Abstract
YB-1 is a multifunctional protein involved in the regulation of transcription, translation, mRNA splicing and probably DNA repair. It contains a conserved cold shock domain and it binds strongly to inverted CCAAT box of different promoters. In this study, we have found that purified YB-1 oligomerizes readily in solutions to form trimers, hexamers and oligomers of 12 molecules. The presence of ATP changed the conformation of YB-1 in such a way that only dimers were detected by gel filtration analyses. Purified YB-1 can separate different DNA duplexes containing blunt ends, 5′ or 3′ recessed ends, or forked structures. This strand separation activity is increased on cisplatin-modified DNA or with duplex molecules containing mismatches. In addition to its exonuclease activity, YB-1 exhibits endonucleolytic activities in vitro. Finally, YB-1 affinity chromatography experiments have indicated that in addition to prespliceosome factors like nucleolin and ALY, YB-1 binds the DNA repair proteins MSH2, DNA polymerase δ, Ku80 and WRN proteins in vitro. Furthermore, immunofluorescence studies have shown that YB-1 re-localizes from the cytoplasm to nuclear areas containing either Ku80 or MSH2 proteins in human 293 embryonic kidney cells. These results suggest that YB-1 is involved in base excision and mismatch repair pathways.
INTRODUCTION
YB-1 is a member of a family of DNA-binding proteins containing a cold shock domain. It was originally identified by its ability to bind to the inverted CCAAT box (Y-box) in the promoter region of MHC class II genes (1). Subsequently, YB-1 has been shown to regulate the expression of a number of genes including proliferating cell nuclear antigen (PCNA) (2), thymidine kinase (3), DNA polymerase α (4), myosin light-chain 2v gene (5), Fas antigen (6), collagen α1(I) gene (7), protein tyrosine phosphatase 1B (PTP1B) gene (8), gelatinase A (9) and the multidrug resistance (MDR1) gene (10,11). In addition to the regulation of transcription, YB-1 is a multifunctional protein that also affects the splicing and the translation of specific mRNAs. Indeed, analysis of the human presplicesome complex has indicated the presence of YB-1 in this complex (12). Furthermore, YB-1 interacts with the splicing factor SRp30c and confers alternative splice site selection in the E1A minigene model system (13). YB-1 is also known to bind A/C-rich exon enhancers and to stimulate splicing of the CD44 alternative exon v4 (14). YB-1 is a component of messenger ribonucleoprotein particles which regulate translation in COS cells (15). Consistent with this observation, YB-1 is capable of specifically regulating the translation of ferritin mRNA (16). Finally, YB-1 is known to increase the stability of IL-2 specific mRNA during T-cell activation (17).
In recent years, several laboratories have demonstrated that YB-1 is directly involved in the cellular response to genotoxic stress. Upon UV irradiation or cisplatin treatments, YB-1 binds to a Y-box element within the MDR1 promoter and increases its expression (11). Moreover, depletion of YB-1 expression protein with anti-sense RNA against YB-1 specific mRNA results in increased sensitivity to cisplatin (11). Interestingly, YB-1 is increased in cultured cell lines resistant to cisplatin. In fact, several studies have indicated that the level of nuclear expression of YB-1 is predictive of drug resistance and patient outcome in breast tumors, ovarian cancers and synovial sarcomas (18–22). Upon UV irradiation, YB-1 translocates from the cytoplasm to the nucleus (23) and is known to bind to modified nucleic acid (24). YB-1 preferentially binds to cisplatin-modified DNA and interacts with PCNA (25), a component of several DNA repair systems (26). In addition, YB-1 stimulates an endonuclease involved in base excision repair (27). All these observations suggest that YB-1 is important in DNA repair and in conferring drug resistance on tumor cells.
It has been reported that YB-1 creates single-stranded regions in the DRA promoter (28) and it is believed that this activity is required in part for the regulation of target promoters (29). In recent years, YB-1 has been shown to bind preferentially to single-stranded nucleic acids and to exhibit 3′-5′ exonuclease activity (30). In this report, we investigated the strand separation activity of human YB-1 against different double-stranded DNA substrates in vitro. Different deletion mutants of YB-1 have indicated that amino acids 39–205 are required for the DNA strand separation activity. We have also found that YB-1 actively promotes strand separation of duplex DNA containing either mismatches or cisplatin modifications independently of the nucleotide sequence. It also exhibits an endonuclease activity on double-stranded DNA. Finally, in vitro YB-1 affinity chromatography and immunofluorescence analyses have shown that several DNA repair proteins can interact with YB-1 reinforcing the notion that this multifunctional protein is involved in the repair of specific DNA damage.
MATERIALS AND METHODS
Cell lines and antibodies
Human 293 embryonic kidney cells were maintained in DMEM supplemented with 10% fetal bovine serum. Polyclonal antibodies against the human WRN were purchased from Novus Biologicals (Littleton, CO). Antibodies against PARP-1 and DNA polymerase δ were purchased from Transduction Laboratories (Lexington, KY). Antibodies against ALY, REF1 and XRCC1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Ku80 were purchased from NeoMarkers (Fremont, CA). Antibodies against DNA-PK, MLH1, MSH2 and PMS2 were purchased from Oncogene Research Products (Boston, MA). Antibodies against nucleolin were purchased from Medical and Biological Laboratories Co. (Watertown, MA). Rabbit polyclonal antibody against human YB-1 and the corresponding pre-immune serum was kindly provided by Dr P. E. DiCorleto (The Cleveland Clinic Foundation, Cleveland, OH). Finally, all horseradish peroxidase-conjugated secondary antibodies were purchased from Amersham Pharmacia. The above antibodies were used as indicated by the manufacturers. Western blots were performed as described previously (31).
Plasmids
Several GST-fusion proteins were constructed for the ‘pull down’ or YB-1 affinity purification assay. Human YB-1 coding sequence was amplified by PCR with appropriate oligonucleotides for subsequent cloning into the BamHI/EcoRI sites of the pGEX-2TK vector. In addition, YB-1 cDNA was cut with SmaI and EcoRI (amino acid residues 39–312 of YB-1), SmaI and SalI (residues 39–205), SalI and EcoRI (residues 205–312) and these fragments were cloned into the appropriate modified restriction sites in the pGEX-2TK vector. A pGEX-2TK construct coding for a GST-fusion peptide containing the exonuclease domain of p53 (p53exo) was kindly provided by the laboratory of Jacques Côté (Centre de Recherche en Cancérologie, Québec City, Canada). ProScan analyses on p53 have indicated that its exonuclease domain is within amino acids 185–290. Plasmids were transfected into BL21 bacteria for fusion protein production. Proteins were visualized by Coomassie staining when indicated.
YB-1 purification and gel filtration
BL21 cells expressing GST–YB-1 fusion proteins were lysed in NETN buffer (0.5% NP-40, 20 mM Tris–HCl pH 8.0, 100 mM NaCl and 1 mM EDTA) and incubated overnight with glutathione–Sepharose beads. The next day, beads were washed with NETN buffer and treated with biotinylated thrombin (Novagen) for 2 h at room temperature in thrombin cleavage buffer (20 mM Tris–HCl pH 8.4, 150 mM NaCl, 2.5 mM CaCl2). Beads were spun down and the supernatant was kept for the next step. Thrombin was captured by incubation with streptavidine agarose (Novagen) for 2 h on a rocking platform at room temperature. Agarose beads were spun down and YB-1 protein from the supernatant was concentrated onto Centricon-30 filters (Amicon). Protein concentration was determined using the Bradford assay. Proteins were then loaded onto a Superdex-200 column for gel filtration analysis using an AKTA-FPLC as indicated by the manufacturer (Amersham Pharmacia). Proteins from each fraction of the column were visualized by Coomassie staining.
Strand separation and endonuclease assays
The DNA substrates used for the strand separation and nuclease assays are depicted in Table 1. One strand was labeled with T4 polynucleotide kinase and [γ-32P]ATP and annealed to its complementary strand as described previously (32). DNA with cisplatin adducts were created by incubating oligonucleotides with cisplatin overnight as described previously (33). DNA substrate was incubated with the recombinant proteins as indicated in the figure legends for 30 min at 37°C in the reaction buffer (40 mM Tris–HCl, pH 7.5, 4 mM MgCl2, 5 mM DTT, 2 mM ATP and 0.1 µg/ml bovine serum albumin). Four microliters of loading buffer was added to the reaction (40% glycerol, 50 mM sodium EDTA, 2% SDS and 1% bromophenol blue, pH 8.0) and the DNA was analysed on native 12% polyacrylamide gel (in TBE buffer). For the endonuclease and exonuclease assays, radioactive DNA substrates were incubated with the indicated purified proteins in the same reaction buffer as indicated for the strand separation reaction. Cleaved DNA products were separated on a denaturing gel (14% polyacrylamide, 8 M urea in TBE) and analyzed by autoradiography.
Table 1. DNA Structures.
YB-1 affinity purification or ‘pull down’ assay
Approximately 108 human 293 cells were lysed in 10 mM Tris–HCl (pH 7.5), 1% (v/v) Triton X-100, 150 mM NaCl, 5 mM EDTA, 50 mM NaF in the presence of a protease inhibitor cocktail (Boehringer Mannheim) at 4°C for 20 min. Cells were sonicated three times for 5 s and cell debris were spun down. Affinity matrices were prepared by immobilizing GST alone or GST–YB-1 fusion proteins on glutathione–Sepharose beads (Amersham Pharmacia) as described (34). Freshly prepared cell lysates were incubated with the affinity matrices overnight at 4°C. After extensive washing with lysis buffer, bound proteins were released by boiling in SDS sample buffer and analyzed by SDS–PAGE and western blotting.
