Abstract
High levels of insulin-like growth factor (IGF)-1 may increase the risk of common cancers in humans. We hypothesized that weight loss induced by diet and/or exercise would reduce IGF-1 in postmenopausal women. Four hundred and thirty nine overweight or obese (BMI ≥25kg/m2) women (50–75 y) were randomly assigned to: i) exercise (N=117), ii) dietary weight-loss (N=118), iii) diet + exercise (N=117), or iv) control (n=87). The diet intervention was a group-based program with a 10% weight loss goal. The exercise intervention was 45 min/day, 5 days/week of moderate-to-vigorous intensity activity. Fasting serum insulin-like growth factor (IGF)-1 and insulin-like growth factor binding protein (IGFBP)-3 were measured at baseline and 12 months by radioimmunoassay. Higher baseline BMI was associated with lower IGF-1 and IGF-1/IGFBP-3 molar ratio. While no significant changes in either IGF-1 or IGFBP-3 were detected in any intervention arm compared to control, the IGF-1/IGFBP-3 ratio increased significantly in the diet (+5.0%, p<0.01) and diet + exercise (+5.4%, p<0.01) groups compared to control. Greater weight loss was positively associated with change in both IGF-1 (ptrend=0.017) and IGF-1/IGFBP-3 ratio (ptrend<0.001) in the diet group, but inversely with change in IGFBP-3 in the diet + exercise group (ptrend=0.01). No consistent interaction effects with baseline BMI were detected. Modified IGF-1 bioavailability is unlikely to be a mechanism through which caloric restriction reduces cancer risk in postmenopausal women.
Keywords: lifestyle, intervention, obesity, energy balance, insulin
INTRODUCTION
Biological and epidemiological evidence suggests that high levels of insulin-like growth factor (IGF)-1 may increase risk for several common cancers (1, 2). IGF-1 has mitogenic and antiapoptotic properties that are modulated through IGF binding proteins (IGFBP) (1). Most circulating IGF-1 is bound to one of six IGFBPs, the most abundant being IGFBP-3, while a small amount remains unbound and biologically active. Modifications in IGF-1 bioavailability have been proposed as a mechanism linking obesity and cancer risk (3, 4) while animal studies suggest that alterations in IGF-1, as well as other cytokines and inflammatory factors, may mediate the antiproliferative, pro-apoptotic, and anticancer effects of negative energy balance (5).
Calorie restriction in rodents and other animal models decreases serum IGF-1 concentrations up to 40% (6, 7) but the effects in humans are equivocal (8–10). One explanation may be that, unlike insulin, human IGF-1 does not appear elevated in obese individuals, but peaks in persons with BMI values of 24–27 kg/m2 (11). Alternately, caloric restriction models in animals may not be relevant to humans because they are typically initiated in young ages, thereby preventing obesity, whereas calorie restriction has been tested in humans who are already obese. It is postulated that obesity-related hyperinsulinemia inhibits IGFBP production and results in elevated levels of free IGF-1. In turn, this exerts a negative feedback on growth hormone secretion, thereby lowering IGF-1 (12). Few studies examining the interrelationships between energy balance, IGF-1 and cancer risk factors have considered differential effects according to baseline weight or body composition.
The purpose of this study was to investigate the independent and combined effects of 12 months of dietary weight loss and/or aerobic exercise on IGF-1, IGFBP-3, and the 1GF-1/IGFBP-3 molar ratio as a proxy for free IGF-1 (13) in overweight and obese postmenopausal women. A secondary purpose was to examine the degree to which any intervention effects were moderated by baseline adiposity. We hypothesized that IGF-1 and the IGF-1/IGFBP-3 molar ratio would be significantly decreased in women randomized to diet and diet + exercise, but that this effect would be stronger among women with baseline BMI <28 kg/m2.
METHODS
The Nutrition and Exercise in Women (NEW) study was a 12-month randomized controlled trial testing caloric restriction and exercise on circulating hormones and other outcomes. Study procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board in Seattle, WA. All participants provided informed written consent.
