Summary
Purinergic signalling has been postulated as a mechanism of cellular signalling since the early 1970s. Cellular responses triggered by extracellular nucleotides and nucleosides occur by defined adenosine (P1) and ATP (P2) receptors, respectively, and play a prominent role in many aspects of health and disease, including those involving the liver. In normal physiology, extracellular nucleotides modulate many of the normal biologic and hepatic metabolic processes such as gluconeogenesis and insulin responsiveness. Further, in multiple disease states, ATP and certain nucleotides serve as danger signals and are involved in heightened purinergic receptor activation in a myriad of pathologic processes. Recently, others and we have shown the regulation of purinergic signalling by ectonucleotidases to play an important role in the acute vascular pathobiology of liver inflammation, regeneration and immunity, as in ischemia reperfusion and transplantation. Increased understanding into mechanisms of extracellular ATP metabolism by such ecto enzymes has also led to novel insights into the exquisite balance of nucleotide P2-receptor and adenosinergic P1-receptor signalling in those chronic hepatic diseases, characterized by steatosis, fibrosis and malignancy. This review will explore the developing role of purinergic signalling in the pathophysiology of liver disease and comment on potential future clinical applications.
Keywords: Ectonucleotidases, P2-receptor, CD39, CD73, T lymphocytes, Liver disease
Introduction
The purinergic signalling hypothesis, i.e. ATP acting as an extracellular signalling molecule, was proposed by one of us (GB) in 1972. Separate families of receptors for adenosine (P1) and ATP (P2) were recognised in 1978. Receptors for purines and pyrimidines were cloned and characterised in the early 1990’s and receptors for ATP separated into P2X ion channel and P2Y G-protein-coupled receptors [1]. It is now known that adenosine has A1, A2A, A2B and A3 subtypes of G-protein-coupled receptors; of these, the A1 adenosine receptor (AR) subtype is well characterized in cardiac, liver and fat tissues.
Later work has defined families of ectonucleotidases e.g. of the ecto-nucleoside triphosphate diphosphohydrolase (ENTPD) CD39 family that hydrolyze extracellular nucleotides and ultimately generate the respective nucleoside derivatives; thereby uniquely regulating this process of cellular signalling. Biological stressors may lead to alteration of release of nucleotides, impact uptake of nucleosides or may decrease enzymatic functions of ectonucleotidases [2].
The liver is of major importance for both systemic nucleotide homeostasis as well as for local intercellular signalling within hepatic lobules, canalicular networks and the biliary systems. The hepatocyte parenchyma, Kupffer, vascular endothelial and smooth muscle cells, stellate cells, myofibroblasts, immune cells and biliary canaliculi (bile duct epithelia) all express purinoreceptors.
Purinergic signalling regulates important hepatic processes such as cell homeostasis, glycogen metabolism, bile secretion and blood flow. Because of space constraints, this review will focus on the pathological roles of purinergic signalling in the liver, as these relate to common disease states.
Inflammatory liver injury
Vascular injury can occur to a native liver during inflammation or with shock-like states, or can be an acute consequence of ischemia reperfusion injury following transplantation. Despite the dual blood supply to the liver, vascular compromise can be catastrophic. Vascular injury induces a wide array of inflammatory responses including the release of adenine nucleotides which drive inflammation and platelet activation [3]. Infusion of ATP under these conditions improves hepatic function and survival after ischemia and following reperfusion. Such interventions are associated with decreased levels of the inflammatory cytokines tumour necrosis factor and interleukin-6 [4].
Vascular CD39 (ENTPD1) ectonucleotidase action seems to have a protective role in hepatic ischemia reperfusion injury, potentially by generating adenosine in concert with CD73/ecto-5′-nucleotidase. However, after hepatic ischemia and reperfusion injury, the hepatic vascular ectonucleotidase activity is lost. Hence, deletion of Entpd1 in mice leads to significantly increased vascular injury and decreased survival under these conditions. Wild type and CD39-deficient mice that receive adenosine are also protected from reperfusion injury [5]. Curiously, deletion of CD39 in natural killer (NK) cells attenuates hepatic ischemia/reperfusion injury in mice suggesting ATP modulates innate cell functions during liver regeneration. In this respect, NK cells that lack the CD39 gene produce less interferon gamma in response to inflammatory mediators [6].