Indirect immunofluorescence
Human 293 embryonic kidney cells were maintained in DMEM supplemented with 10% fetal bovine serum. They were plated on glass coverslips the day before the immunofluorescence experiments. Cells were fixed in cold methanol for 10 min and permeabilized with 0.15% Triton X-100 at 4°C for 10 min. After washing with PBS, cells were blocked with 2% milk at 4°C for 30 min. After blocking, antibodies (1:250 for anti-Ku80, 1:50 for anti-MSH2, 1:2000 for anti-WRN or 1:500 for anti-YB-1) diluted in blocking buffer were applied to the coverslips and incubated overnight at 4°C. Coverslips were washed with PBS and incubated for 1 h at room temperature with rhodamine-secondary antibody for WRN or YB-1 detection (Santa Cruz), and FITC-secondary antibody (Santa Cruz) for Ku80 or MSH2 detection. After washing, coverslips were mounted on glass slides. Slides were viewed at 60× magnification (1.4NA oil-immersion 60× objective) and zoomed 2× for image acquisition on a Nikon inverted diaphot confocal microscope equipped with Krypton/Argon lasers (488 and 568 nm). Images were captured with a BioRad MRC1024 confocal microscopy system. Finally, images were colored and merged in Adobe photoshop.
RESULTS
Purified human YB-1 forms oligomers in solution
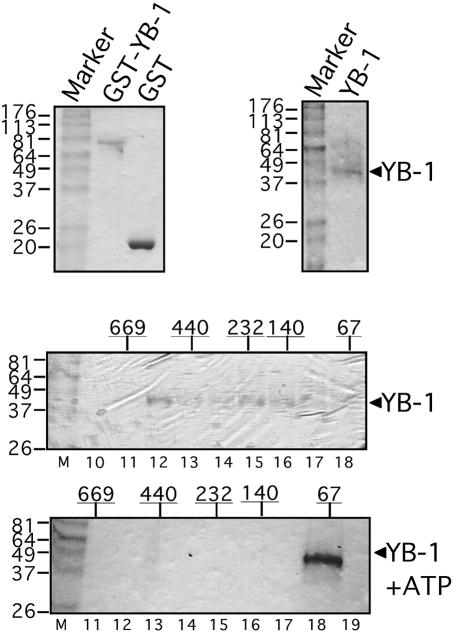
Recent studies have indicated that YB-1 may act as multimers and that two C-tail domains of YB-1 are required for homodimerization (28,30). To determine whether YB-1 can oligomerize in solution, the GST–YB-1 fusion protein was produced in bacteria and purified with glutathione–Sepharose beads (Fig. 1, top left). As the GST portion of this fusion protein could modify the behavior of YB-1 in vitro, the GST portion was cleaved off with thrombin (see Materials and Methods). This purified YB-1 protein has a molecular weight of ∼42–44 kDa (Fig. 1, top right). Purified YB-1 was loaded onto a Superdex-200 column and the eluted proteins were analyzed on a 10% SDS–polyacrylamide gel. Coomassie staining revealed that YB-1 eluted in fractions 12–18. In a parallel experiment, molecular weight protein markers were also eluted from a Superdex-200 column and analyzed on a gel. As indicated in Figure 1, the YB-1 peptide was recovered in multiple peaks that co-eluted with molecular weight markers of 530, 232, 140 and 67 kDa (bottom). However, YB-1 co-eluted with the molecular weight marker of 67 kDa upon addition of 2 mM ATP in the solution. A dimer of YB-1 would be ∼80 kDa which elutes with the 67 kDa molecular weight marker. These results indicate that in addition to monomers and dimers, YB-1 can form trimers, hexamers and oligomers of 12 YB-1 molecules in solution. In the presence of ATP, only dimers are formed.
Figure 1.
Characterization of purified human YB-1 on gels stained by Coomassie. (Top, left) This gel contains GST–YB-1 and GST proteins purified on glutathione–Sepharose beads. (Top, right) GST–YB-1 was treated with thrombin to remove the GST portion of the fusion protein as described in Materials and Methods. The size of YB-1 is ∼42–44 kDa. (Bottom) These gels contain YB-1 proteins eluted from a Superdex-200 gel filtration column with or without 2 mM ATP in the buffer. The numbers above each gel represent the position of marker proteins eluted from such a column. The numbers below the gel represent the fraction number.
Human YB-1 separates double-stranded DNA in the presence or absence of ATP
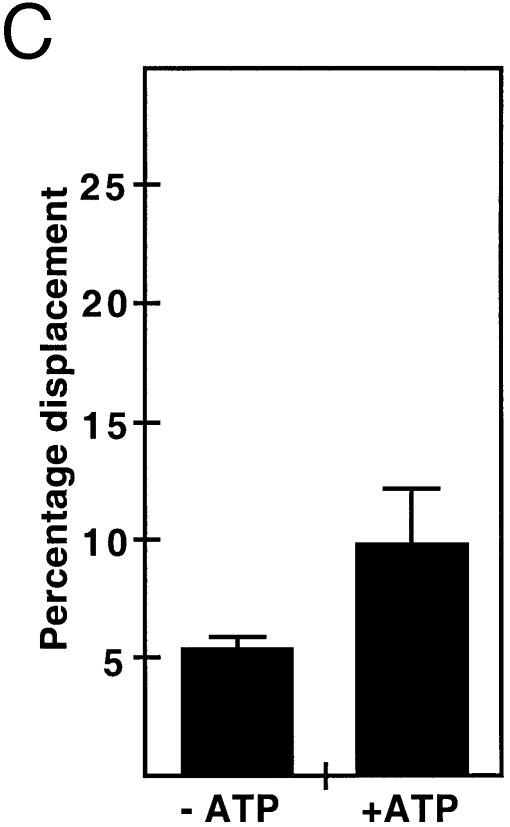
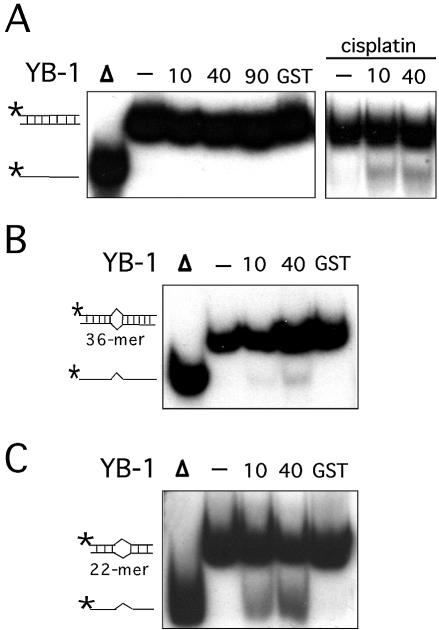
YB-1 is known to create single strand regions in the DRA promoter (28). We thus tested whether purified YB-1 can separate the strands of different DNA duplexes (Table 1). A forked DNA structure was first tested in the presence or absence of ATP. ATP was also tested in these reactions because PROSCAN analysis of YB-1 protein has indicated that residues 28–58 form a motif with 69% homology to an ATP-binding signature region. As shown in Figure 2A, purified YB-1 is capable of separating the strands like a DNA helicase enzyme. The amount of displaced radioactive strand was YB-1 concentration dependent (Fig. 2A). YB-1 proteins from fractions 12, 15, 16 and 18 of the Superdex-200 column (Fig. 1) were tested. All these fractions showed similar strand displacement activities (data not shown). YB-1 was capable of separating the strands of a forked DNA structure without ATP (Fig. 2B). However, this activity was doubled in the presence of 2 mM ATP in the reaction (Fig. 2B and C). The percentage of displacement was calculated from several reactions by excising the corresponding bands from the gels and counting the amount of radioactivity with a β-counter. Similar results were obtained with different DNA structures (data not shown). These observations indicate that purified YB-1 can separate the strands of a forked DNA structure even in the absence of ATP. Finally, ATPase assays were performed and no ATP hydrolysis was detected with our purified YB-1 in the presence or absence of DNA molecules (data not shown).
Figure 2.