Participants & Interventions
The study methods have been previously described (14). Briefly, participants were postmenopausal women (50–75 y) with a BMI ≥25.0 (≥23.0 kg/m2 if Asian-American), recruited through media and mass mailings. Exclusion criteria included: >100 min/week of moderate physical activity; diagnosed diabetes or fasting blood glucose ≥126 mg/dL; serious medical condition(s); postmenopausal hormone use; >2 alcoholic drinks/day; current smoking; participation in another structured weight loss program; contraindication to participation (e.g. abnormal exercise tolerance test).
Eligible women were randomized to one of: i) reduced-calorie dietary modification (N=118); ii) moderate-to-vigorous intensity aerobic exercise (N=117); iii) combined diet and exercise (N=117); or iv) control (no intervention) (N=87). Computerized random assignment, using permuted blocks randomization to achieve a proportionally smaller control group, was stratified according to BMI (≥ or <30kg/m2) and race/ethnicity.
The dietary intervention was a modification of the Diabetes Prevention Program (DPP)(15) and Look AHEAD (Action for Health in Diabetes)(16) lifestyle behavior change programs with goals of: 1200–2000 kcal/day, <30% daily calories from fat, and 10% weight loss. The exercise intervention goal was 45 minutes of moderate-to-vigorous (≥4 metabolic equivalents [METs]) intensity exercise at a target heart rate of 70–85% observed maximum, 5 days/week. Participants attended three facility-based supervised sessions/week, and exercised 2 days/week at home.
Measures
All study measures were obtained and analyzed by trained personnel who were blinded to participants’ randomization status.
At baseline (pre-randomization) and 12 months, demographic information, height, weight, medical history, dietary intake, supplement use and physical activity data were collected (14). Body composition was measured by DXA whole-body scanner (GE Lunar, Madison, WI, USA).
Twelve-hour fasting venous blood (50 mL) was collected during clinic visits (no exercise within 24 hours, no alcohol within 48 hours). Blood was processed within 1 hour and samples were stored at −70°C. Blood samples were analyzed in batches such that each participant’s samples were assayed simultaneously, the number of samples from each group were approximately equal, participant randomization dates were similar, and sample order was random.
IGF-1 and IGFBP-3 assays were performed at the Jewish General Hospital (Montreal, Canada) using reagents from the Diagnostic Systems Laboratory, Beckman Coulter (Webster, TX, USA). The intra-assay coefficients of variation (CV) were 4.2% and 3.8% for IGF-1 and IGFBP-3, respectively; inter-assay CVs were 5.4% and 4.9% for IGF-1 and IGFBP-3, respectively. The molar ratio of IGF-1/IGFBP-3 was calculated as a proxy for free IGF-1: [IGF-1 (ng/mL) × 0.130]/ [IGFBP-3 (ng/mL) × 0.036] (13).
Statistical Analysis
For the primary analysis, no change from baseline was assumed in cases of missing data. Age-adjusted partial correlation coefficients were performed between baseline measures. The mean 12-month change in IGF-1, IGFBP-3, and the IGF-1/IGFBP-3 ratio in each intervention group was compared to controls using the generalized estimating equations (GEE) modification of linear regression to account for intra-individual correlation over time. Assuming 80% power to make three primary pairwise comparisons (diet+exercise vs. exercise; diet+exercise vs. diet; and diet vs. exercise), the minimum detectable differences in IGF-1, IGFBP-3, and the IGF-1/IGFBP-3 ratio were estimated at 14.36, 305, and 0.009, respectively (type I error=0.05). Bonferroni adjustment for multiple comparisons was made (two-sided alpha=0.05/3=0.016). Intervention effects were examined based on assigned treatment at randomization, regardless of adherence or study retention (i.e. intent-to-treat).
