Abstract
Background
The anterior hippocampus is associated with emotional functioning and hippocampal volume is reduced in depression. More women are clinically depressed than men, yet the depressed female brain is little studied. We reported reduced anterior hippocampal volume in behaviorally depressed adult female cynomolgus macaques; the mechanisms contributing to that reduction are unknown. The present study represents the first systematic morphological investigation of the entire hippocampus in depressed female primates.
Methods
Cellular determinants of hippocampal size were examined in subregions of anterior and posterior hippocampus in antidepressant-naive, adult female monkeys characterized for behavioral depression and matched on variables that influence hippocampal size (n=8 depressed, 8 nondepressed). Unbiased stereology was used to estimate neuronal and glial numbers, neuronal soma size, and regional and layer volumes.
Results
Neuropil and cell layer volumes were reduced in cornu ammonis (CA)1 and dentate gyrus (DG) of the anterior but not the posterior hippocampus of depressed compared to nondepressed monkeys. Glial numbers were 30% lower in anterior CA1 and DG of depressed monkeys, with no differences observed in the posterior hippocampus. Granule neuron number tended towards a reduction in anterior DG; pyramidal neuron number was unchanged in any region. Size of pyramidal neurons and glial densities tended to be reduced throughout the whole hippocampus of depressed monkeys, whereas neuronal densities were unchanged.
Conclusions
The reduced size of the anterior hippocampus in depressed females appears to arise from alterations in numbers of glia and extent of neuropil, but not numbers of neurons, in CA1 and DG.
Keywords: unbiased stereology, macaca fascicularis, hippocampal volume, glial number, neuron number, soma size
INTRODUCTION
Hippocampal morphometric alterations are implicated in the pathophysiology of depression, as evidenced by numerous reports of reduced hippocampal volume in depressed patients (1). The mechanisms contributing to these reductions are poorly understood. Given that standard pharmacotherapeutics help less than half of patients, treating depression poses a substantial challenge (2). Increased understanding of the underlying neurobiology is essential to generating novel therapeutic interventions.
The therapeutic effects of some antidepressants appear to be associated with hippocampal neurogenesis under certain conditions (3). Elevated glucocorticoid levels are associated with suppressed neurogenesis and gliogenesis, increased apoptosis, dendritic retraction, and reduced dendritic spines in the hippocampus (4, 5, 6). However, postmortem analyses of hippocampi from depressed patients did not reveal substantial neuronal and glial loss (7, 8), but instead demonstrated decreased density of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes (9), increased neuronal and glial packing densities, and decreased neuronal size (10). These studies suggest that alterations in neuropil and glia may contribute more to volume reductions in depression than changes in neuronal number (10). However, most human hippocampal studies are based on relatively few sections from patients with histories of antidepressant exposure. In animal studies, antidepressant treatment prevents stress-induced glial reductions in mood-related brain regions (11, 12), suggesting that antidepressant exposure in human subjects might prevent glial reductions in depression. Glial deficits have been reported in depressed patients in brain regions that share functional connectivity with the hippocampus, including the anterior cingulate (13, 14) and prefrontal cortices (15), providing evidence for glial abnormalities in the neurocircuitry of depression.
Despite the greater prevalence of depression in women (16), little is known about the depressed female brain. Experimental studies of depression in females are critical because contributing mechanisms likely differ between sexes. Sex-dependent differences in behavioral and neurobiological responses to stress have been observed in both humans and rodents (17, 18). Ovarian steroid-induced changes in hippocampal morphology have been described in rodents (19), and volume alterations in the anterior hippocampus are associated with menstrual cycle phase in women (20). Moreover, glial deficits associated with depression-like behavior in prenatally stressed mice are limited to the hippocampus of female and not male offspring (21), and estrogen protects against neuronal loss in the hippocampus of chronically stressed female rats (22). Notably, these reproductive system influences on rodent hippocampus are confined to the anterior region, that is, the portion of the hippocampus that is associated with emotional functioning (23). Thus, estrogen-influenced structural alterations in the hippocampus in response to stress may be central to the neurobiology of depression in females.
In order to promote understanding of the depressed female primate brain, we have studied social stress-associated depressive behavior and accompanying physiological and neurobiological factors in adult female cynomolgus macaques (Macaca fascicularis) for 25 years (24, 25). Briefly, depressive behavior in female cynomolgus monkeys is naturally occurring in captivity and not induced by experimental manipulation. It may be observed in relation to social environmental challenges such as social isolation and social subordination. Social stress-associated behavioral depression in adult female cynomolgus macaques is similar to human depression in physiological, neurobiological, and behavioral characteristics, including reduced body mass, hypothalamic–pituitary–adrenal axis perturbations, autonomic dysfunction, increased cardiovascular disease risk, reduced hippocampal volume, altered serotonergic function, decreased activity levels, and increased mortality. In addition, behaviorally depressed monkeys also have low ovarian steroid concentrations, even though they continue to have menstrual cycles. The menstrual cycles of cynomolgus macaques are like those of women in length and hormonal fluctuations, and the macaque hippocampus more closely parallels the cellular organization and connectivity patterns of the human hippocampus than does that of the rat (26).