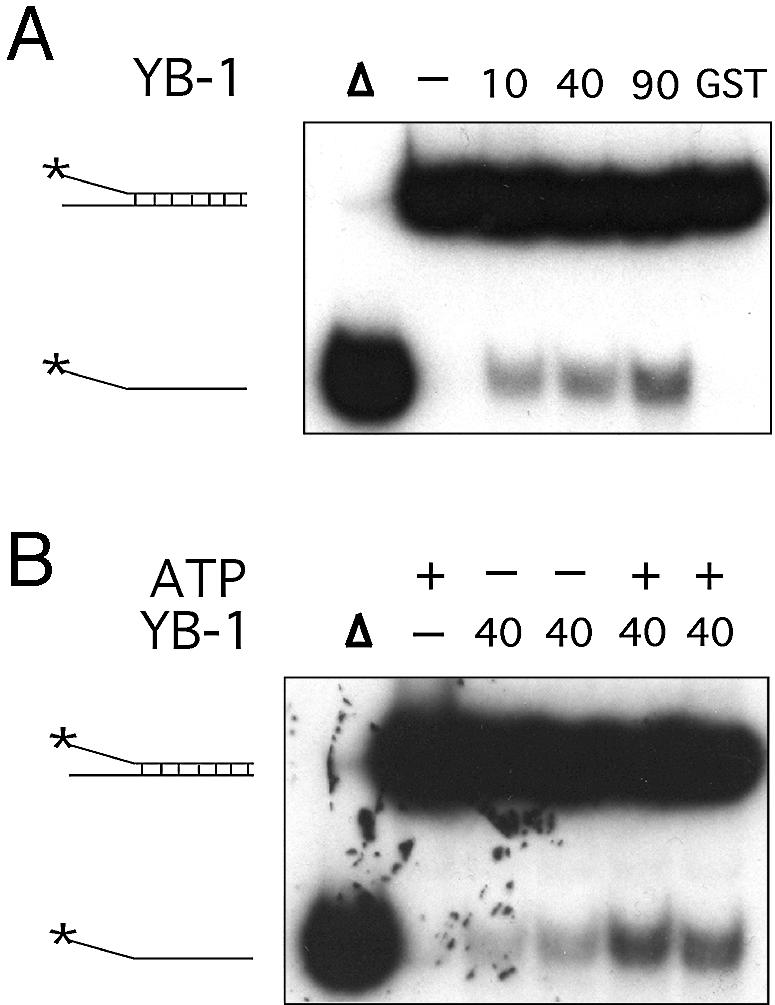
Strand separation activity of human YB-1 on a forked DNA duplex structure. (A) Increasing amounts of purified YB-1 proteins (indicated in ng) or 40 ng of GST were incubated with a radioactive 22 bp forked duplex under standard conditions for helicase activity (see Materials and Methods) for 30 min at 37°C. Reactions were stopped in the appropriate dye buffer and the DNA products were run on a 12% native polyacrylamide gel. The double-stranded and single-stranded structures are depicted on the left. The asterisk represents the labeled strand at its 5′ end. The triangle represents heat denatured DNA. (B) YB-1 (40 ng) was incubated with a radioactive 22 bp forked duplex as in (A), in the presence or absence of 2 mM ATP. (C) Histogram representation of the YB-1 strand separation reactions performed in (B). All experiments were done in duplicates with 40 ng of purified human YB-1. The percentage strand displacement is calculated by using the formula: c.p.m. of displaced strand/(c.p.m. of displaced strand + c.p.m. of double-stranded DNA) × 100. The c.p.m. was calculated for each band of the gel as indicated in Materials and Methods.
Human YB-1 can separate the strands of either blunted, 5′ or 3′ recessed DNA structures
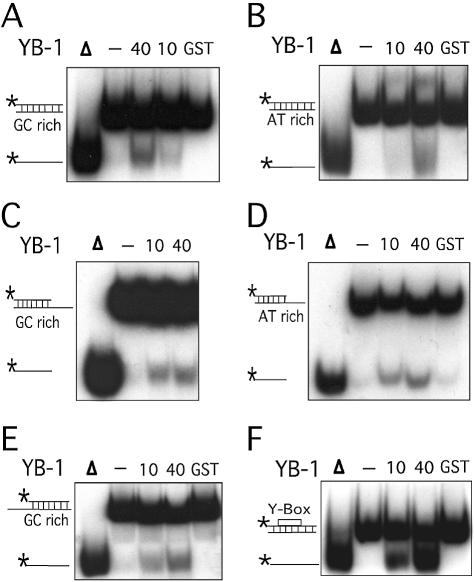
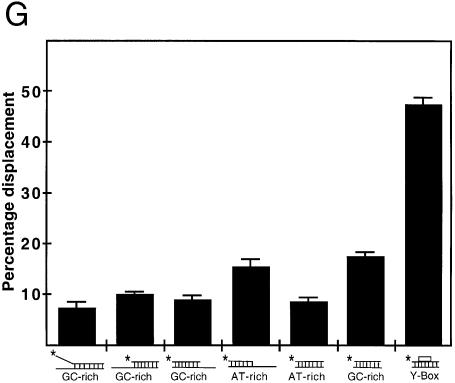
The capacity of YB-1 to separate DNA strands was examined with different duplex structures (see Table 1). As shown in Figure 3A–E, YB-1 was equally capable of separating DNA strands from a 22mer duplex containing either blunt ends, a 5′ recessed or a 3′ recessed end. In addition, YB-1 displaced GC-rich or AT-rich radioactive strands equally well (see Table 1 for oligonucleotide sequences). Overall, an 8–16% strand displacement was observed with different duplex sequences and this independently of the structures at the ends of the DNA substrates (Fig. 3C).
Figure 3.
Strand separation activity of YB-1 on different duplex structures. (A–F) The indicated amounts (in ng) of purified human YB-1 proteins were incubated with the indicated radioactive DNA substrates under standard conditions for helicase activity (see Materials and Methods) for 30 min at 37°C. Again, 40 ng of GST proteins were used as negative controls when indicated. Reactions were stopped in the appropriate dye buffer and the DNA products were run on a 12% native polyacrylamide gel. The double-stranded and single-stranded DNA structures with their sequence characteristics are depicted on the left of each autoradiogram. The 5′-labeled strand of the duplex is represented by an asterisk. (A) and (B) represent reactions with blunt-ended DNA. (C) and (D) represent reactions with a structure having a 3′ recessed end. (E) represents reactions with a structure having a 5′ recessed end. In (F), the Y-box sequence is an inverted CCAAT sequence. All DNA substrates contain 22 paired bases. (G) Histogram representation of the YB-1 strand separation reactions performed in (A–F). The strand displacement data were obtained from experiments done in duplicate with 40 ng of the indicated purified proteins. The percentage strand displacement is calculated by using the formula: c.p.m. of displaced strand/(c.p.m. of displaced strand + c.p.m. of double-stranded DNA) × 100. The c.p.m. was calculated for each band of the gel as indicated in Materials and Methods.
YB-1 is a transcription factor that binds strongly to an inverted CCAAT box (1). A 22mer duplex containing this sequence (Y-box sequence) was also examined. As shown in Figure 3F, YB-1 separated the two strands of the Y-box duplex very efficiently. Close to 50% of total duplex was separated by YB-1 (Fig. 3G). All these results indicate that YB-1 can separate a 22mer duplex DNA independently of the nucleotide sequence. However, YB-1 has a very strong preference for a duplex DNA containing an inverted CCAAT box.
Human YB-1 does not separate a 36mer DNA duplex with no inverted CCAAT box sequence
The DNA substrates used in the strand separation reactions in the preceding sections contained <23 bp. We therefore asked whether the length of the DNA duplex affects YB-1 strand separation activity. As indicated in Figure 4A (top), YB-1 was unable to separate the strands of a 36 bp DNA duplex.
Figure 4.
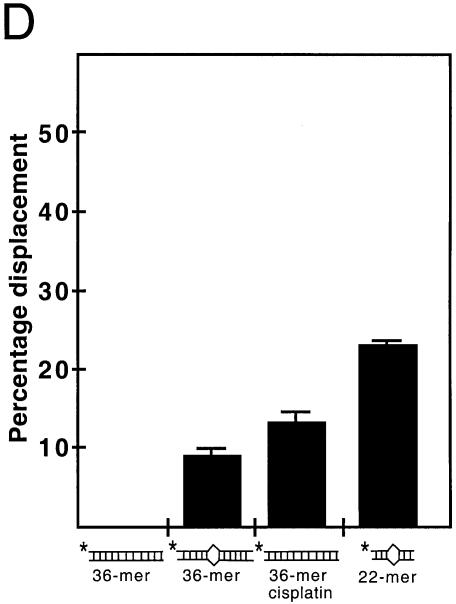
Strand separation activity of YB-1 on duplex structures containing mismatches or cisplatin cross-links. (A–C) The indicated amounts (in ng) of purified human YB-1 proteins were incubated with the indicated radioactive DNA substrates under standard conditions for helicase activity (see Materials and Methods) for 30 min at 37°C. Forty nanograms of GST were used as a negative control. The panels in (A) represent strand separation reactions performed with a 36 bp duplex. The panel on the right contains a reaction performed with a 36mer duplex treated with cisplatin. In (B), reactions were performed with a 36mer duplex containing one base mismatch. In (C), reactions were performed with a 22mer duplex structure containing two nucleotide mismatches. (D) Histogram representation of the YB-1 strand separation reactions performed in (A). The strand displacement data were obtained from experiments done in duplicate with 40 ng of the indicated purified proteins. The percentage strand displacement is calculated by using the formula: c.p.m. of displaced strand/(c.p.m. of displaced strand + c.p.m. of double-stranded DNA) × 100. The c.p.m. was calculated for each band of the gel as indicated in Materials and Methods.
Human YB-1 separates 36mer DNA duplex treated with cisplatin or containing mismatches
It has been shown that YB-1 binds preferentially to cisplatin-modified DNA (25). Thus, the 36mer duplex structure was incubated overnight with cisplatin and the resulting modified DNA was used in YB-1-mediated strand separation reaction. As shown in Figure 4A, YB-1 was capable of separating the strands of the 36mer DNA substrate containing cisplatin modifications. Approximately 14% of cisplatin-modified strands were separated by YB-1 (Fig. 4D).