The effect of weight loss on IGF-1, IGFBP-3, and the IGF-1/IGFBP-3 ratio was examined using a stratified analysis (<5%, 5–10%, and >10% weight loss) performed within each arm, without imputation for missing values. These analyses were repeated after stratification by baseline BMI (<28 vs. ≥28 kg/m2) using the cut-point around which IGF-1 peaks in humans (11). The potential moderating effect by baseline BMI was tested by including an appropriate interaction term in each model.
All statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
At 12 months, 399 participants completed a physical exam, a DXA scan, and provided a blood sample; 40 did not complete the study. Participant characteristics, intervention adherence, 12-month weight and body composition changes have been previously published (14). Mean age and BMI were 58.0 years and 30.9 kg/m2. Most women (65%) were college graduates and 85% were non-Hispanic white. Mean weight changes were −2.4% (p=0.03) in the exercise group, −8.5% (p<0.001) in the diet group, and −10.8% (p<0.001) in the diet + exercise group, compared to −0.8% among controls.
Exercise groups participated in moderate-to-vigorous activity for a mean (SD) 163.3 (70.6) mins/wk (exercise), and 171.5 (62.9) mins/wk (diet + exercise). Both groups significantly increased average pedometer steps/day (+2416 and +3471 steps/d, respectively) compared to baseline. In both diet groups, women attended an average of 27 diet counseling sessions (86%).
Baseline Associations
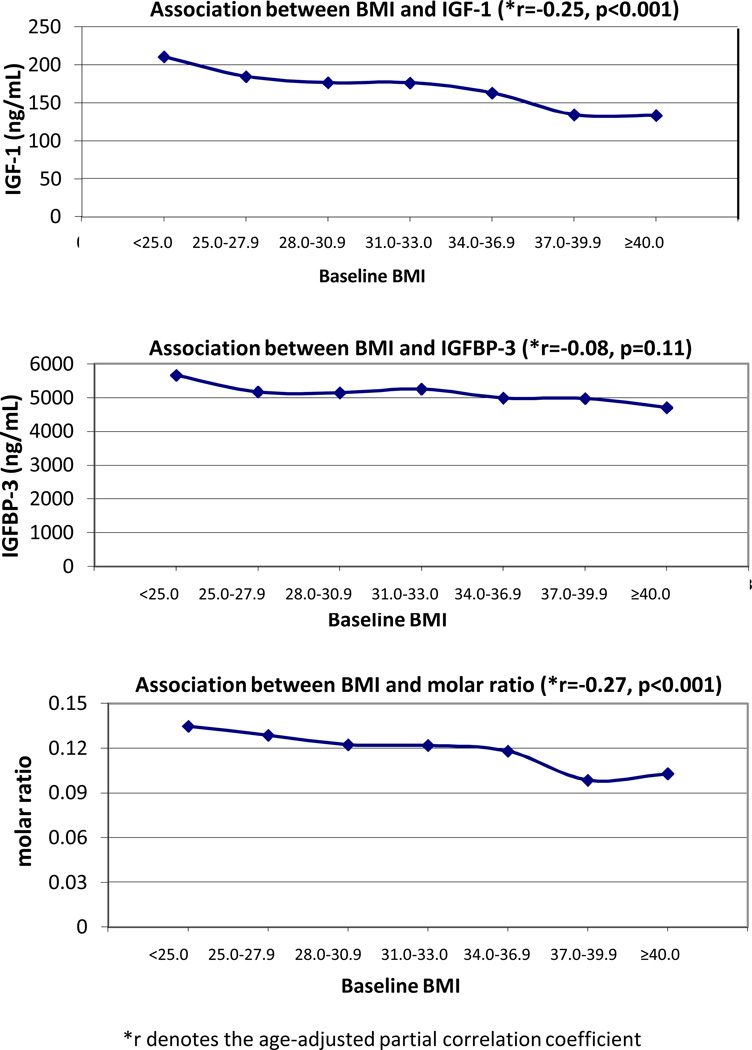
The age-adjusted partial correlations between BMI and IGF-1, IGFBP-3, and the IGF-1/IGFBP-3 ratio at baseline are shown in Figure 1. Both IGF-1 and the IGFBP-3 molar ratio were inversely associated with BMI, while IGBP-3 showed no association. In addition to age (−0.13, p=0.008), baseline IGF-1 concentrations were also significantly inversely associated with, waist circumference (−0.25, p<0.0001), and % body fat mass (−0.20, p<0.0001). IGFBP-3 was significantly inversely associated with % body fat (−0.11, p=0.02). The IGF-1/IGBP-3 molar ratio was significantly inversely associated with age (−0.10, p=0.04), waist circumference (−0.33, p<0.0001), and % body fat (−0.17, p<0.001).