Previously, we reported reduced anterior hippocampal volume in antidepressant-naïve, behaviorally depressed females (27). The goal of the present study was to elucidate the cellular components of anterior hippocampal volume reduction by systematic investigation of the entire hippocampus in depressed female primates, while controlling for ovarian steroids and eliminating the confounding influence of treatment. To accomplish this, design-based stereologic methods were used to estimate neuronal and glial numbers, neuronal soma size, and laminar volumes in all subregions of the anterior and posterior hippocampus, in tissue derived from a carefully matched sample of antidepressant-naïve, adult, female monkeys characterized for behavioral depression. Based on previous studies, we hypothesized that alterations in glia and neuropil would contribute more than neuronal alterations to differences in hippocampus size, and that these effects would be limited to the anterior hippocampus.
METHODS AND MATERIALS
Subjects
Twenty-eight reproductive-aged female cynomolgus macaques (Macaca fascicularis), were obtained (from Institut Pertanian Bogor, Bogor, Indonesia) and housed for 24 months in stable social groups of four animals each. As part of a study investigating the comorbidity of depression and cardiovascular disease risk factors, the animals consumed a diet designed to mimic that typical in North America, containing 0.28 mg cholesterol/Cal and 42% of calories as fat (28, 29, 30). All procedures involving primates were conducted in compliance with institutional (IACUC #A99-102), state, and federal laws for the usage of primates in laboratory settings.
Behavior
Social status hierarchies, determined monthly by recording outcomes of agonistic interactions (28, 31, 32), were stable over time as in previous experiments (33). Behavioral depression is operationally defined as a slumped or collapsed body posture (head lower than shoulders), in which an animal’s eyes are open but the animal lacks interest in or responsivity to environmental stimuli (28, 31, 32, Figure S1 in Supplement 1). Behavioral depression in captive cynomolgus macaques appears spontaneously and is not induced by an experimental manipulation. Each monkey was observed for 15 minutes/week × 52 weeks/year × 2 years for a total of 26 hours/monkey. Percent time spent behaviorally depressed was calculated as [time spent in behavioral depression/total observation time] × 100. Interobserver reliability was ≥92%. Detailed characteristics of the behavioral and peripheral physiology of these animals have been described (28, 29, 30, 34).
Hypothalamic-Pituitary-Adrenal (HPA) Axis Function
One month prior to necropsy, a dexamethasone suppression test (DST) was used to assess the sensitivity of the HPA axis to glucocorticoid negative feedback (28, 31, 32). Morning blood samples were taken for baseline cortisol determinations, dexamethasone (130 μg/kg body weight, I.M.) was administered later in the evening, and then another blood sample was taken the following morning for cortisol assay. Percent suppression of cortisol, calculated as [baseline - second morning cortisol levels]/baseline cortisol] × 100, was used as an indicator of sensitivity to glucocorticoid negative feedback (35).
Steroid Hormone Assays
Estradiol and progesterone were assayed in blood collected at necropsy. Ovarian steroids and cortisol concentrations were determined using commercially available radioimmunoassays (Diagnostic Products Corporation, Los Angeles, California).
Subject Selection
Given that depressive behavior is variably expressed among individuals, the sample used in the present study roughly represented the upper and lower tertiles of the distribution of depressive behavior. From the parent population of 28 animals, eight animals from the upper 32 % of the distribution of time spent in the depressed posture were matched with eight animals from the lower 39 % of the distribution on body weight, age, social status, basal cortisol levels, cortisol response in the DST, % suppression of cortisol, and estradiol and progesterone levels at the time of necropsy (Table 1), for a final sample of N=16 animals. This matching was done to reduce variance in the relatively small sample, thus assuring that behavioral depression was the only statistically significant difference between the two groups with respect to characteristics known to affect hippocampal structure.
Table 1.