YB-1-mediated strand separation reaction was also examined on duplex DNA containing one or two mispaired bases. Figure 4B indicates that YB-1 can separate the strands of a 36mer duplex DNA containing one mismatch. Approximately 9% of the total DNA duplex was separated (Fig. 4D). Finally, a 22mer DNA duplex structure (GC rich) with two mismatches was tested. Up to 25% of this DNA substrate was separated into single-stranded DNA (Fig. 4C and D). All these results indicate that YB-1 can separate duplex DNA with either mispaired bases or cisplatin-modified lesions.
The DNA strand separation activity of human YB-1 requires amino acids 40–205
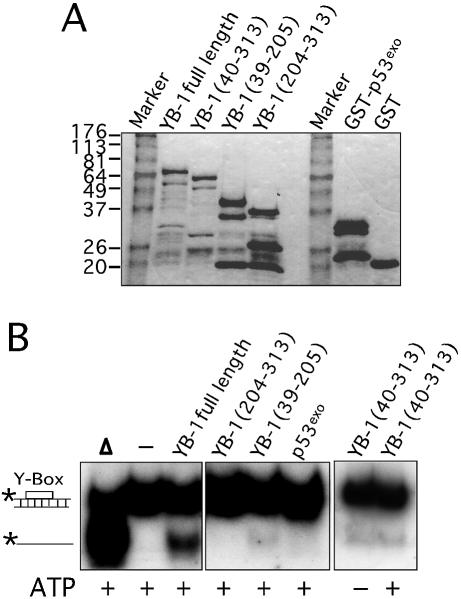
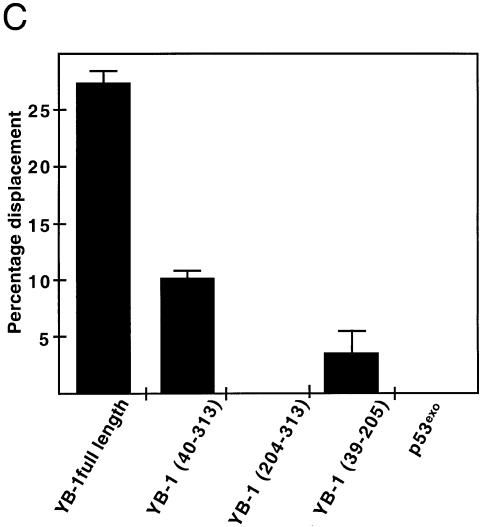
To demonstrate that the strand separation activity was intrinsic to YB-1, a series of GST–YB-1 fusion proteins were analyzed (Fig. 5A). Although we did see some protein degradation, all fusion proteins were treated with thrombin and the purified YB-1 fragments were assayed on the Y-box duplex. These experiments were repeated three times. As seen in Figure 5B, full-length YB-1 separated the two strands as shown before. YB-1 proteins lacking the first 40 amino residues (including part of the potential ATP-binding site) showed weak activity on the gel. Approximately 10% of the DNA duplex was separated by this mutant compared with 27% for the full-length YB-1 (Fig. 5C). The strand separation activity of this mutant YB-1 (amino acids 40–313) was similar in the presence or absence of ATP (Fig. 5B, last panel on the right). In contrast, the C-terminus part of YB-1 (amino acids 204–313) did not exhibit strand separation activity. Finally, a YB-1 fragment corresponding to residues 39–205 (Fig. 5B, middle) exhibited a very weak activity (<5% strand displacement in Fig. 5C). As a negative control, a GST-fusion peptide containing the exonuclease domain of p53 (p53exo) was assayed under the same conditions and no strand separation activity was detected (Fig. 5B and C). These results indicate that the strand separation activity is localized to the region of YB-1 between amino acids 40 and 205. However, both the N-terminus and C-terminus portions of YB-1 are obviously required for optimal activity. Similar results were obtained with a forked DNA substrate (data not shown).
Figure 5.
Strand separation activity of different mutant YB-1 on a DNA duplex structure. (A) Characterization of the different mutant GST–YB-1 fusion proteins (before thrombin treatments) on gels stained by Coomassie. The YB-1 amino acids present in the fusion proteins are indicated above each lane. (B) The different GST-fusion proteins were cleaved with thrombin as described in the Materials and Methods and the cleaved products were used in strand displacement reactions. Approximately 40 ng of the purified YB-1, mutant YB-1 proteins or p53exo peptide (exonuclease domain) were incubated with a radioactive 22 bp duplex containing a Y-box sequence under standard conditions for helicase activity (see Materials and Methods) for 30 min at 37°C in the absence or presence of 2 mM ATP. Reactions were stopped in the appropriate dye buffer and the DNA products were analyzed on a 12% native polyacrylamide gel. The double-stranded and single-stranded structures are depicted on the left. The asterisk represents the labeled strand at its 5′ end. The triangle represents heat denatured DNA. (C) Histogram representation of the strand separation reactions performed in (B). All experiments were done in duplicate with 40 ng of purified YB-1, mutant YB-1 proteins or p53exo (exonuclease domain) in reaction buffer containing 2 mM ATP. The percentage of strand displacement is calculated by using the formula: c.p.m. of displaced strand/(c.p.m. of displaced strand + c.p.m. of double-stranded DNA) × 100. The c.p.m. was calculated for each band of the gel as indicated in Materials and Methods.
Human YB-1 exhibits endonucleolytic activities on double-stranded DNA
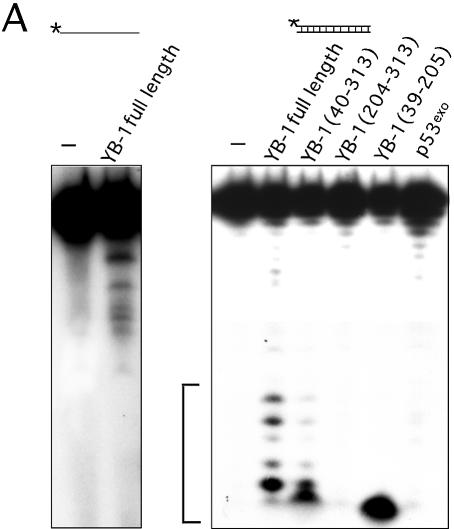
Exposition of the native gels in Figure 5B for an additional 3 days showed some degradation of the DNA substrates (data not shown). This suggests that nucleolytic activity was also present in the strand separation reactions. Consistent with this observation, a recent report has indicated that GST–YB-1 chimeric proteins exhibit 3′ to 5′ exonuclease activity (30). However, this activity was described only for single-stranded DNA molecules. Similar analyses were thus performed with our purified YB-1 proteins on different duplex substrates. To determine if our purified YB-1 protein exhibited any exonuclease activity, a single-stranded DNA substrate was first analyzed. As shown in Figure 6A (left), YB-1 degraded the single-stranded DNA. However, the autoradiogram had to be exposed for 3 days to detect the exonuclease activity.
Figure 6.
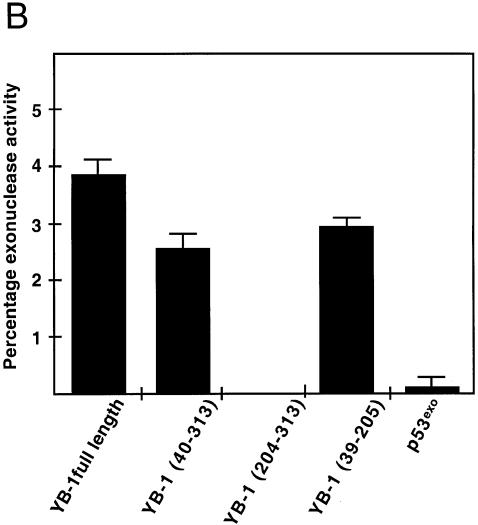
Exonuclease and DNA nicking activity of YB-1. (A) Forty nanograms of full-length, mutant YB-1 or p53exo (exonuclease domain of p53) were incubated for 30 min at 37°C as described in Materials and Methods with the different DNA substrates indicated above each gel. Reactions were stopped in the appropriate dye buffer and cleaved DNA products were analyzed on 14% denaturing polyacrylamide gels to analyze the exonuclease activity. (B) Histogram representation of the YB-1 nuclease activity performed in (A) with the duplex DNA substrate. The percentage nuclease activity was calculated by scanning the figures and using the formula: intensities of cleaved products/(intensities of cleaved products + intensity of uncleaved probe) × 100. Results were calculated from duplicate experiments.
A double-stranded DNA substrate was incubated with YB-1 to examine the exonuclease activity. As shown in the second panel of Figure 6A, the pattern of degradation was different from the single-stranded substrate. To demonstrate that the exonuclease activity was intrinsic to YB-1, a series of purified YB-1 fragments were analyzed. Full-length YB-1 exhibited nucleolytic activity on duplex DNA (Y-box duplex) (Fig. 6A, right). The absence or presence of ATP did not affect the nucleolytic activities of full-length YB-1 (data not shown). A YB-1 protein lacking the first 40 amino acid residues also exhibited similar nucleolytic activity but at a lower efficiency (Fig. 6B). The C-terminus portion of YB-1 (residues 204–313) did not show nuclease activity. A peptide fragment comprising residues 39–205 of YB-1 cleaved the 5′ radioactive nucleotide of the duplex. Finally, the exonuclease domain of p53 (p53exo) showed very little nuclease activity on this Y-box DNA duplex. These results indicate that YB-1 introduces nicks or breaks into double-stranded DNA molecules.