Figure 1.
Associations between IGF-1, IGFBP-3 and the IGF-1/IGFBP-3 molar ratio with body mass index (BMI) at baseline among overweight or obese postmenopausal women.
Intervention Effects
Compared to controls, neither IGF-1 nor IGFBP-3 changed significantly in either group receiving dietary intervention (Table 1). The IGF-1/IGFBP-3 molar ratio increased significantly in the diet group (p=0.008) and in the diet + exercise group (p=0.004). No significant change in IGF-1, IGFBP-3 or their molar ratio was detected among exercisers compared to controls.
Table 1.
Baseline, 12-mo (mean, SD), and change in serum IGF-1, IGFBP-3 and IGF-1/IGFBP-3 molar ratio, stratified by intervention arm.
| Mean (SD) | Change | Change | p | ||
|---|---|---|---|---|---|
| Baseline | 12 month | % | |||
|
CONTROL | |||||
| IGF-1 (ng/mL) | 178.7 (53.4) | 174.0 (53.1) | −4.7 | −2.6 | ref |
| IGFBP-3 (ng/mL) | 5214 (1102) | 5110 (1240) | −104 | −2.0 | ref |
| Molar Ratioł | 0.124 (0.028) | 0.123 (0.027) | −0.001 | −0.4 | ref |
|
EXERCISE | |||||
| IGF-1 (ng/mL) | 172.8 (47.3) | 169.0 (46.2) | −3.8 | −2.2 | 0.81 |
| IGFBP-3 (ng/mL) | 5137 (943) | 4991 (958) | −146 | −2.8 | 0.63 |
| Molar Ratioł | 0.121 (0.025) | 0.122 (0.024) | 0.001 | 0.8 | 0.53 |
|
DIET | |||||
| IGF-1 (ng/mL) | 167.9 (46.0) | 170.3 (45.5) | 2.4 | 1.4 | 0.06 |
| IGFBP-3 (ng/mL) | 5081 (951) | 4921 (1022) | −160 | −3.2 | 0.56 |
| Molar Ratioł | 0.119 (0.024) | 0.125 (0.024) | 0.006 | 5.0 | 0.008 |
|
DIET + EXERCISE | |||||
| IGF-1 (ng/mL) | 177.1 (57.3) | 179.6 (58.5) | 2.6 | 1.4 | 0.06 |
| IGFBP-3 (ng/mL) | 5165 (1051) | 4969 (929) | −197 | −3.8 | 0.31 |
| Molar Ratioł | 0.123 (0.026) | 0.130 (0.028) | 0.007 | 5.4 | 0.004 |
Results from GEE model, comparing change from baseline to 12-months between each intervention group and controls.
Molar ratio = [IGF-1 (ng/mL) × 0.130]/ [IGFBP-3 (ng/mL) × 0.036]
Greater weight loss was associated with an increase in IGF-1 and the IGF-1/IGFBP-3 molar ratio among women randomized to diet alone (Table 2), while we observed an inverse trend greater between weight loss and IGFBP-3 in the diet + exercise group.
Table 2.