Group Characteristics
| Depressed (N=8) | Nondepressed (N=8) | t(14) | p | |
|---|---|---|---|---|
| % Time Behaviorally Depressed | 4.25 ± 0.48 | 0.64 ± 0.16 | 7.10 | 0.000005 |
| Age (yrs) | 18.4 ±1.38 | 20.5 ± 1.10 | 1.21 | 0.25 |
| Body Weight (kg) | 3.19 ± 0.14 | 3.51 ± 0.21 | 1.71 | 0.11 |
| Progesterone (ng/ml) | 19.07 ± 4.69 | 18.74 ± 4.05 | 0.05 | 0.96 |
| Estradiol (pg/ml) | 0.32 ± 0.12 | 0.45 ± 0.16 | 0.67 | 0.52 |
| Baseline Cortisol (μg/ml) | 42.05 ± 5.13 | 42.39 ± 3.82 | 0.02 | 0.99 |
| Cortisol Response to Dexamethasone | 10.99 ± 1.41 | 11.30 ± 2.10 | 0.12 | 0.90 |
| % Suppression of Cortisol | 73.65 ± 2.16 | 73.16 ± 3.93 | 0.11 | 0.92 |
| Relative Social Status* | 0.33 ± 0.09 | 0.57 ± 0.15 | 1.40 | 0.18 |
Values represent means ± SEM.
Range 0–1, 0=subordinate, 1=dominant.
Tissue Preparation and Cell Labeling
Procedures for tissue collection and cell labeling are provided in Supplement 1 and summarized here. Hemisected brains were frozen and stored at −80°C. Temporal lobes were dissected, fixed, and cut coronally at 50μm. To control for laterality, experimental groups were counterbalanced for hemisphere (27). Sections were processed in batches equally representing behavioral depression status. Pyramidal neurons were labeled by NeuN immunohistochemistry visualized using avidin-biotin-peroxidase and diaminobenzidine (Figure S2A in Supplement 1). Granule neurons and glia were labeled by histochemistry for Nissl substance (cresyl violet, Figure S2B–D in Supplement 1).
Quantitative Stereological Analyses
Total numbers of neurons and glia were estimated using the optical fractionator technique (36), and the Stereo Investigator system (MicroBrightfield, Williston, VT). The anterior hippocampus was delineated from the posterior hippocampus by the presence of the uncus (27, 37). Within each region (anterior, posterior), the following subregions were assessed: 1) DG, 2) proximal CA3 (portion within the blades of the DG), 3) distal CA3 (portion deep to the DG) + CA2, and 4) CA1 + subiculum (Figure 1B). The distal CA3 and adjacent CA2 are difficult to differentiate (26), and were combined. The boundary between the CA1 and subiculum is oblique (26) and subicular structure may be altered in depressed patients (38); thus, the CA1 region was expanded to include the subiculum. Glia were counted in combined cell and neuropil layers in each subregion (e.g., molecular layer, granule cell layer, polymorphic cell layer in the DG) regardless of glial subtype; neurons were analyzed in the principle cell layers only (e.g., granule cell layer of the DG).
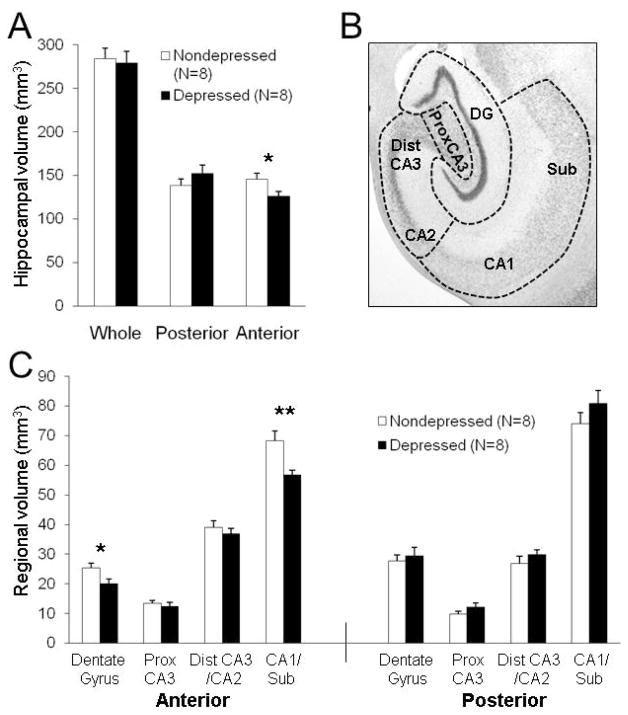
Figure 1.
Specific subregions of the anterior hippocampus were smaller in behaviorally depressed female monkeys. (A) Whole and posterior hippocampal volumes were not different, but anterior hippocampal volumes were smaller (t(14) = 2.32, p < 0.05) in depressed compared to nondepressed monkeys, similar to those reported in our previous study (Willard et al., 2009). (B) Nissl-stained coronal section depicting the delineations used for volumetric assessment of subregions. (C) In the anterior hippocampus, subregion volumes were smaller in depressed monkeys (F(1,14) = 5.36, p < 0.05) and differed by subregion (F(3,42) = 6.91, p < 0.001), with significantly smaller anterior CA1/subiculum (p < 0.01) and DG (p < 0.05) volumes. No effects were found in the posterior hippocampus. *p < 0.05; **p < 0.01.
