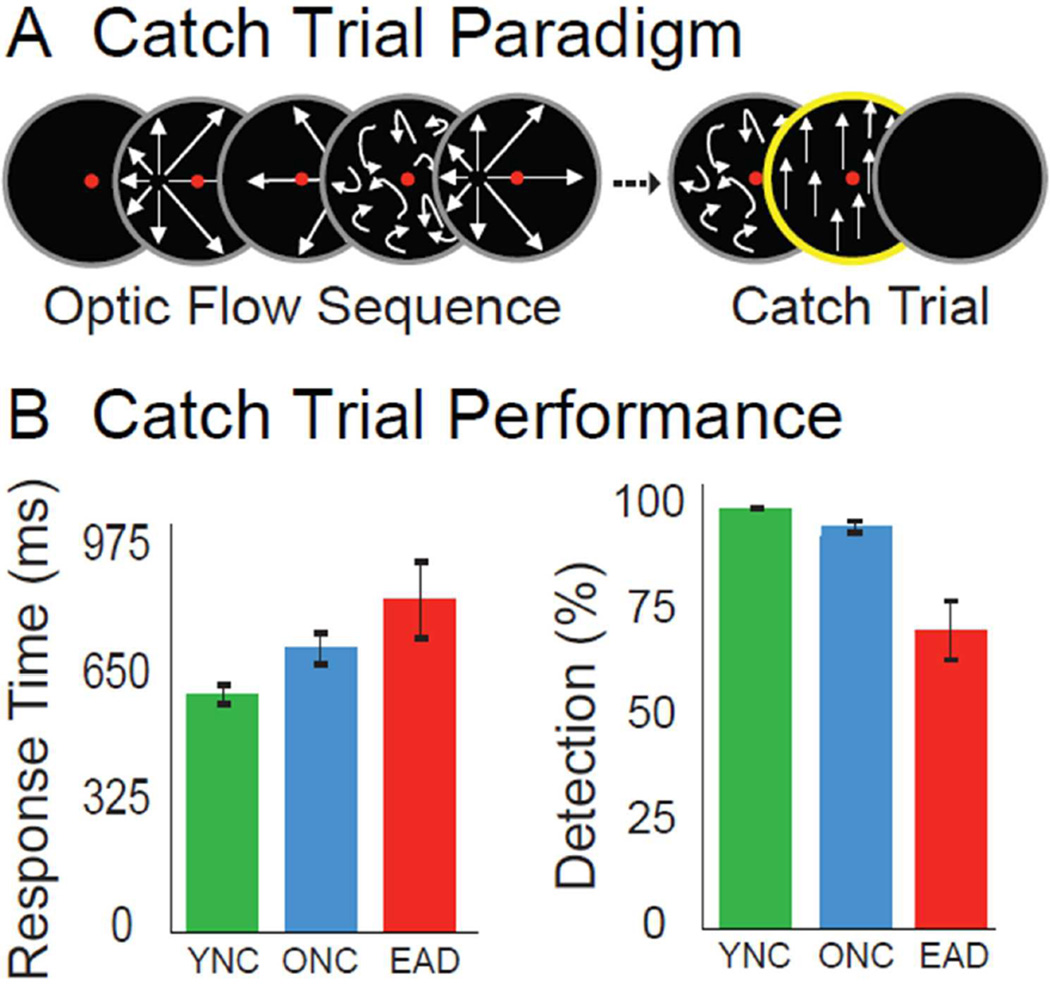
Each trial’s random number of interleaved random motion and radial motion stimuli finished with a vertical motion stimulus (Figure 2A). The vertical motion cued subjects’ to press an up or down button indicating the direction of the vertical motion and verifying attention to the stimuli.
Figure 2.
Vertical motion, catch trial stimuli and behavioral task performance. A. Catch-trial stimuli consisting of vertically moving dots were presented at the end of each trial sequence, and cued subjects to make an up or down button press response based on the direction of planar motion. B. Button press data were used to measure catch trial performance. Response times differentiated only the YNC from EAD (p= .016), while the percentage of detected catch was significantly lower in the EAD group (p= .004).
The button press data were used to calculate each subject’s percent detection and response times. Vertical motion catch trial response times are slower in EADs than in YNCs (F2,36 = 4.71, p = .016; Figure 2B, left) with percent catch trial detection being higher in YNCs than in EADs (F2,36 = 6.56, p =.004; Fig. 2B, right).