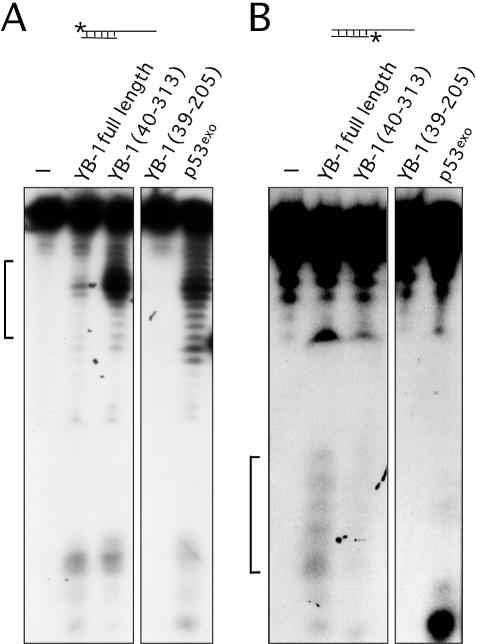
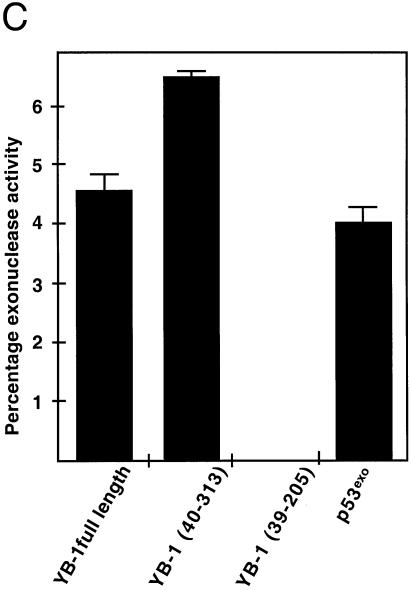
Additional experiments were performed with YB-1 fragments (fragments exhibiting nicking activities in the previous section) on DNA substrates labeled on either strand to compare the cleavage patterns. As shown in Figure 7, full-length YB-1 also cleaved a DNA duplex containing a 3′ overhang. However, the cleavage pattern was different from the patterns obtained with duplex DNA possessing blunt ends (compare Figs 6A with 7A). Interestingly, the cleavage pattern was stronger with a YB-1 molecule lacking the first 40 amino acid residues. These results indicate that the first 40 amino acid residues of YB-1 will affect its nicking activity. In contrast, a YB-1 fragment comprising residues 39–205 did not exhibit nuclease activity on this DNA substrate. As expected, the exonuclease domain of p53 exhibited a strong nuclease activity with a cleavage pattern similar to the N-terminus truncated YB-1 peptide (Fig. 7A). Similar experiments were performed on the same DNA substrate except that the opposite strand was labeled at the 5′ end (Fig. 7B). Although labeling of the short strand did show some heterogeneity of the probe, only the full-length YB-1 exhibited a weak nucleolytic activity. Less than 2% of the probe was cleaved with 40 ng of YB-1. The cleavage pattern indicated nicking near the labeled 5′ end of the strand. None of the mutated YB-1 peptide showed nicking on the shorter stand of the duplex. The p53exo peptide cleaved the labeled 5′ of this substrate (Fig. 7B, last lane on the right panel). These results suggest that YB-1 can introduce double-stranded breaks and exhibit endonucleolytic activities. However, this activity was weak as the autoradiograms had to be exposed for 3 days to detect the cleavage products.
Figure 7.
Endonucleolytic activity of YB-1. (A and B) Forty nanograms of full-length, mutant YB-1 or p53exo (exonuclease domain of p53) peptides were incubated with the indicated DNA substrates in reaction buffer for 30 min at 37°C as described in Materials and Methods. DNA products were analyzed on 14% denaturing polyacrylamide gels. The brackets on the right side of each gel indicate the cleavage products of each probe by the proteins. Experiments were repeated twice. (C) Histogram representation of the YB-1 nuclease activity performed in (B). The percentage nuclease activity was calculated by scanning the figures and using the formula: intensities of cleaved products/(intensities of cleaved products + intensity of uncleaved probe) × 100. Results were calculated from duplicate experiments.
Several DNA repair proteins bind to human YB-1 immobilized on a matrix
YB-1 binds to cisplatin-modified DNA (25) and can separate the strands of such modified DNA (preceding sections). It also interacts with PCNA (25) and an endonuclease involved in base excision repair (27). These observations suggest that YB-1 may be involved in certain aspects of DNA repair. To identify other DNA repair proteins that may interact with YB-1, whole cell extracts from the human 293 embryonic kidney cell line were loaded on affinity column containing the GST–YB-1 fusion protein immobilized on glutathione–Sepharose beads. GST immobilized on glutathione–Sepharose beads was used as a control. Bound proteins were analyzed by western blots with antibodies specific to different DNA repair proteins. Western blots were first examined with antibodies against proteins known to interact with YB-1 or known to be in the same cellular complex. Nucleolin and ALY were analyzed as both are found in prespliceosomes with YB-1 (35). As shown in Figure 8, both nucleolin and ALY bound to the GST–YB-1 beads but not to the GST control beads. These results indicate that the GST–YB-1 fusion construct can be used for affinity chromatography experiments.
Figure 8.
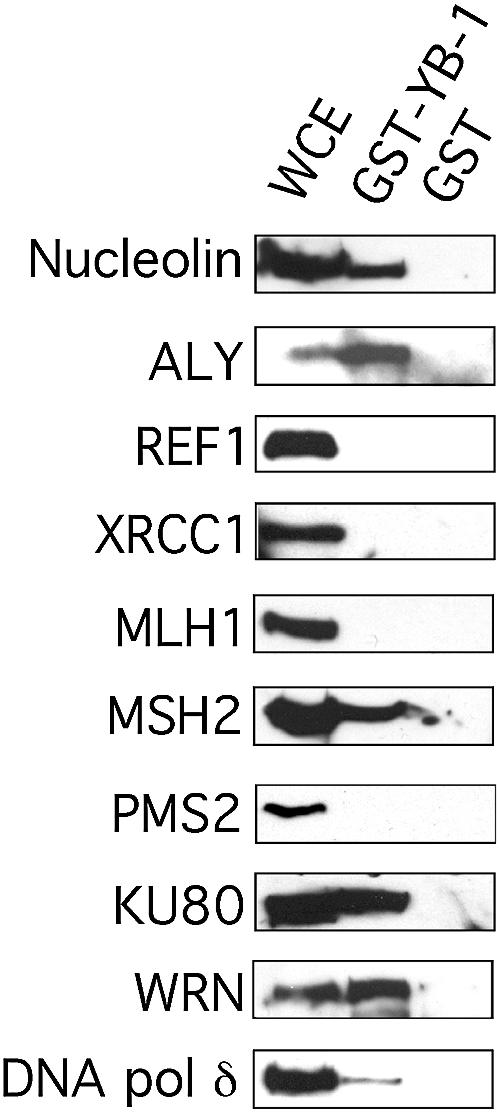
Immunoblots against DNA repair proteins bound to GST–YB-1 affinity Sepahrose beads. Human 293 embryonic kidney whole cell extracts (WCE) were incubated with either 50 µg of GST–YB-1 or GST linked glutathione–Sepharose beads overnight. Proteins bound to the affinity beads were analyzed by SDS–PAGE with antibodies against the indicated proteins on the left of each blot.
Poly(ADP-ribose) polymerase-1 (PARP-1), REF1 endonuclease (also known as APE1), and XRCC1 are proteins involved in short or long patch excision repair pathways (36–38). Western blot analyses revealed that none of these proteins bound to the GST–YB-1 matrix (Fig. 8 and data not shown).
As YB-1 can separate the strands of a duplex DNA containing mismatches, antibodies against proteins known to be involved in the mismatch repair pathway were also tested. The presence of MLH1, MSH2 and PMS2 was examined. As indicated in Figure 8, only MSH2 bound to the GST–YB-1 beads but not to the GST control beads.
Proteins involved in homologous recombination and non-homologous end-joining were also examined. These include RAD51, RAD51C and RAD52 for the homologous recombination pathway and DNA-PK, Ku80 and WRN for the non-homologous end-joining pathway (39–41). RAD51, RAD51C, RAD52 and DNA-PK did not bind the GST–YB-1 beads (data not shown). In contrast, Ku80 and WRN proteins bound strongly to the GST–YB-1 matrix but not to the GST control. Finally, an antibody against DNA polymerase δ was also tested on the western blots. As indicated in Figure 8, DNA polymerase δ bound to the GST–YB-1 matrix with low affinity as suggested by the weak signal observed on the blot. Altogether, these results indicate that several proteins involved in DNA repair bind YB-1 in vitro. These findings strongly support the involvement of YB-1 in specific DNA repair pathways.