Stereological parameters were empirically derived for use in macaque tissue, as described in Supplement 1, and the precision of each estimate indicated by the Schmitz-Hof (39) coefficient of error (CE). Parameters are described in detail for each ROI in Table S1 in Supplement 1. The section sampling interval was determined separately for each region of the hippocampus, since the anatomy of the anterior hippocampus changes significantly along the anterior-posterior extent, whereas the posterior hippocampus is more homogenous. Thus, systematically random series at intervals of 1-in-12 were used for the anterior hippocampus and 1-in-24 for the posterior hippocampus, resulting in an average total of 20 sections per animal. Volumes for each subregion and layer were determined using the planimetric measures from the optical fractionator probe, and validated by applying the Cavalieri method probe on a subset of animals (40). Whole hippocampal volumes were assessed by summing all subregions (DG, proximal CA3, distal CA3/CA2, and CA1) within the anterior or posterior region (Figure 1B). Average cell densities were calculated for each ROI (total number of cells/planimetric volume). Soma size was estimated in every fifth neuron counted using the nucleator probe (40, 41). Analyses were performed blinded to the experimental condition.
Statistical Analyses
Two-tailed t tests were used to verify that depressed and nondepressed animals did not differ in matching characteristics and to compare whole, anterior and posterior hippocampal volumes between groups (as in our previous postmortem study, 27). Two-group (depressed, nondepressed) × four-subregion (DG, proximal CA3, distal CA3/CA2, CA1/subiculum) mixed-models ANOVA was used to assess the main effect of depression and depression by subregion interactions. Given the size differences among subregions, main effects of subregion were expected and are not reported. Neuronal soma size was analyzed separately for granule and pyramidal neurons due to the large difference in their size. Student-Newman-Keuls was used for post hoc comparisons between groups. F and t ratios and degrees of freedom are listed in the figure legends. The α-level was set at 0.05 for all analyses; data are depicted as means ± SEMs.
RESULTS
Small anterior hippocampal volume is characteristic of behaviorally depressed female monkeys
There were no differences between depressed and nondepressed animals in body weight, age, social status, basal cortisol levels, cortisol response to DST, % suppression of cortisol, or estradiol or progesterone levels at the time of necropsy (Table 1). There were no significant differences between depressed and nondepressed animals in whole or posterior hippocampal volumes (Figure 1A). Anterior hippocampal volumes were 13.5% smaller (p < 0.05) in depressed animals compared to their nondepressed counterparts. These results are nearly identical to those observed in our previous postmortem evaluation of hippocampal volume (27).
Differences in anterior hippocampal volume are restricted to the CA1 and DG, and are greatest in the neuropil layers of behaviorally depressed female monkeys
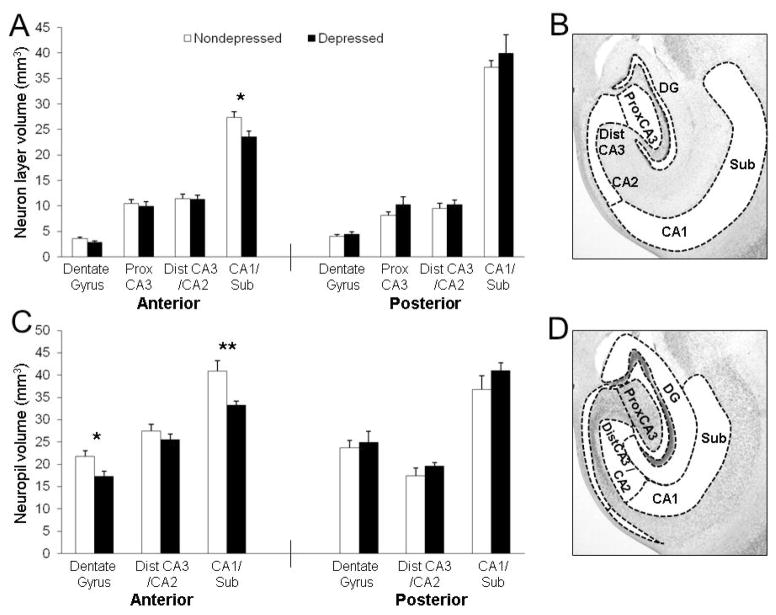
No significant effects were observed in subregion, neuropil, or principle cell layer volumes in the posterior hippocampus. In the anterior hippocampus, a significant main effect of depression (p < 0.05) and a depression × subregion interaction (p < 0.001) were observed for subregion volumes (Figure 1C). The volumes of the anterior CA1/subiculum (p < 0.01) and DG (p < 0.05) were smaller in depressed compared to nondepressed monkeys. While no main effect of depression was observed for the volumes of the principle cell layers in the anterior hippocampus, there was a significant depression × subregion interaction (p < 0.01; Figure 2A–B). The volume of the pyramidal cell layer in the anterior CA1/subiculum was smaller in depressed compared to nondepressed monkeys (p < 0.05). A main effect of depression was observed for neuropil volumes in the anterior hippocampus (p < 0.05; Figure 2C–D), but the depression × subregion interaction did not reach significance (p = 0.055). The volumes of the neuropil layers in the anterior CA1/subiculum (p < 0.01) and DG (p < 0.05) were smaller in depressed animals.