Pharmacologic pre-conditioning is a potential mechanism to protect against hepatic ischemic reperfusion injury. Stimulation of ARs has been associated with liver protection from ischemia. Interestingly hepatic ischemic preconditioning is associated with upregulation of CD39. This is likely mediated by transcription factor Sp1, a potential therapeutic target for the treatment of liver ischemia [7].
Regeneration
After partial hepatectomy adenine nucleotides have been noted to undergo rapid decreases in the remnant liver. The onset of liver regeneration occurs very rapidly potentially related to this immediate loss of intracellular nucleotides. ATP activates cell cycle progression and proliferation of hepatocytes in vitro and in vivo and modulates growth factor activities probably via P2Y2 receptors via paracrine signalling. In fact 30 seconds after partial hepatectomy the ATP content of a remnant liver has been shown to decrease by 50% compared to control. Increased energy demands can account for some of the decrease, however it is postulated that ATP is an immediate sensing mechanism relaying information to the remnant liver tissue to start to regenerate [8].
Hepatocellular proliferation is impaired in P2Y2 receptor knockout mice, establishing a trophic role for ATP in hepatocyte proliferation with implications for liver regeneration and growth after injury [9]. Regulated catalysis of extracellular nucleotides by vascular CD39 (NTPDase1) is required for both hepatocyte and endothelial cell proliferation (sinusoidal angiogenesis) during liver regeneration [10].
Fibrotic liver disease and cirrhosis
Caffeine is the most common pharmacologically active agent ingested worldwide and has major effects as an AR antagonist. Caffeine may be related to the progression of hepatic fibrosis. Epidemiologically, enthusiastic coffee drinking lessens the likelihood of death and hospitalization from chronic liver diseases [11].
In addition adenosine partially reverses cirrhosis induced by carbon tetrachloride in rats and impacts established micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation [12].
How does this occur at the cellular level? Stellate cells are present in the perisinusoidal space of Disse. They are thought to represent the main hepatic source of extracellular matrix components. Stellate cells exhibit responsiveness to metabolic needs imposed by liver growth and repair by impacting extracellular matrix content. These cells interact closely with other myofibroblastic type cells in autocrine and paracrine purinergic signaling responses involving both nucleosides and nucleotides.
Stellate cells express functional A2A as well as P2Y receptors; furthermore, activated cells alter the expression of P2Y receptor subtypes. Moreover, these cells regulate ectonucleotidase expression markedly after activation. As activation of P2Y receptors in these activated hepatic stellate cells might also regulate procollagen-1 transcription, these may be attractive targets to prevent or treat liver fibrosis [13].
Although much interest has been directed at CD39 on sinusoidal endothelial cells and stellate cells, the related ectonucleotidase CD39L1/NTPDase 2 may be more relevant to less common forms of liver disease. This latter ectonucleotidase is preferentially expressed by portal myofibroblasts and is selectively down-regulated in biliary cirrhosis [14].
Portal hypertension, hepato-renal syndrome and hepatic encephalopathy
As cirrhosis progresses the normal response to increased portal venous vascular resistance of decreasing hepatic artery vascular resistance is decreased. The process is mediated by adenosine in normal livers. In cirrhotic livers the adenosine-mediated vasodilatation of the hepatic artery is exaggerated, leading to a greater impact on the hepatic arterial flow after exposure to adenosine [15].
There is evidence for aberrant purinergic signaling in the hepato-renal syndrome. Intra-hepatic caffeine administered via the portal vein has been shown to increase urine output in rats. This effect was not seen with intra-venous caffeine or after the liver was denervated [16]. This effect appears to be mediated by hepatic A1 AR. Chronic selective blockade of putative hepato-renal reflexes via SLV329 (A1 receptor antagonist) displays both diuretic and natriuretic effects without a change of creatinine clearance in rat models of cirrhosis [17].