Baseline and 12 mo (Mean, SD), and % change in IGF-1, IGFBP-3 and the IGF-1/IGFBP-3 molar ratio, stratified by weight loss.
| IGF-1 | IGFBP-3 | Molar Ratioł | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | 12-mo | % Change |
p | Baseline | 12-mo | % Change | p | Baseline | 12-mo | % Change | p | |
|
CONTROL |
|||||||||||||
| weight loss <5% |
67 | 181.0 (52.5) |
172.5 (50.9) |
−4.7 | ref | 5262 (1064) |
5126 (1159) |
−2.6 | ref | 0.124 (0.027) |
0.122 (0.026) |
−2.0 | ref |
| 5−10% | 9 | 174.1 (57.6) |
187.0 (69.7) |
7.4 | 0.008 | 5029 (1205) |
4855 (1164) |
−3.5 | 0.84 | 0.125 (0.036) |
0.137 (0.037) |
9.6 | 0.0003 |
| >10% | 4 | 173.7 (66.3) |
183.8 (52.9) |
5.8 | 0.45 | 5628 (1180) |
6026 (2340) |
7.1 | 0.55 | 0.110 (0.024) |
0.114 (0.16) |
3.4 | 0.36 |
| ptrend=0.13 | ptrend=0.59 | ptrend=0.03 | |||||||||||
|
EXERCISE |
|||||||||||||
| weight loss <5% |
75 | 172.9 (48.8) |
167.0 (46.6) |
−3.4 | ref | 5242 (983) |
5055 (1007) |
−3.6 | ref | 0.119 (0.024) |
0.119 (0.022) |
0.2 | ref |
| 5−10% | 26 | 181.0 (45.7) |
182.8 (49.2) |
1.0 | 0.33 | 5086 (837) |
5041 (850) |
−0.9 | 0.23 | 0.129 (0.027) |
0.131 (0.029) |
2.0 | 0.63 |
| >10% | 4 | 189.8 (57.4) |
167.3 (46.7) |
−11.8 | 0.03 | 5166 (918) |
4665 (1103) |
−9.7 | 0.13 | 0.132 (0.027) |
0.130 (0.022) |
−1.1 | 0.81 |
| ptrend=0.88 | ptrend=0.89 | ptrend=0.77 | |||||||||||
|
DIET |
|||||||||||||
| weight loss <5% |
28 | 167.2 (48.9) |
163.3 (50.6) |
−2.3 | ref | 5065 (955) |
5044 (1286) |
−0.4 | ref | 0.119 (0.026) |
0.118 (0.029) |
−0.9 | ref |
| 5−10% | 27 | 175.9 (49.1) |
172.2 (39.5) |
−2.1 | 0.97 | 5190 (962) |
4997 (761) |
−3.7 | 0.4 | 0.122 (0.026) |
0.125 (0.023) |
1.9 | 0.43 |
| >10% | 49 | 161.0 (41.8) |
171.8 (45.1) |
6.7 | 0.03 | 5030 (837) |
4767 (871) |
−5.2 | 0.22 | 0.116 (0.023) |
0.130 (0.022) |
12.3 | <0.001 |
| ptrend=0.017 | ptrend=0.22 | ptrend<0.001 | |||||||||||
|
DIET + EXERCISE |
|||||||||||||
| weight loss <5% |
18 | 158.2 (47.9) |
163.2 (50.1) |
3.2 | ref | 4962 (973) |
4983 (979) |
0.4 | ref | 0.115 (0.027) |
0.118 (0.027) |
2.8 | ref |
| 5−10% | 21 | 167.7 (53.8) |
173.7 (46.2) |
3.6 | 0.93 | 4702 (985) |
4708 (890) |
0.1 | 0.95 | 0.129 (0.028) |
0.134 (0.025) |
3.9 | 0.75 |
| >10% | 69 | 188.6 (60.1) |
189.8 (64.2) |
0.7 | 0.68 | 5342 (1072) |
5004 (938) |
−6.3 | 0.04 | 0.127 (0.025) |
0.136 (0.027) |
7.1 | 0.19 |
| ptrend=0.59 | ptrend=0.01 | ptrend=0.16 | |||||||||||
Molar ratio = [IGF-1 (ng/mL) × 0.130]/ [IGFBP-3 (ng/mL) × 0.036]
In the stratified analysis (BMI ≥28 vs.<28) the IGF-1/IGFBP-3 ratio was significantly increased among women with BMIs ≥28 kg/m2 randomized to diet + exercise compared to controls. No significant differences were detected in women with BMIs <28 kg/m2. A greater decrease in IGFBP-3 was observed among women with BMI ≥28 vs.<28 in the diet + exercise group (pinteraction= 0.005) (supplementary table).