Figure 2.
CA1 and DG neuropil and principle cell layers were smaller in the anterior hippocampus of depressed monkeys. (A) No main effect of depression was found, but there was an interaction by subregion (F(3,42) = 4.32, p < 0.01) for principle cell layer volumes in the anterior hippocampus, with reduced volume of the pyramidal cell layer in the CA1/subiculum (p < 0.05) of depressed monkeys. No effects were observed in the posterior hippocampus. (B) The subregion delineations for principle cell layers are depicted. (C) Neuropil volumes were smaller in depressed monkeys (F(1,14) = 8.41, p < 0.05). The depression × subregion interaction was suggestive but did not reach significance (F(3,42) = 3.22, p < 0.06). Smaller neuropil extent was observed in the anterior CA1/subiculum (p < 0.01) and DG (p < 0.05). No effects were found in the posterior hippocampus. (D) The subregion delineations for neuropil layers are depicted. *p < 0.05; **p < 0.01.
Neuronal number, density and soma size are minimally altered in the hippocampi of behaviorally depressed female monkeys
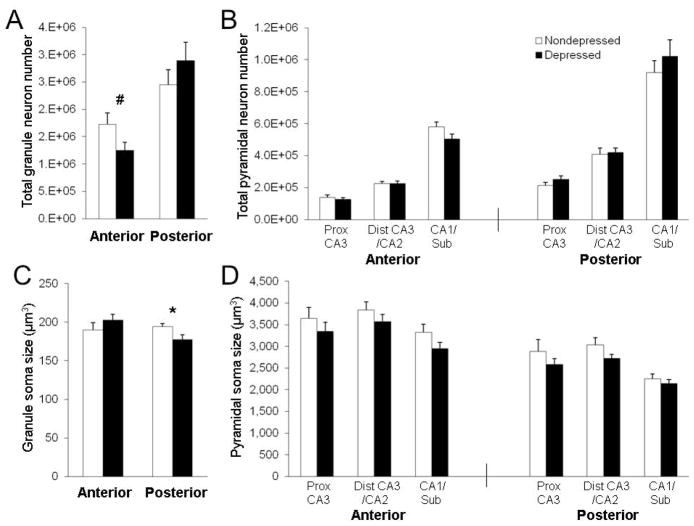
No significant effects were observed for neuronal density in the anterior or posterior hippocampus (Figure S3 in Supplement 1). In the anterior hippocampus, depressed animals had fewer neurons than nondepressed animals, on average, but the main effect of depression did not reach significance (p < 0.10). However, there was a significant depression × subregion interaction (p < 0.05; Figure 3). There appeared to be fewer anterior DG granule neurons on average, although this difference was not significant (p < 0.10; Figure 3A). No significant effects were observed for neuronal number in the posterior hippocampus. While there was no difference between groups in anterior DG granule neuron soma size, posterior DG granule neuron soma size (p < 0.05) was significantly smaller in depressed compared to nondepressed monkeys (Figure 3C). Pyramidal neuron soma size was, on average, lower in depressed compared to nondepressed animals across all subregions throughout the extent of the whole hippocampus (Figure 3D), but no significant effects were observed in either the anterior or posterior hippocampus.
Figure 3.
Alterations in hippocampal neurons were minor in behaviorally depressed female monkeys. (A–B) In the anterior hippocampus, neuron number tended to be lower in depressed monkeys (F(1,14) = 4.12, p < 0.07), but did differ by subregion (F(3,42) = 3.23, p < 0.05), with a trend for fewer DG granule neurons (p < 0.09). No effects were found in the posterior hippocampus. (C) Posterior, but not anterior, DG granule neuron soma size (t(1,14) = 5.05, p < 0.05) was smaller in depressed monkeys. (D) The average pyramidal neuron soma size was lower in depressed animals across all subregions, but no effects were found in the anterior or posterior hippocampus. #p < 0.10; *p < 0.05.