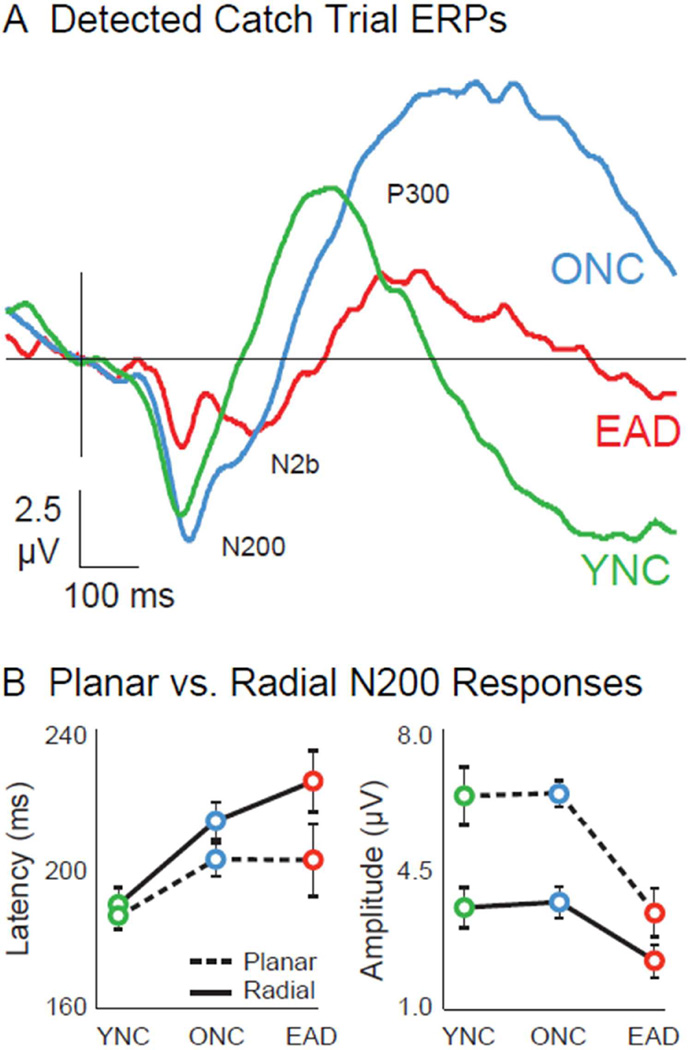
N200s from the vertical motion stimulus, and its catch trial task (Figure 3A), show shorter latencies (F1, 36 = 4.29, p=.046; Fig. 3B, left) and larger amplitudes than those to radial motion (F1, 36 = 33.04, p<.001; Fig. 3B, right). The shorter latencies to vertical motion suppress group differences (F2,38 = .97, p=.39). However, EAD’s vertical motion N200s are much smaller than those of YNCs or ONCs, more significantly (F2, 38 = 8.12, p = .001) than with radial motion.
Figure 3.
Vertical motion ERPs to catch trial stimuli and comparisons to radial motion responses. A. Responses evoked by detected catch trials revel three distinct wave components: a larger N200 wave, followed by a smaller N2b that is absent in YNCs, and a late P300. Vertical motion evokes N200s of equal latency in all groups, but of significantly smaller amplitude in EADs compared to ONCs (p= .01). The N2b component is delayed in the EAD (p= .009), and the P300 is delayed (p=.001) in ONCs and EADs, and diminished in EADs (p=.017). B. N200 responses evoked by planar motion catch trials are faster (p=__) in all groups, suppressing the age-related latency effect seen with optic flow. Planar motion also evokes larger N200s in all groups (p< .001), but this enhancement effect was much smaller in the EAD, making the AD-related amplitude effect more significant (p= .001).
The vertical motion ERPs show two additional components in ONCs and EADs: N2bs that are delayed in EADs compared to ONCs (F1,26 = 9.34, p=.004). P300s that are delayed (F2,38 = 9.04, p=.001) in ONCs and EADs, and diminished in EADs (F2,38 = 4.55, p=.017).