DISCUSSION
No significant changes in IGF-1 or its main binding protein, IGFBP-3, were detected in any intervention arm compared to control; however, the IGF-1/IGFBP-3 molar ratio increased significantly in the diet and diet + exercise groups. This suggests that lifestyle-based weight loss of 8–10% may increase IGF-1 bioavailability in overweight and obese postmenopausal women - an effect that is counter-intuitive to the anticipated effect of weight loss on cancer risk, and opposite to the effects consistently observed in animal models (5). However, as noted above, the majority of published animal studies have not tested caloric restriction weight loss in already obese animals, and therefore do not serve as true models for overweight/obese persons undergoing weight loss. Furthermore, by comparison to animal models in which caloric restriction does alter IGF-1 and impede tumor growth, the degree of change in energy balance was relatively modest in this study (17, 18). Equivalently severe exercise and/or caloric restriction may not be practical in most human populations.
The equivocal effect of dietary weight loss on IGF-1 in our trial is consistent with a limited number of other studies in women and men (19–23). In the only other study of exclusively postmenopausal women (n=99, mean BMI 26.9 kg/m2), 5 months of dietary intervention yielding a mean BMI decrease of 1.62 kg/m2 did not significantly alter serum IGF-1 compared to weight-stable controls, although IGFBP-1 and -2 were increased (22); IGFBP-3 was not measured. In another 6 month trial comparing intermittent vs. continuous caloric restriction in premenopausal women (mean BMI 30.6 ± 5.1 kg/m2), negligible changes in total and free IGF-1 were observed in response to mean weight changes of −6.4 and −5.6 kg in each group, respectively (23). However, both groups experienced similar significant increases in IGFBP-1 and -2.
In contrast, IGF-1 increased significantly after a mean 9.7 ± 4.3% weight loss in obese women with a mean BMI of 37.2 kg/m2(19). Likewise, IGF-1 increased significantly in a sample of obese men and women (mean BMI 32.6 kg/m2) during a 6-month weight loss period, and levels continued to increase despite partial weight regain during the subsequent 6 months (20). In another sample of premenopausal women (mean BMI of 29.2 ±6.2 kg/m2), intentional weight loss ≥5% body weight (vs. weight stable/gain) was associated with a significant increase in serum total IGF-1, while free IGF-1 and IGFBP-3 remained unchanged (21).
Strengths of our study include its large sample and adequate power to examine changes in IGF-1 and IGFBP-3 in three separate lifestyle interventions compared to controls. However, the IGF-1 axis is complex and circulating levels may not reflect local tissue levels, which we were unable to assess. Furthermore, we relied on the IGF-1/IGFBP-3 molar ratio as a surrogate of bioactive IGF-1. Although this ratio is a reasonable approximation of free IGF-1 under normal physiological conditions (13), free IGF-1 is also influenced by levels of other IGFBPs (24). Finally, IGF-1 and IGFBP-3 levels are affected by multiple factors, including gender and race/ethnicity (25); therefore, these results may not be generalizable to men or to populations that are not predominantly Caucasian.
The findings of the current study, plus those of previous studies, suggest that animal models of caloric restriction on IGF-1 and IGFBP-3 are not confirmed with caloric restriction and weight loss in overweight/obese women. They also suggest that modified IGF-1 bioavailability is unlikely to represent a major mechanistic link through which caloric restriction impacts obesity-associated cancer risk in humans.