Glial number is substantially lower in the anterior CA1 and DG of behaviorally depressed female monkeys
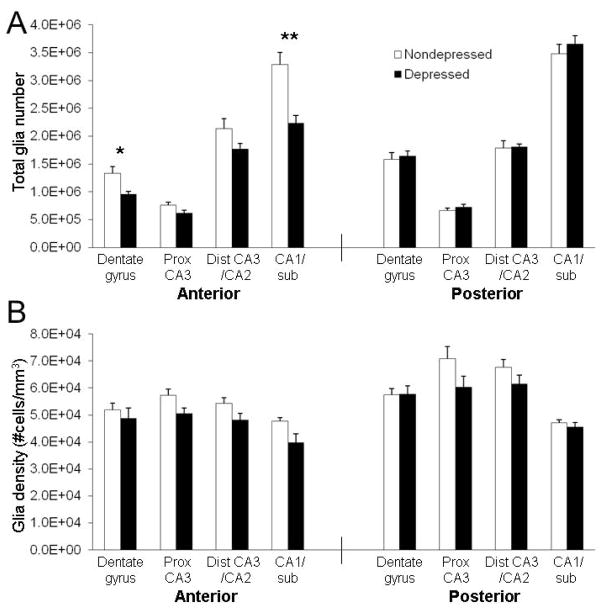
No significant effects were observed for glial number or density in the posterior hippocampus. A significant main effect of depression (p < 0.01) and an interaction effect between depression × subregion (p < 0.001) were observed for the number of glia in the anterior hippocampus (Figure 4A). Depressed animals had 30% fewer glia in both the anterior CA1 (p < 0.01) and DG (p < 0.05) than nondepressed animals. While the main effect of depression was suggestive, it did not reach significance for glial density in the anterior hippocampus (p = 0.06), and no depression × subregion interaction effect was found (Figure 4B).
Figure 4.
Behaviorally depressed female monkeys had reduced numbers of glia in the anterior CA1 and DG. (A) Depressed monkeys had fewer glia in the anterior hippocampus (F(1,14) = 11.14, p < 0.01) and the numbers differed by subregion (F(3,42) = 8.99, p < 0.0001), with 30% fewer glia in the anterior CA1 (t(1,14) = 3.99, p = 0.001) and DG (t(1,14) = 2.85, p = 0.01). No effects were observed in the posterior hippocampus. (B) Glial density tended to be lower in the anterior hippocampus of depressed monkeys (F(1,14) = 4.15, p < 0.07), but no interaction by subregion was observed. No main effect of depression was observed for glial density in the posterior hippocampus, but the interaction by subregion was suggestive, although it did not reach significance (F(3,42) = 2.66, p = 0.06). *p < 0.05; **p < 0.01.
DISCUSSION
To our knowledge, this is the first study to systematically investigate the morphological characteristics of the entire hippocampus in depressed female primates with no prior antidepressant exposure. The results indicate profound alterations in anterior hippocampal morphology specifically within the CA1 and the DG in depressed compared to nondepressed animals, with few alterations in the posterior hippocampus. Moreover, it appears that the number of glia and the extent of the neuropil contributed more than neuronal alterations to anterior hippocampus size. These observations are compelling because many of the characteristics that differentially affect hippocampal structure in human studies are controlled in this primate model. The animals lived in the same housing conditions, consumed the same diet, and were not exposed to antidepressant pharmacotherapy, recreational drugs, or alcohol. Depressive behavior was objectively documented over the course of two years, and depressed and nondepressed monkeys were carefully matched on body weight, age, social status, ovarian steroids and cortisol levels.
A major strength of this study lies in the methodology. Design-based stereology produces robust outcomes because sources of systematic errors are removed from the calculations (42). This is likely why the hippocampal volume measurements reported in the present study precisely replicated our previous postmortem unbiased stereological study (27). We found nearly the same magnitude and level of significance for reduced anterior hippocampal volume in depressed monkeys in both studies, even though the studies used different subjects, were conducted years apart, and used different stereological software. These replicated results further suggest that smaller anterior hippocampal volume may be characteristic of behaviorally depressed adult female monkeys. In addition, the smaller CA1 volume we report in the present study is similar to shape deformation analyses via MRI that showed deficits in the CA1 and subiculum of depressed patients (38, 43), specifically in the anterior hippocampus (44).
There are a number of potential limitations in the study. The sample size was relatively small. We were unable to distinguish glial subtypes, although glial subtypes have distinct functions and may be altered differentially in depression. Whether animals were exhibiting behavioral depression at the time of necropsy is unknown, thus prohibiting a discussion of state versus trait characteristics. Finally, the delineation between anterior and posterior hippocampus is largely arbitrary. While anterior and posterior regions have different efferent and afferent connectivity, it is likely that the observed differences exist along a gradient rather than representing discrete subregions (45).