Re-localization of YB-1 to nuclear areas containing DNA repair proteins in cisplatin-treated cells
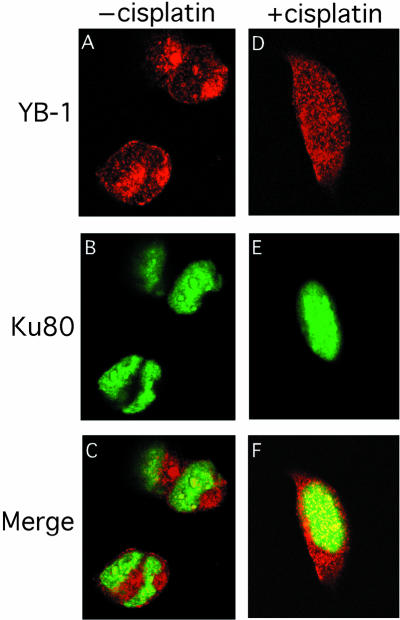
Co-localization experiments with antibodies against Ku80 and YB-1 were performed on intact 293 cells that had been treated with or without 7.5 mg/ml of cisplatin for 2 h. FACS analyses revealed no evidence of an increase in apoptotic figures in cisplatin-treated cultures compared with untreated cells at the 2 h time point (data not shown). Thus, we were analyzing cells before the onset of apoptosis. As shown in Figure 9A, YB-1 is found mainly in the cytoplasm of untreated 293 cells with some nuclear localization. In contrast, Ku80 is only localized to the nucleus of untreated cells in a diffuse pattern (Fig. 9B). Immunofluorescence images for both antibodies were captured in separate channels and overlaid to see the extent of co-localization. As shown in Figure 9C, few nuclear foci contained both proteins. In the presence of cisplatin, some YB-1 proteins translocated to the nucleus (Fig. 9D). The Ku80 distribution pattern was similar in both cisplatin-treated and untreated cells (compare Fig. 9B with E). Interestingly, >90% of YB-1 proteins found in the nucleus of cisplatin-treated cells co-localized with Ku80 (yellow fluorescence in Fig. 9F). These results indicate that upon cisplatin treatment, YB-1 re-localizes into multiple nuclear foci containing Ku80 proteins. Pre-immune serum from the YB-1 immunized rabbit did not show such results (data not shown). Western analysis on 293 whole cell lysates indicated that the rabbit immune serum used in the immunofluorescence study recognizes only one band specific to YB-1 (Fig. 9G). However, the anti-YB-1 antibody immunoprecipitated YB-1 from 293 cell lysate only poorly (data not shown). Hence, co-immunoprecipitation could not be performed with this antibody.
Figure 9.
Co-localization of YB-1 and Ku80 by immunofluorescence. Human 293 embryonic kidney cells were untreated (A–C) or incubated with 7.5 µg/ml of cisplatin for 2 h (D–F), then fixed and subsequently incubated simultaneously with rabbit anti-YB-1 and mouse anti-Ku80 as described in Materials and Methods. Anti-rabbit rhodamine-labeled and anti-mouse FITC-conjugated secondary antibodies were used to visualize YB-1 and Ku80 by confocal microscopy at 568 and 488 nm, respectively. Images depict representative cells from each of the non-treated and cisplatin-treated cultures. In the merged images (C and F) a yellow color appears where YB-1 (red) and Ku80 (green) fluorescence signals coincide. In (G), western analysis of 293 total cell lysate with anti-YB-1 and pre-immune sera.
Co-localization experiments were also performed with antibodies against MSH2 and YB-1 (Fig. 10). Again, YB-1 is found in the cytoplasm of untreated 293 cells. There are few foci in the nucleus containing YB-1 (Fig. 10A). Upon cisplatin treatment, YB-1 re-localizes into the nucleus of 293 cells (Fig. 10D). As shown in Figure 10B and E, MSH2 is found in the nucleus of untreated and cisplatin-treated 293 cells in a weak diffuse pattern. Merging the images shows that there are few nuclear foci containing both YB-1 and MSH2 proteins (yellow fluorescence in Fig. 10C) in untreated 293 cells. However, the number of nuclear foci containing both MSH2 and YB-1 proteins increased substantially in cisplatin-treated cells (Fig. 10F). Approximately 30% of MSH2 nuclear foci contained YB-1 proteins upon cisplatin treatment. These results indicate that upon cisplatin treatment, a proportion of cellular YB-1 re-localizes into multiple nuclear areas containing MSH2 proteins.
Figure 10.
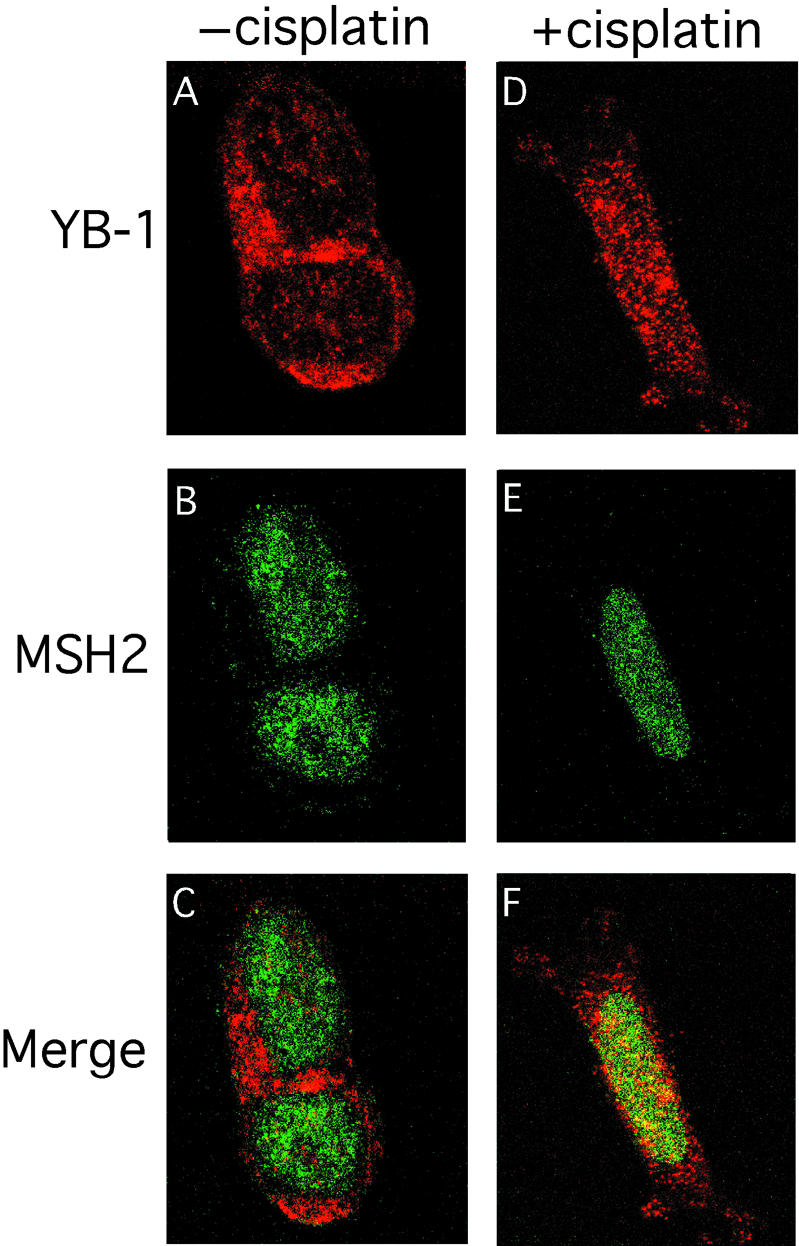
Co-localization of YB-1 and MSH2 by immunofluorescence. Human 293 embryonic kidney cells were untreated (A–C) or incubated with 7.5 µg/ml of cisplatin for 2 h (D–F), then fixed and subsequently incubated simultaneously with rabbit anti-YB-1 and mouse anti-MSH2 as described in Materials and Methods. Anti-rabbit rhodamine-labeled and anti-mouse FITC-conjugated secondary antibodies were used to visualize YB-1 and MSH2 by confocal microscopy at 568 and 488 nm, respectively. Images depict representative cells from each of the non-treated and cisplatin-treated cultures. In the merged images (C and F) a yellow color appears where YB-1 (red) and MSH2 (green) fluorescence signals coincide.
Both anti-WRN and YB-1 antibodies were generated in rabbits. Consequently, to detect co-localization of YB-1 with WRN proteins in treated 293 cells, a specific epitope-tagged version of the proteins will be constructed. This is part of another study. Finally, the antibody against DNA polymerase δ used in this study does not work in immunofluorescence.
DISCUSSION
Recent studies have implicated YB-1 in genome stability as it interacts with proteins involved in DNA repair such as PCNA (25), human endonuclease III (27) and p53 (42,43). YB-1 also exhibits exonuclease activities on single-stranded DNA (30). In this report, we investigated the strand separation and nucleolytic properties of YB-1 on different DNA duplex substrates in vitro. For such analysis, we produced GST–YB-1 in bacteria and designed a purification scheme involving the removal of the GST portion of the fusion construct with thrombin and a gel filtration step. Elution from the Superdex-200 gel filtration column has indicated that YB-1 can readily form trimers, hexamers and oligomers of 12 molecules in solution. However, the presence of ATP changed the conformation of the protein as YB-1 mainly formed dimers, a complex more likely to be present in vivo. This confirms earlier reports suggesting that YB-1 is acting as an oligomer in vivo (28,30). PROSCAN analyses of YB-1 amino acid sequence have indicated that it possesses an ATP-binding signature at its N-terminus (residues 28–58). ATPase assays were performed with our purified YB-1 and it did not hydrolyze ATP even in the presence of DNA.