Supplementary Material
Acknowledgements
Funding/Support: This study was funded through NIH R01 CA102504 and U54-CA116847. CM and KLC were supported by fellowships from the Canadian Institutes of Health Research (CIHR). KFS received support from NIH 5KL2RR025015-03. AK was supported by NCI R25 CA094880 and is now supported by NCI 2R25CA057699-16. While working on the trial, CMA was employed at the Ohio State University, and located to NCI following completion of her effort on the NEW trial.
Footnotes
Author Contributions: Drs Mason and McTiernan had access to all of the study data and take responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Mason, Foster-Schubert, Alfano, Blackburn, McTiernan; Acquisition of data: Kong, Campbell, Duggan, Blackburn, Pollack, McTiernan; Analysis and interpretation of data: Mason, Xiao, Imayama, McTiernan; Drafting of the manuscript: Mason Critical revision of the manuscript for important intellectual content: Foster-Schubert, Duggan, Imayama, Kong, Campbell, Blackburn, Alfano, Pollack, McTiernan; Statistical analysis: Mason, Xiao, Obtained funding: McTiernan; Administrative, technical, or material support: Xiao; Study supervision: McTiernan.
Financial disclosures: The authors have no disclosures.
References
- 1.Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinology and metabolism clinics of North America. 2012;41:335–350. doi: 10.1016/j.ecl.2012.04.014. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. European journal of human genetics. 2009;17:1668–1675. doi: 10.1038/ejhg.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends in endocrinology and metabolism. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature reviews. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 5.Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- 6.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer research. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 7.Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, et al. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–89. [PubMed] [Google Scholar]
- 9.DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1444–1451. [PubMed] [Google Scholar]
- 10.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calle E, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 12.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. The Proceedings of the Nutrition Society. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 13.Frystyk J. Free insulin-like growth factors -- measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14:337–375. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Foster-Schubert KE, Alfano CM, Duggan C, Xiao L, Campbell KL, Kong A, et al. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity. 2011;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clinical Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 17.Rogozina OP, Bonorden MJ, Grande JP, Cleary MP. Serum insulin-like growth factor-I and mammary tumor development in ad libitum-fed, chronic calorie-restricted, and intermittent calorie-restricted MMTV-TGF-alpha mice. Cancer prevention research (Philadelphia, Pa. 2009;2:712–719. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- 18.Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr Cancer. 2011;63:389–401. doi: 10.1080/01635581.2011.535968. [DOI] [PubMed] [Google Scholar]
- 19.Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Kocelak P, Janowska J, Semik-Grabarczyk E. The effect of weight reduction on plasma concentrations of ghrelin and insulin-like growth factor 1 in obese women. Polish Journal of Endocrinology. 2008;59:301–304. [PubMed] [Google Scholar]
- 20.Lien LF, Haqq AM, Arlotto M, Slentz CA, Muehlbauer MJ, McMahon RL, et al. The STEDMAN project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. Omics : a journal of integrative biology. 2009;13:21–35. doi: 10.1089/omi.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvie M, Renehan AG, Frystyk J, Flyvbjerg A, Mercer T, Malik R, et al. Increase in serum total IGF-1 and maintenance of free IGF-1 following intentional weight loss in premenopausal women at increased risk of breast cancer. Open Obes J. 2010;2:63–70. [Google Scholar]
- 22.Kaaks R, Bellati C, Venturelli E, Rinaldi S, Secreto G, Biessy C, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. European Journal of Clinical Nutrition. 2003;57:1079–1088. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 23.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. International journal of obesity (2005) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frystyk J. Utility of free IGF-I measurements. Pituitary. 2007;10:181–187. doi: 10.1007/s11102-007-0025-y. [DOI] [PubMed] [Google Scholar]
- 25.Berrigan D, Potischman N, Dodd KW, Hursting SD, Lavigne J, Barrett JC, et al. Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2009;19:146–155. doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.