We assessed the behavioral relevance of the three vertical motion ERP components of ONCs and EADs using stepwise multiple linear regression of vertical motion response times and percent detections. In ONCs and EADs, vertical motion response times relied only on N2b amplitudes (F1,26 = 7.26, p=.012, βstd = .47). In contrast, vertical motion percent detections relied only on N200 amplitudes (F1,26 = 14.43, p=.001, βstd = .60). This implies that the effect of aging on response times is mainly related to attentional factors, whereas the effect of AD on motion detection is mainly related to sensory responsiveness.
DISCUSSION
Optic flow simulating observer self-movement evokes robust posterior ERPs that reflect dorsal extrastriate cortical specialization for the representation of heading direction [20, 21]. We find comparable N200 response amplitudes and latencies to a variety of heading directions (Figure 1A–B), consistent with a homogenous distribution of heading directions among neurons in dorsal stream cortex [22, 23].
These findings suggest an unbiased representation of heading directions, so that no direction is more readily seen than any other. It also suggests close parallels between ERPs and underlying cellular mechanisms of visual motion processing for heading representation, navigation, and steering in dorsal extrastriate cortex. This is consistent with the initial emergence of cortical dysfunction in areas other than those linked to verbal memory, that is, beyond mesial temporal structures [9].
Optic flow ERPs reveal clear distinctions between the physiological impact of aging and early AD. The most robust differentiation of aging and AD effects in optic flow ERPs is that aging delays N200 responses, whereas AD reduces N200 amplitude (Figure 1C). These effects belie the apparent continuum of functional impairments of optic flow based heading perception and navigation across these subject populations [10, 13].
The delay of N200 responses in ONs is consistent with decreased cortico-cortical conduction velocities in aging, the underlying mechanisms likely relating to aging-related changes in cortical white matter integrity [24, 25]. Diffusion-tensor MRI has provided new evidence of such changes [26] [27], and we have previously correlated DTI-MRI measures of white matter dissolution to optic flow perceptual impairments [28]. Still more severe white matter changes are seen in AD [29] [30], potentially creating virtual cortico-cortical disconnection [31] that could contribute to the loss of function seen in AD, and evidenced by the loss of neural responsiveness in these studies.
Our findings are consistent with earlier results on the effects of motion speed and coherence on optic flow N200s [32]. In the earlier work, we found that motion speed has little effect on responses in YNCs, ONCs, or EADs. However, reducing optic flow coherence by adding randomly moving dots, increases the latency and decreases the amplitude of N200s in all subject groups. Together, these studies suggest that optic flow responses are maintained across naturalistic self-movement cues such as heading and speed. In contrast, the less naturalistic motion incoherence cue degrades optic flow responses in a manner that imitates aspects of aging and AD. The apparent additivity of motion noise and aging effects supports the view that aging and AD increase the intrinsic noise in cortical processing [33].
Vertical motion stimuli yielded N200s with shorter latencies than those evoked by radial motion, which is consistent with dorsal extrastriate organization. Planar motion activates middle temporal cortex (MT), which projects to radial motion centers in posterior parietal areas [34, 35]. The ~4 ms difference between planar and radial N200 latencies in YNCs matches the latency of cortico-cortical conduction between neurons in adjacent visual areas [36]. The successively larger differences between planar and radial N200 latencies in ONCs (~12 ms) and EADs (~25 ms) suggest declines in cortico-cortical conduction velocities [31] [30].
Vertical motion, linked to the catch trial detection task, also yielded N200s with larger amplitudes than those evoked by radial motion. We previously found that planar motion evokes the same amplitudes as radial motion [37], prompting us to attribute the larger planar motion N200s in these data to the concurrent behavioral task. This is consistent with enhanced cortical neuronal responses to motion stimuli that are linked to behavioral tasks [38, 39]. Thus, the absence of larger vertical motion N200s in EADs suggests that AD impairs task-related response enhancement.
The absence of task enhancement in EADs may be linked to the delay of late ERP components. The vertical motion N200s of ONCs and EADs were followed by N2b and P300 responses (Figure 3). The N2b is thought to reflect top-down activation related to attentional orientation to a task [40], with the subsequent triggering a behavioral response reflected in the P300 [41]. The substantial delay of the N2b in EADs may prevent its additive interactions with the N200. The more synchronous N200 and N2b of ONCs, and the indistinct (possibly synchronous) waveforms of YNCs, might be a source of N200 enhancement in those groups. Likewise the distinctly delayed N2b of EADs could account for their lack of N200 enhancement in the vertical motion catch trial responses, and their impaired performance in the motion detection task.