Our purified YB-1 can separate the strands of different DNA duplex molecules under buffer conditions used for helicase assays (44). The strand separation activity of YB-1 is increased 2-fold in the presence of ATP (Fig. 2). Importantly, a mutant YB-1 lacking part of the potential ATP-binding domain did not exhibit increased strand separation activity in the presence of ATP (Fig. 5B). Thus, our data indicate that binding of ATP changes the conformation of YB-1 and affects its strand separation activities. YB-1 does not seem to have a preferred directionality like an helicase. It can separate the strands from a duplex molecule with either a 3′ or 5′ recessed end, blunt-ended DNA, or a forked DNA structure equally well. The strongest strand separation activity was observed with DNA duplexes containing an inverted CCAAT box. Additional analyses with deletion mutants have indicated that the DNA strand separation activity is intrinsic to YB-1 protein and the active site is situated between amino acid residues 40 and 205. Importantly, this region of YB-1 is known to contain the DNA-binding domain (30). The length of the duplex structure affects YB-1 strand separation activities. YB-1 can separate a 22 bp molecule but not a 36 bp molecule. However, it will separate a 36mer containing one or two base mismatches or cisplatin modifications. This strongly suggests that YB-1 will bind and process DNA molecules with such lesions.
YB-1 is not only capable of separating the strands, but it can also cleave double-stranded DNA molecules. However, this YB-1 nicking activity is weaker than the strand separation activities. Unlike the strand separation activity, it takes several days of exposition to see the nucleolytic cleavage pattern on an autoradiogram. This suggests that the DNA strand separation activity of YB-1 is stronger than its nucleolytic activity under the in vitro conditions employed in this study. Our preliminary observations indicate that YB-1 cleavage activities seem to be sequence and/or structure dependent. Noticeably, the exonucleolytic activity on a DNA duplex substrate containing a 3′ overhang increased dramatically when YB-1 lacked the first 40 N-terminus amino acid residues. This increased nucleolytic activity was not observed on blunt duplex. The reason for the enhanced nucleolytic activity with the mutant YB-1 (40–313) peptide is unknown. Presumably the deletion of the first 40 amino acids of YB-1 has affected its interaction with different DNA structures in vitro.
Our data on the enzymatic activities of YB-1 toward duplex with mismatches and cisplatin modifications suggest that YB-1 might be involved in base excision repair and mismatch repair pathways. It was thus logical to ask whether YB-1 can bind to proteins involved in such DNA repair pathways. Accordingly, affinity chromatography experiments were performed on a YB-1 matrix with 293 whole cell lysates. The only repair proteins that bound the YB-1 matrix in our study were DNA polymerase δ, MSH2, Ku80 and WRN. In addition, immunofluorescence studies have indicated re-localization of YB-1 to regions of the nucleus containing either Ku80 or MSH2 proteins in cisplatin-treated cells (Figs 9 and 10).
The MSH2/YB-1 interaction is an interesting observation as cancer cell lines with a mutation in the MSH2 gene are also resistant to platinum-containing compounds (45). This provides a link between the activity of YB-1 and the mismatch repair protein MSH2 in the processing of cisplatin-modified DNA. Future experiments with purified MSH2 will determine whether it can bind directly to YB-1 and alter its activities. DNA polymerase δ is another factor (with PCNA and EXO1) associated with mismatch repair (46). It is interesting to note that DNA polymerase δ bound our GST–YB-1 matrix and that PCNA interacts with YB-1 in vivo (25). Again, these results point to a potential role for YB-1 in the mismatch repair pathway.
Ku80 has been reported to have a role in transcriptional reinitiation by RNA polymerase II in addition to double-strand break repair (47). As YB-1 is a transcription factor, it is possible that the observed interaction with Ku80 is in the context of transcription in cells and not DNA repair. However, Ku antigen has also been shown to down regulate the activity of the base excision repair protein endonuclease III in the context of DNA molecules with closely opposed lesions (48). Thus, Ku antigen will also affect some aspects of base excision repair in cells. Additional analyses on a Ku/YB-1 complex are required to distinguish between these possibilities.
It is believed that WRN protein is a dual exonuclease/helicase enzyme not only involved in non-homologous end-joining, but also in long patch base excision repair (42). Mutations in the WRN gene are responsible for the human progeroid disorder Werner syndrome. Importantly, WRN protein translocates to specific nuclear foci upon UV treatment (49). These foci are believed to be sites of DNA repair. The co-localization of WRN and YB-1 and their direct interaction at sites of DNA repair upon UV treatment require experiments with good antibodies against both proteins.
In summary, we have found that YB-1 can separate different DNA duplex structures and this activity is increased when duplex molecules contain either mispaired bases or cisplatin modifications. YB-1 exhibits endonucleolytic activity in addition to its exonuclease. Finally, YB-1 binds in vitro proteins involved in both base excision repair and mismatch repair pathways suggesting an involvement of YB-1 in these repair pathways. Additional in vitro as well as in vivo analyses are required to confirm the functional interactions between YB-1 and these DNA repair proteins.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Dr P. E. DiCorleto from the Cleveland Clinic Foundation (Cleveland, OH) for the rabbit pre-immune and anti-YB-1 antibodies and to Jacques Côté from the Centre de Recherche en Cancérologie (Québec City) for the GST-p53 construct. We thank F. Deschênes for technical assistance and Dr P. O. de Campos-Lima for his helpful discussion. This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) to M.L. M.L. is a scholar of the CIHR.
REFERENCES
- 1.Didier D.K., Schiffenbauer,J., Woulfe,S.L., Zacheis,M. and Schwartz,B.D. (1988) Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc. Natl Acad. Sci. USA, 85, 7322–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travali S., Ku,D.-H., Rizzo,M.G., Ottavio,L., Baserga,R. and Calabretta,B. (1989) Structure of the human gene for the proliferating cell nuclear antigen. J. Biol. Chem., 264, 7466–7472. [PubMed] [Google Scholar]
- 3.Lipson K.E., Chen,S.-T., Koniecki,J., Ku,D.-H. and Baserga,R. (1989) S-phase specific regulation by deletion mutants of the human thymidine kinase promoter. Proc. Natl Acad. Sci. USA, 86, 6848–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson B.E., Nasheuer,H.P. and Wang,T.S.-F. (1991) Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol. Cell. Biol., 11, 2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y. and Chien,K.R. (1995) EFIA/YB-1 is a component of cardiac HF-1A binding activity and positively regulates transcription of the myosin light-chain 2v gene. Mol. Cell. Biol., 15, 2972–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasham A., Lindridge,E., Rudert,F., Onrust,R. and Watson,J. (2000) Regulation of the human fas promoter by YB-1, Puralpha and AP-1 transcription factors. Gene, 252, 1–13. [DOI] [PubMed] [Google Scholar]
- 7.Norman J.T., Lindahl,G.E., Shakib,K., En-Nia,A., Yilmaz,E. and Mertens,P.R. (2001) The Y-box binding protein YB-1 suppresses collagen alpha 1(I) gene transcription via an evolutionarily conserved regulatory element in the proximal promoter. J. Biol. Chem., 276, 29880–29890. [DOI] [PubMed] [Google Scholar]
- 8.Fukada T. and Tonks,N.K. (2003) Identification of YB-1 as a regulator of PTP1B expression: implications for regulation of insulin and cytokine signaling. EMBO J., 22, 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertens P.R., Alfonso-Jaume,M.A., Steinmann,K. and Lovett,D.H. (1999) YB-1 regulation of the human and rat gelatinase A genes via similar enhancer elements. J. Am. Soc. Nephrol., 10, 2480–2487. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith M.E, Madden,M.J., Morrow,C.S. and Cowan,K.H. (1993) A Y-box consensus sequence is required for basal expression of the human multidrug resistance (mdr1) gene. J. Biol. Chem., 268, 5856–5860. [PubMed] [Google Scholar]
- 11.Ohga T, Uchiumi,T., Makino,Y., Koike,K., Wada,M., Kuwano,M. and Kohno,K. (1998) Direct involvement of the Y-box binding protein YB-1 in genotoxic stress-induced activation of the human multidrug resistance 1 gene. J. Biol. Chem., 273, 5997–6000. [DOI] [PubMed] [Google Scholar]