Hepatic encephalopathy is a neuropsychiatric syndrome with either acute or chronic impaired liver function. Adenosine influences the high-affinity uptake of glutamate and aspartate under conditions of hepatic encephalopathy, which has therapeutic implications for the treatment of this disease [18]
Cancer
Liver cancer is typically associated with chronic inflammatory states, which are linked to immune dysregulation, disordered metabolism, and aberrant cell proliferation. However, in established or disseminated metastatic malignancy ATP plays an important role as an early danger signal to the immune system. The catalytic properties of CD39, expressed by either immune suppressive T regulatory cells or endothelium, appear key in abrogating this process. In generating adenosine, CD39 ectonucleotidase activities inhibit T cell proliferation and impact immune responses while promoting angiogenesis, thus being permissive for the growth of transplanted tumors [19] (See Fig. 1).
Figure 1.
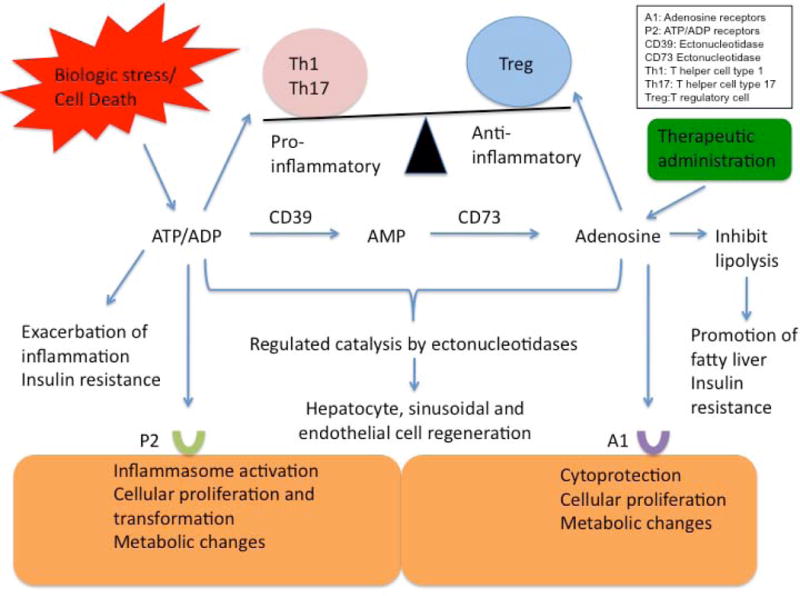
An overview of the complex interplay between extracellular ATP and adenosine. As illustrated, purinergic signalling influences both the process of inflammation as well as cellular regeneration. Extracellular ATP from biologic stress can incite inflammation. As ATP is metabolized by ectonucleotidases on endothelial surfaces to adenosine, the inflammatory response is regulated through induction of immune suppressive T regulatory cells. This has important implications for potential therapies in malignancy and liver transplantation.
Both ATP and adenosine in concert alter the balance of apoptosis and proliferation deviating cells towards malignancy. ATP infusions into the intraperitoneal space of a two-stage rat model of hepatocarcinogenesis increase numbers of preneoplastic foci in the liver. These manifestations are comparable to what has been observed with Entpd5 knock out mice [20]. ENTPD5/CD39L4 is a related ectoenzyme to CD39, and is a soluble endoplasmic reticulum UDPase involved in intracellular purine metabolism, which promotes glycolysis as well as proliferation in cancer cells via the PTEN signaling pathway. Interestingly, there are contrasting roles of this ectonucleotidase as well as CD39 in the suppression of liver cancer development vs. the promotion of transplanted tumor growth in mice [21].
Hepatic steatosis/steatohepatitis, alcoholic liver disease and toxins
Type 2 diabetes is associated with non-alcoholic steatohepatitis and insulin resistance. Others, and we, have proposed that extracellular nucleotides and nucleosides serve as “metabolokines”, suggesting functional links between inflammation and associated metabolic derangements. Adenosine has been shown to activate A1 AR in adipocytes, which decreases adenylate cyclase activity leading to inhibition of lipolysis. This discovery was made using several selective A1 AR agonists as well as A1 AR knockout mice. Modulation of the A1 AR and impacts on the regulation of lipolysis in pathological conditions where free fatty acids play an important role and have great potential importance in many common diseases e.g. insulin resistance, diabetes, and dyslipidemia.