The suggestion that delayed top-down enhancement of motion evoked N200s plays an important role in AD effects is supported by parallels between our behavioral and neurophysiological findings. Vertical motion behavioral response times increased with aging, but only EADs showed impaired detection (Figure 2). Likewise, aging increased N200 and N2b latency, the latter being linked to delays in our data. In contrast, only the EADs showed the reduced N200 amplitudes that were linked to poor performance in our detection task.
Thus, our findings link age-related delays in a behavioral tasks [42] to prolonged ERP latencies [43], and AD related perceptual impairments [44] to reductions in ERP amplitudes [45]. As a result, we must conclude that aging and AD are distinguishable processes. This leads to the critical question of whether AD represents a separate process that is superimposed on aging, or an extension of the aging process that is caused by aging. If the latter is the case, neurophysiological studies may define a sub-group of our older adults who are at immediate risk of progression to AD and the best population for the study of potentially disease altering interventions.
Acknowledgements
We gratefully acknowledge William Vaughn’s expertise in the development of the software used in these studies. We are also grateful for Teresa Steffenella’s contributions to data acquisition. This work was supported by AG17596, EY10287, and NS06574.
REFERENCES
- 1.Cogan DG. Visual disturbances with focal progressive dementing disease. American Journal of Ophthalmology. 1985;100:68–72. doi: 10.1016/s0002-9394(14)74985-2. [DOI] [PubMed] [Google Scholar]
- 2.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Archives of Neurology. 1988;45:789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- 3.Levine DN, Lee JM, Fisher CM. The Visual Variant of Alzheimer's Disease: A Clinicopathologic Case Study. Neurology. 1993;43:305–313. doi: 10.1212/wnl.43.2.305. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein NM, Flaherty G, Tobin TS. Dementia and wandering behavior: concern for the lost elder. New York: Springer Publishing Company; 2002. [Google Scholar]
- 5.Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- 6.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild Cognitive Impairment Represents Early-Stage Alzheimer's Disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 8.Pietrini P, Furey ML, Graff-Radford N, Freo U, Alexander GE, Grady CL, Dani A, Mentis MJ, Schapiro MB. Preferential Metabolic Involvement of Visual Cortical Areas in a Subtype of Alzheimer's Disease: Clinical Implications. American Journal Psychiatry. 1996;153:1261–1268. doi: 10.1176/ajp.153.10.1261. [DOI] [PubMed] [Google Scholar]
- 9.Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical,genetic, and neuropathological characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien HL, Tetewsky SJ, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in Alzheimer's disease. Cereb Cortex. 2001;11:1083–1092. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- 11.Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: Getting lost between aging and AD. Neurology. 2003;60:802–808. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- 12.Mapstone M, Duffy CJ. Approaching objects cause confusion in patients with Alzheimer's disease regarding their direction of self-movement. Brain. 2010;133:2690–2701. doi: 10.1093/brain/awq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Spatial disorientation in Alzheimer's disease: the remembrance of things passed. Neurology. 2003;61:1491–1497. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- 14.Kavcic V, Fernandez R, Logan DJ, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer's disease. Brain. 2006;129:736–746. doi: 10.1093/brain/awh727. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez R, Kavcic V, Duffy CJ. Neurophysiologic analyses of low- and high-level visual processing in Alzheimer disease. Neurology. 2007;68:2066–2076. doi: 10.1212/01.wnl.0000264873.62313.81. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conference Proceedings. 1982;2:1–10. [Google Scholar]
- 18.Rosenfeld VR. Enumerating De Bruijn sequences. Match-Communications in Mathematical and in Computer Chemistry. 2002:71–83. [Google Scholar]
- 19.Rosenfeld VR. Enumerating Kautz sequences. Kragujevac J Math. 2002;24:41. [Google Scholar]
- 20.Ungerleider LG. The cortical pathways for object recognition and spatial perception. In: Chagas C, Gattass R, Gross C, editors. Pattern Recognition Mechanisms. New York: Springer-Verlag; 1985. pp. 21–38. [Google Scholar]