The total numbers of neurons that we observed within each hippocampal subregion for nondepressed monkeys are comparable to those previously reported for adult macaques (46). In agreement with postmortem studies of depressed patients (7, 8, 9, 10), we found no strong evidence for neuron loss in the hippocampus of depressed monkeys. There was a trend for fewer granule neurons in the anterior hippocampus, which may reflect reduced DG cytogenesis as observed in the anterior (ventral) hippocampus of rodents under chronic mild stress (47). This could only be verified only with additional studies. Decreased pyramidal soma size was reported in depressed patients (10), and we observed a trend for this effect. We also observed a reduction in the size of the anterior DG granule and CA1 pyramidal cell layers, and reduced neuropil layer volumes within the DG and CA1. Reduced neuron layer volume without reduced neuron number provides further evidence for alterations in the substance between neuronal cell bodies (i.e. neuropil), which may implicate mechanisms relating to water content and synaptic complexity. No differences were observed between groups in the extent of tissue shrinkage during histological processing, a measure suggested to reflect water content (10). Thus, tissue from these same animals is being used to investigate mechanisms related to synaptic complexity. Nevertheless, neuronal alterations reported here were minor in depressed monkeys and likely contributed little to the observed anterior volume reduction.
The most striking outcome of the present study was the 30% reduction of total glia in the anterior CA1 and DG. Growing evidence suggests that alterations in cell populations in depression may be due to inflammatory, oxidative and nitrosative stress (48). This is the first study in which glial numbers were assessed over all layers and not just the principle cell layer within each subregion of the hippocampus in female primates, and in the absence of antidepressant exposure, thus the results are not easily compared to previous studies. For example, increased Nissl-stained glial packing densities within the principle cell layers of the depressed human hippocampus, measured in a few sections per subject, has been reported (10), whereas we observed decreased glial packing density in the CA1/subiculum and proximal CA3 measured over all layers within those subregions. More recently, no differences in neuronal and glial number between depressed patients and controls were reported from a more comprehensive study of mostly men, half of which were taking antidepressants at the time of death (8). While not significant, glia number was, on average, lower in all subregions of the anterior but not posterior hippocampus in that study.
Although glial subtypes were not distinguished in this study, it is known that astrocytes are by far the most abundant glial cell type in the hippocampus. Astrocytes play many critical roles that support neurons, such as regulating energy and metabolism, neurotransmitter modulation, and synaptic plasticity (49). Glial pathologies, including astrocyte alterations, have long been implicated in depression (50). Two studies of human tissue reported decreased GFAP-immunoreactive astrocytes in the hippocampus of depressed patients (7), specifically in the CA1 and CA2 (9). Likewise, in the hippocampus of male tree shrews, psychosocial stress increased apoptosis in non-neuronal cells (51) and reduced GFAP-immunoreactive astrocytes (12). Although this evidence suggests that the change in total glia observed here may reflect a reduction in astrocytes, it is important to note that oligodendrocyte deficits have been reported in other mood-related brain regions in depressed patients, including the amygdala and prefrontal cortex (52). Likewise, white matter deficits have been reported throughout mood disorder neurocircuitry (52). Thus, reduced numbers of oligodendrocytes in mood-related brain regions such as the hippocampus may be contributing to the reported alterations in connectivity.
Dendritic atrophy in the CA3 is commonly reported in male rodents in response to stress (6, 53), however we observed no significant differences between groups in CA3 neuropil volume. Unlike rodent stress models, behavioral depression in adult female monkeys is not induced by the manipulation of stress or administration of glucocorticoids. Although subordinates are socially-stressed, only 60% exhibit depressive behavior (24, 25, 28, 31, 32). The depressed and nondepressed groups in this study were balanced on social status, cortisol levels and HPA-axis function, so that the results obtained would more likely reflect depressive behavior than stress. However, behaviorally depressed animals in this model typically exhibit HPA-axis dysfunction (24, 25, 28, 31, 32). Moreover, insensitivity to glucocorticoid negative feedback in a DST has long been considered a feature of certain subtypes of human depression (54). Although HPA activity was matched between the two groups in this study, this does not necessarily preclude HPA influences on hippocampal morphology. Indeed, glucocorticoid receptor immunoreactivity was recently shown to be increased in the CA1 of depressed women compared to depressed men, even though no differences were observed between depressed patients and controls (55). Future evaluations of glucocorticoid receptors in the CA1 of behaviorally depressed monkeys are warranted to investigate this potential sex-specific sensitivity to stress in primates.
It is important to note that sex differences also exist in animal models of stress and depression. Prolonged stress in male vervet monkeys resulted in neuronal loss in the CA3 compared to controls, but no difference in neuronal number was observed in females under the same conditions (56). In rodent models, estrogen protects chronically stressed female rodents from the dendritic retraction observed in CA3 pyramidal neurons in males (57). In addition, estradiol increased dendritic spines in the CA1 in chronically stressed female rodents (58) and decreased CA1 spines in acutely stressed females (59). The CA1 appears to be more stress-sensitive in female rodents, and the greatest differences between depressed and nondepressed female monkeys in this study were observed in the CA1. Across the whole hippocampus of prenatally-stressed rodents, reduced glial number and a trend for reduced granule neuron number was reported for females and not males (21), further suggesting female-specific sensitivities to stress in the hippocampus. Although matched on ovarian steroids in the present study, behaviorally depressed female monkeys have suppressed ovarian function and are thus estrogen-deficient (24, 25, 28, 31, 32). Additional studies are necessary to determine whether ovarian dysfunction contributes to morphological deficits in the anterior hippocampus of depressed female monkeys.