- 12.Hartmuth K., Urlaub,H., Vornlocher,H.P., Will,C.L., Gentzel,M., Wilm,M. and Luhrmann,R. (2002) Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl Acad. Sci. USA, 99, 16719–16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raffetseder U., Frye,B., Rauen,T., Jurchott,K., Royer,H.D., Jansen,P.L. and Mertens,P.R. (2003) Splicing factor SRp30c interaction with Y-box protein-1 confers nuclear YB-1 shuttling and alternative splice site selection. J. Biol. Chem., 278, 18241–18248. [DOI] [PubMed] [Google Scholar]
- 14.Stickeler E., Fraser,S.D., Honig,A., Chen,A.L., Berget,S.M. and Cooper,T.A. (2001) The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4. EMBO J., 20, 3821–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davydova E.K., Evdokimova,V.M., Ovchinnikov,L.P. and Hershey,J.W. (1997) Overexpression in COS cells of p50, the major core protein associated with mRNA, results in translation inhibition. Nucleic Acids Res., 25, 2911–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashizuka M., Fukuda,T., Nakamura,T., Shirasuna,K., Iwai,K., Izumi,H., Kohno,K., Kuwano,M. and Uchiumi,T. (2002) Novel translational control through an iron-responsive element by interaction of multifunctional protein YB-1 and IRP2. Mol. Cell. Biol., 22, 6375–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C.Y., Gherzi,R., Andersen,J.S., Gaietta,G., Jurchott,K., Royer,H.D., Mann,M. and Karin,M. (2000) Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev., 14, 1236–1248. [PMC free article] [PubMed] [Google Scholar]
- 18.Bargou R.C., Jurchott,K., Wagener,C., Bergmann,S., Metzner,S., Bommert,K., Mapara,M.Y., Winzer,K.J., Dietel,M., Dorken,B. and Royer,H.D. (1997) Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nature Med., 3, 447–450. [DOI] [PubMed] [Google Scholar]
- 19.Janz M., Harbeck,N., Dettmar,P., Berger,U., Schmidt,A., Jurchott,K., Schmitt,M. and Royer,H.D. (2002) Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int. J. Cancer, 97, 278–282. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein D.B., Stortchevoi,A., Boosalis,M., Ashfaq,R. and Guillaume,T. (2002) Overexpression of DNA-binding protein B gene product in breast cancer as detected by in vitro-generated combinatorial human immunoglobulin libraries. Cancer Res., 62, 4985–4991. [PubMed] [Google Scholar]
- 21.Yahata H., Kobayashi,H., Kamura,T., Amada,S., Hirakawa,T., Kohno,K., Kuwano,M. and Nakano,H. (2002) Increased nuclear localization of transcription factor YB-1 in acquired cisplatin-resistant ovarian cancer. J. Cancer Res. Clin. Oncol., 128, 621–626. [DOI] [PubMed] [Google Scholar]
- 22.Oda Y., Ohishi,Y., Saito,T., Hinoshita,E., Uchiumi,T., Kinukawa,N., Iwamoto,Y., Kohno,K., Kuwano,M. and Tsuneyoshi,M. (2003) Nuclear expression of Y-box-binding protein-1 correlates with P-glycoprotein and topoisomerase II alpha expression and with poor prognosis in synovial sarcoma. J. Pathol., 199, 251–258. [DOI] [PubMed] [Google Scholar]
- 23.Koike K., Uchiumi,T., Ohga,T., Toh,S., Wada,M., Kohno,K. and Kuwano,M. (1997) Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett., 417, 390–394. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa H., Uchiumi,T., Fukuda,T., Ashizuka,M., Kohno,K., Kuwano,M. and Sekiguchi,M. (2002) Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry, 41, 12739–12744. [DOI] [PubMed] [Google Scholar]
- 25.Ise T., Nagatani,G., Imamura,T., Kato,K., Takano,H., Nomoto,M., Izumi,H., Ohmori,H., Okamoto,T., Ohga,T., Uchiumi,T., Kuwano,M. and Kohno,K. (1999) Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res., 59, 342–346. [PubMed] [Google Scholar]
- 26.Jonsson Z.O. and Hubscher,U. (1997) Proliferating cell nuclear antigen: more than a clamp for DNA polymerases. Bioessays, 19, 967–975. [DOI] [PubMed] [Google Scholar]
- 27.Marenstein D.R., Ocampo,M.T., Chan,M.K., Altamirano,A., Basu,A.K., Boorstein,R.J., Cunningham,R.P. and Teebor,G.W. (2001) Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor. J. Biol. Chem., 276, 21242–21249. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald G.H., Itoh-Lindstrom,Y. and Ting,J.P. (1995) The transcriptional regulatory protein, YB-1, promotes single-stranded regions in the DRA promoter. J. Biol. Chem., 270, 3527–3533. [DOI] [PubMed] [Google Scholar]
- 29.Swamynathan S.K., Nambiar,A. and Guntaka,R.V. (1998) Role of single-stranded DNA regions and Y-box proteins in transcriptional regulation of viral and cellular genes. FASEB J., 12, 515–522. [DOI] [PubMed] [Google Scholar]
- 30.Izumi H., Imamura,T., Nagatani,G., Ise,T., Murakami,T., Uramoto,H., Torigoe,T., Ishiguchi,H., Yoshida,Y., Nomoto,M., Okamoto,T., Uchiumi,T., Kuwano,M., Funa,K. and Kohno,K. (2001) Y box-binding protein-1 binds preferentially to single-stranded nucleic acids and exhibits 3′→5′ exonuclease activity. Nucleic Acids Res., 29, 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebel M., Spillare,E.A., Harris,C.C and Leder,P. (1999) The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J. Biol. Chem., 274, 37795–37799. [DOI] [PubMed] [Google Scholar]
- 32.Bennett R.J., Sharp,J.A. and Wang,J.C. (1998) Purification and characterization of the Sgs1 DNA helicase activity of Saccharomyces cerevisiae. J. Biol. Chem., 273, 9644–9650. [DOI] [PubMed] [Google Scholar]
- 33.Moggs J.G., Yarema,K.J., Essigmann,J.M. and Wood,R.D. (1996) Analysis of incision sites produced by human cell extracts and purified proteins during nucleotide excision repair of a 1,3-intrastrand d(GpTpG)-cisplatin adduct. J. Biol. Chem., 271, 7177–7186. [DOI] [PubMed] [Google Scholar]
- 34.Banin S., Truong,O., Katz,D.R., Waterfield,M.D., Brickell,P.M. and Gout,I. (1996) Wiskott-Aldrich syndrome protein (WASp) is a binding partner for c-Src family protein-tyrosine kinases. Curr. Biol., 6, 981–988. [DOI] [PubMed] [Google Scholar]
- 35.Hartmuth K., Urlaub,H., Vornlocher,H.P., Will,C.L., Gentzel,M., Wilm,M. and Luhrmann,R. (2002) Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl Acad. Sci. USA, 99, 16719–16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkle A. (2001) Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays, 23, 795–806. [DOI] [PubMed] [Google Scholar]
- 37.Marenstein D.R., Chan,M.K., Altamirano,A., Basu,A.K., Boorstein,R.J., Cunningham,R.P. and Teebor,G.W. (2003) Substrate specificity of human endonuclease III (hNTH1). Effect of human APE1 on hNTH1 activity. J. Biol. Chem., 278, 9005–9012. [DOI] [PubMed] [Google Scholar]
- 38.Vidal A.E., Boiteux,S., Hickson,I.D. and Radicella,J.P. (2001) XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein–protein interactions. EMBO J., 20, 6530–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumann P., Benson,F.E. and West,S.C. (1996) Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell, 87, 757–766. [DOI] [PubMed] [Google Scholar]
- 40.Masson J.Y., Tarsounas,M.C., Stasiak,A.Z., Stasiak,A., Shah,R., McIlwraith,M.J., Benson,F.E. and West,S.C. (2001) Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev., 15, 3296–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opresko P.L., Cheng,W.H., Von Kobbe,C., Harrigan,J.A. and Bohr,V.A. (2003) Werner syndrome and the function of the Werner protein; what they can teach us about the molecular aging process. Carcinogenesis, 24, 791–802. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto T., Izumi,H., Imamura,T., Takano,H., Ise,T., Uchiumi,T., Kuwano M. and Kohno,K. (2000) Direct interaction of p53 with the Y-box binding protein, YB-1: a mechanism for regulation of human gene expression. Oncogene, 19, 6194–6202. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y.F., Homer,C., Edwards,S.J., Hananeia,L., Lasham,A., Royds,J., Sheard,P. and Braithwaite,A.W. (2003) Nuclear localization of Y-box factor YB1 requires wild-type p53. Oncogene, 22, 2782–2794. [DOI] [PubMed] [Google Scholar]
- 44.Gray M.D., Shen,J.C., Kamath-Loeb,A.S., Blank,A., Sopher,B.L., Martin,G.M., Oshima,J. and Loeb,L.A. (1997) The Werner syndrome protein is a DNA helicase. Nature Genet., 17, 100–103. [DOI] [PubMed] [Google Scholar]
- 45.Fink D., Aebi,S. and Howell,S.B. (1998) The role of DNA mismatch repair in drug resistance. Clin. Cancer Res., 4, 1–6. [PubMed] [Google Scholar]
- 46.Marti T.M., Kunz,C. and Fleck,O. (2002) DNA mismatch repair and mutation avoidance pathways. J. Cell. Physiol., 191, 28–41. [DOI] [PubMed] [Google Scholar]
- 47.Woodard R.L., Lee,K.J., Huang,J. and Dynan,W.S. (2001) Distinct roles for Ku protein in transcriptional reinitiation and DNA repair. J. Biol. Chem., 276, 15423–15433. [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto M., Donald,C.D., Yannone,S.M., Chen,D.J., Roy,R. and Kow,Y.W. (2001) A possible role of Ku in mediating sequential repair of closely opposed lesions. J. Biol. Chem., 276, 12827–12831. [DOI] [PubMed] [Google Scholar]
- 49.Blander G., Zalle,N., Daniely,Y., Taplick,J., Gray,M.D. and Oren,M. (2002) DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J. Biol. Chem., 277, 50934–50940. [DOI] [PubMed] [Google Scholar]