We have previously established that genetic deletion of Cd39/Entpd1 worsens insulin resistance and has a major impact on hepatic glucose metabolism. Here the effects appear preferentially related to aberrant effects of extracellular nucleotide signaling [22]. Excessive alcohol gestion may be associated with fatty liver. The mechanisms by which ethanol stimulates these morphological changes remain unknown. Recent studies from the laboratory of Cronstein and co-workers indicate that excessive adenosine generated by ethanol metabolism plays an important role in ethanol-induced hepatic steatosis. This effect appears dependent upon both A1 and A2B receptors. Hence, pharmacological targeting of these ARs may be effective in the prevention of alcohol-induced steatosis and early liver disease [23].
Hepatic inflammation with stimulation of immune cells contributes to acetaminophen (APAP) hepatotoxicity in mice and is triggered by P2X7 receptor activation. In this model, Entpd1 null mice exhibit enhanced P2X7 signaling and show increased APAP-induced hemorrhage and mortality. As the use of soluble ectonucleotidases (e.g. apyrase) also decrease APAP-induced mortality, this suggests a potential future therapeutic intervention for this condition [25].
Liver transplantation
Assessment of graft viability and post-transplant instability using a combination of liver ATP levels and serum hyaluronic acid has been proposed [26]. Although human liver has been successfully maintained under hypothermic conditions with high concentrations of adenosine, as in University Wisconsin preservation solutions, for up to 10–14 hrs, fully overcoming ischemic damage is a major obstacle to liver transplantation. Infused ATP also promotes cellular recovery after ischemic injury; this action is enhanced by the synergistic effect of superoxide dismutase [27].
Another development from purinergic signaling is the ability to monitor and predict rejection in the post transplant allograft. The ImmuKnow assay is a measure of peripheral blood CD4+ total ATP, which can be used to monitor immune response. In patients with hepatitis C virus (HCV) after orthotopic liver transplant, low ATP levels in CD4+ T cells based on the ImmuKnow assay were associated with progression to fibrosis. Thus the greater suppression of cellular immunity, the greater the risk of development of fibrosis. Recipients with recurrent HCV have significantly lower immune responses compared to those with acute cellular rejection [28].
Conclusions and future directions
There are now substantial data implicating extracellular nucleotides and nucleosides in a variety of normal metabolic liver functions. Aberrant or disordered purinergic signalling are also components of many disease states of the liver. In terms of therapeutic strategies, modulation of purinergic signalling via changes in nucleotide fluxes, inducing or inhibiting ectonucleotidase actions or otherwise scavenging nucleosides may prove useful for limiting and controlling pathological immune responses. Further, unravelling the role of adenosine in ischemia reperfusion injury may lead to therapies for the donor liver that result in prolonged graft survival. The development of selective agonists and antagonists for purinoceptor subtypes that are orally bioavailable and stable in vivo might be employed for these and other pathological conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998:412–492. [PubMed] [Google Scholar]
- 2.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 3.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, et al. Regulated Catalysis of Extracellular Nucleotides by Vascular CD39/ENTPD1 Is Required for Liver Regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P, Ba ZF, Morrison MH, Ayala A, Dean RE, Chaudry IH. Mechanism of the beneficial effects of ATP-MgCl2 following trauma-hemorrhage and resuscitation: downregulation of inflammatory cytokine (TNF, IL-6) release. J Surg Res. 1992;52:364–371. doi: 10.1016/0022-4804(92)90117-i. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, Imai M, Nowak-Machen M, Guckelberger O, Enjyoji K, Wu Y, et al. Liver damage and systemic inflammatory responcses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signalling. 2011;7:427–434. doi: 10.1007/s11302-011-9239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crumm S, Cofan M, Juskeviciute E, Hoek JB. Adenine nucleotide changes in the remnant liver: An early signal for regeneration after partial hepatectomy. Hepatology (Baltimore Md) 2008;48:898–908. doi: 10.1002/hep.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thevananther S, Sun H, Hernandez A, Awad SS, Karpen SJ. Impaired hepatocellular proliferation in P2Y2 purinergic receptor knockout mice: mitogenic role of extrcellular ATP. Hepatology. 2008(44):206A. [Google Scholar]