- 21.Duffy CJ, Wurtz RH. Response of monkey MST neurons to optic flow stimuli with shifted centers of motion. Journal of Neuroscience. 1995;15:5192–5208. doi: 10.1523/JNEUROSCI.15-07-05192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page WK, Duffy CJ. Heading representation in MST: sensory interactions and population encoding. Journal of Neurophysiology. 2003;89:1994–2013. doi: 10.1152/jn.00493.2002. [DOI] [PubMed] [Google Scholar]
- 23.Logan DJ, Duffy CJ, Logan DJ, Duffy CJ. Cortical area MSTd combines visual cues to represent 3-D self-movement. Cerebral Cortex. 2006;16:1494–1507. doi: 10.1093/cercor/bhj082. [DOI] [PubMed] [Google Scholar]
- 24.Leuchter AF, Dunkin JJ, Lufkin RB, Anzai Y, Cook IA, Newton TF. Effect of white matter disease on functional connections in the aging brain. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57:1347–1354. doi: 10.1136/jnnp.57.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Double KL, Halliday GM, Kril JJ, Harasty JA, Cullen K, Brooks WS, Creasey H, Broe GA. Topography of brain atrophy during normal aging and Alzheimer's disease. Neurobiology of Aging. 1996;17:513–521. doi: 10.1016/0197-4580(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 26.Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol.Aging. 2002;23:433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 27.Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating Imaging Indices of White Matter Integrity and Volume in Healthy Older Adults. Cereb.Cortex. 2007 doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- 28.Kavcic V, Ni H, Zhu T, Zhong J, Duffy CJ. White matter integrity linked to functional impairments in aging and early Alzheimer's disease. Alzheimers Dement. 2008;4:381–389. doi: 10.1016/j.jalz.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann.Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 30.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol.Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Hof PR, Bouras C, Constantinidis J, Morrison JH. Selective disconnection of specific visual association pathways in cases of Alzheimer's disease presenting with Balint's syndrome. Journal of Neuropathology & Experimental Neurology. 1990;49:168–184. doi: 10.1097/00005072-199003000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez R, Duffy CJ. Early Alzheimer's disease blocks responses to accelerating self-movement. Neurobiol Aging. 2012;33:2551–2560. doi: 10.1016/j.neurobiolaging.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salthouse TA, Lichty W. Tests of the neural noise hypothesis of age-related cognitive change. J Gerontol. 1985;40:443–450. doi: 10.1093/geronj/40.4.443. [DOI] [PubMed] [Google Scholar]
- 34.Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. Journal of Comparative Neurology. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orban GA, Vanduffel W. Functional Mapping of Motion Regions. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge: MIT Press; 2004. pp. 1229–1246. [Google Scholar]
- 36.Girard P, Hupe JM, Bullier J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. J Neurophysiol. 2001;85:1328–1331. doi: 10.1152/jn.2001.85.3.1328. [DOI] [PubMed] [Google Scholar]
- 37.Kavcic V, Fernandez R, Logan D, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer's disease. Brain. 2006;129:736–746. doi: 10.1093/brain/awh727. [DOI] [PubMed] [Google Scholar]
- 38.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 39.Dubin MJ, Duffy CJ. Behavioral influences on cortical neuronal responses to optic flow. Cereb.Cortex. 2007;17:1722–1732. doi: 10.1093/cercor/bhl083. [DOI] [PubMed] [Google Scholar]
- 40.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DE. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salthouse TA, Toth J, Daniels K, Parks C, Pak R, Wolbrette M, Hocking KJ. Effects of aging on efficiency of task switching in a variant of the trail making test. Neuropsychology. 2000;14:102–111. [PubMed] [Google Scholar]
- 43.Celesia GG, Daly RF. Effects of aging on visual evoked responses. Arch Neurol. 1977;34:403–407. doi: 10.1001/archneur.1977.00500190037005. [DOI] [PubMed] [Google Scholar]
- 44.Silverman SE, Tran DB, Zimmerman KM, Feldon SE. Dissociation between the detection and perception of motion in Alzheimer's disease. Neurology. 1994;44:1814–1818. doi: 10.1212/wnl.44.10.1814. [DOI] [PubMed] [Google Scholar]
- 45.Kubova Z, Kremlacek J, Valis M, Langrova J, Szanyi J, Vit F, Kuba M. Visual evoked potentials to pattern, motion and cognitive stimuli in Alzheimer's disease. Doc Ophthalmol. 2010;121:37–49. doi: 10.1007/s10633-010-9230-5. [DOI] [PubMed] [Google Scholar]