In conclusion, the data reported here suggest that alterations in glia and neuropil, but not necessarily neurons, in the anterior CA1 and DG may contribute to the hippocampus size differences observed in behaviorally depressed female monkeys compared to their nondepressed counterparts. The study was conducted using carefully matched, antidepressant-naïve, adult female monkeys in which depressive behavior was continually documented over two years, and not induced by the experimental manipulation of stress. The macaque hippocampus closely resembles the human hippocampus in nuclear organization and efferent and afferent connectivity. Future studies in rodents and primates are needed to determine whether the observed reductions in the anterior hippocampus of depressed female monkeys are sex or primate-specific. Sex-specific assessments of hippocampal morphology in depression are critical to illuminate factors that contribute to the increased prevalence of depression in women.
Supplementary Material
Acknowledgments
The authors would like to thank Beth Uberseder for her technical assistance. This research was funded by R21MH086731 (to CAS), and institutional funds from the Wake Forest University Graduate School of Arts and Sciences.
Footnotes
Supplemental information: Supplemental Methods and Materials, Supplemental Table S1, Supplemental Figures S1–S3
FINANCIAL DISCLOSURES
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeroff CB. The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41:189–206. doi: 10.1016/j.jpsychires.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 7.Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb JA, Simpson J, Mahajan GJ, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res. 2012 Nov 29;2012 doi: 10.1016/j.jpsychires.2012.10.020. pii: S0022-3956(12)00343–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 10.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 13.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 15.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 16.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 20.Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 21.Behan AT, van den Hove DL, Mueller L, Jetten MJ, Steinbusch HW, Cotter DR, Prickaerts J. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 2011;21:71–79. doi: 10.1016/j.euroneuro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, et al. 173-Estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience. 2007;146:60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2012;74:528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- 25.Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2012;233:87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. New York: Oxford UP; 2007. pp. 37–114. [Google Scholar]
- 27.Willard SL, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology. 2009;34:1469–1475. doi: 10.1016/j.psyneuen.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 30.Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70:637–645. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shively CA, Laber-Laird K, Anton RF. The behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 32.Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- 33.Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- 34.Shively CA, Mussselman DL, Willard SL. Stress, depression, and coronary artery disease: Modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalin NH, Carnes M. Biological correlates of attachment bond disruption in humans and nonhuman primates. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:459–469. [PubMed] [Google Scholar]
- 36.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 37.Willard SL, Daunais JB, Cline JM, Shively CA. Hippocampal volume in postmenopausal cynomolgus macaques with behavioral depression. Menopause. 2011;18:582–586. doi: 10.1097/gme.0b013e3181fcb47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bearden CE, Thompson PM, Avedissian C, Klunder AD, Nicoletti M, Dierschke N, et al. Altered hippocampal morphology in unmedicated patients with major depressive illness. ASN Neuro. 2009;1(4):art:e00020. doi: 10.1042/AN20090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 40.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz C, Schuster D, Niessen P, Korr H. No difference between estimated mean nuclear volumes of various types of neurons in the mouse brain obtained on either isotropic uniform random sections or conventional frontal or sagittal sections. J Neurosci Methods. 1999;88:71–82. doi: 10.1016/s0165-0270(99)00019-9. [DOI] [PubMed] [Google Scholar]
- 42.West MJ. Design-based stereological methods for counting neurons. Prog Brain Res. 2002;135:43–51. doi: 10.1016/S0079-6123(02)35006-4. [DOI] [PubMed] [Google Scholar]
- 43.Cole J, Toga AW, Hojatkashani C, Thompson P, Costafreda SG, Cleare AJ, Williams SC, Bullmore ET, Scott JL, Mitterschiffthaler MT, Walsh ND, Donaldson C, Mirza M, Marquand A, Nosarti C, McGuffin P, Fu CH. Subregional hippocampal deformations in major depressive disorder. J Affect Disord. 2010;126:272–277. doi: 10.1016/j.jad.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 47.Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 48.Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–59. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 49.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucassen PJ, Fuchs E, Czéh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry. 2004;55:789–796. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011;132:26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 53.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 54.Carroll, Curtis GC, Mendels J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Arch Gen Psychiatry. 1976;33:1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q, Joels M, Swaab DF, Lucassen PJ. Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology. 2012;62:527–533. doi: 10.1016/j.neuropharm.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 58.McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, et al. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.