- 10.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, et al. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Frontiers in bioscience: a journal and virtual library. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadden Ish, Partovi N, Yoshida EM. Review article: possible beneficial effects of coffee on liver disease and function. Alimentary Pharmacology and Therapeutics. 2007;26:1–8. doi: 10.1111/j.1365-2036.2007.03319.x. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Muñoz R, Díaz-Muñoz M, Suárez-Cuenca JA, Trejo-Solís C, López V, Sánchez-Sevilla L, et al. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology (Baltimore Md) 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- 13.Dranoff JA, Ogawa M, Kruglov EA, Gaça MDA, Sévigny J, Robson SC, et al. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G417–424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dranoff JA, Kruglov EA, Toure J, Braun N, Zimmermann H, Jain D, et al. Ectonucleotidase NTPDase2 is selectively down-regulated in biliary cirrhosis. J Investig Med. 2004;52:475–482. doi: 10.1136/jim-52-07-42. [DOI] [PubMed] [Google Scholar]
- 15.Zipprich A, M WZ, Ripoll C, Groszmann RJ. A distinct nitric oxide and adenosine A1 receptor dependent hepatic artery vasodilatory responcse in the CCL-cirrhotic liver. Liver Int. 2010;30:988–994. doi: 10.1111/j.1478-3231.2010.02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ming Z, Lautt WW. Caffeine-induced natriuresis and diuresis via blockade of hepatic adenosine-mediated sensory nerves and a hepatorenal reflex. Can J Physiol Pharmacol. 2010;88:1115–1121. doi: 10.1139/y10-090. [DOI] [PubMed] [Google Scholar]
- 17.Hocher B, Heiden S, von Websky K, Arafat AM, Rahnenfuhrer J, Alter M, et al. Renal effects of the novel selective adenosine A1 receptor blocker SLV329 in experimental liver cirrhosis in rats. PLoS ONE. 2011;6:e17891. doi: 10.1371/journal.pone.0017891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt W, Wolf G, Grüngreiff K, Linke K. Adenosine influences the high-affinity uptake of transmitter glutamate and aspartate under conditions of hepatic encephalopathy. Metab Brain Dis. 1993;8:73–80. doi: 10.1007/BF00996890. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Müller CE, et al. CD39/ENTPD1 Expression by CD4+Foxp3+ Regulatory T Cells Promotes Hepatic Metastatic Tumor Growth in Mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, et al. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia (New York, NY) 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW, et al. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell. 2010;143:711–724. doi: 10.1016/j.cell.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Enjyoji K, Kotani K, Thukral C, Blumel B, Sun X, Wu Y, et al. Deletion of Cd39/Entpd1 Results in Hepatic Insulin Resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Borea PA, Wilder T, Yee H, Chiriboga L, Blackburn MR, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. The Journal of clinical investigation. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, et al. P2X7 Receptor-Mediated Purinergic Signaling Promotes Liver Injury in Acetaminophen Hepatotoxicity in Mice. AJP: Gastrointestinal and Liver Physiology. 2012 doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudo Y, Takaya S, Kobayashi M, Fukuda A, Harada O, Suto T, et al. Assessment of graft viability using hyaluronic acid and adenosine triphosphate in orthotopic liver transplantation from non-heart-beating donors. Transplant Proc. 2000;32:2114–2115. doi: 10.1016/s0041-1345(00)01594-3. [DOI] [PubMed] [Google Scholar]
- 26.Flye S. The synergistic effect of superoxide dismutase and adenosine triphosphate-MgCl2 on acute hepatic ischemia. Transplant Proc. 1987;19:1324–1326. [PubMed] [Google Scholar]
- 27.Mizuno S, Hamada T, Nakatani K, Kishiwada M, Usul M, Sakurai H, et al. Monitoring peripheral blood CD4+ adenosine triphosphate activity after living donor liver transplantation: impact of combination assays of immune function and CYP3A5 genotype. J Hepatobiliary Pancreat Sci. 2011;18:226–232. doi: 10.1007/s00534-010-0335-8. [DOI] [PubMed] [Google Scholar]


