Introduction
Hepatocellular carcinoma(HCC) is the 6th most common malignancy diagnosed worldwide(1). Late stage presentation, co-morbidities, and limited donor availability enables only 10% of patients to receive curative therapies. Hence, there exists a critical need for novel treatments addressing HCC at all stages. During the last decade, several transarterial locoregional therapies have been developed. One of these, Yttrium-90(Y90) radioembolization, has matured into a recognized treatment option, with a demonstration of a clear palliative role by inducing necrosis and delaying progression(2-8). This overview will describe the biological rationale for Y90, highlight seminal data, propose research questions and discuss the future role of Y90 in HCC.
Biological Rationale
HCC is a tumor that arises almost exclusively in cirrhosis caused by viruses, alcohol or non-alcohol related steatohepatitis, insulin-resistant metabolism, autoimmunity and others. Therefore, survival of HCC patients is related to the tumor and underlying liver condition. In fact, genomic data coded in non-tumoral cirrhotic tissue have shown the ability to determine risk of tumor recurrence following resection/ablation; this explains the tendency of HCC to recur and develop de-novo tumor foci that are usually confined within the liver during the history of the disease(9). The observation that HCC is mostly a liver-limited cancer has allowed the development of a wide range of therapeutic strategies aimed at loco-regional approaches and organ replacement by means of transplantation(10).
The experience gained in recent years indicates that HCC is truly a radiosensitive tumor. External irradiation (electrons, protons, carbon) produces significant tumor responses in patients with HCC(11). Limitations to its clinical applicability are determined by the co-existing intense radiosensitivity of normal liver tissue, precluding the irradiation of large liver volumes with doses >35-40 Gy(12).
Intra-arterial(IA) radiation therapies were developed in an attempt to capitalize on the arterial perfusion of HCC, with the aim of delivering tumoricidal doses to liver tumors irrespective of number, size, and location(sparing normal parenchyma). Radioembolization is a term proposed by a panel of experts to define those procedures in which radioactive microspheres are injected intra-arterially for internal radiation purposes(13). It is the artery in which microspheres are injected that defines the volume of liver tissue exposed to radiation(intravascular brachytherapy). Contrary to transarterial chemoembolization(TACE), in which a combination of drug and ischemia are likely to drive the antitumor effect, Y90 effects are predominantly caused by the radiation effect, with a minor contribution from microembolization(14). Given this mechanism of action, patients with macrovascular invasion may be treated.
The commercially available microspheres include resin(SIR-Spheres®, Sirtex Medical, Australia) or glass(TheraSphere®, Nordion, Canada); both are loaded with yttrium-90(Y90), a pure beta emitter(no isolation/radioprotection). Y90 is a high-energy radiation source with a short half-life(2.67 days) and a short tissue penetration(2.5 mm). Within 2 weeks following injection, >95% of the radiation has been deposited. Glass and resin microspheres differ in several characteristics(specific activity, number of spheres). Despite these differences, clinical outcomes appear equivalent(15).
The biological effects of radiotherapy are mediated by the absorbed dose(energy absorbed/unit mass). With Y90, absorbed dose may be heterogeneous depending on hemodynamics and variable intratumoral vessel density within each liver tumor(16). Despite this heterogeneity, most injected microspheres are preferentially absorbed into the tumor microvasculature in a 3:1 to 20:1 ratio compared with the normal liver, with a preferential deposition in the periphery of nodules (dose >500 Gy)(17, 18). Exposure to radiation then produces irreversible cell damage in tumor epithelial, stromal and endothelial cells that ultimately leads to compromised tumor growth. In mouse models, radiation preferentially damages endothelial cells of the gut microvasculature, suggesting that endothelial cells may represent the principal targets for radiation and that the death of epithelial stem cells may be a secondary event in gastrointestinal toxicity(19). Similarly, it has been proposed that tumor cell death in response to radiotherapy may represent a secondary event after death of endothelial cells(20). High doses to hyper-perfused tissue suggest that vessel damage may be key to antitumoral effect of Y90.
The heterogeneous deposition of microspheres results in variability of dosimetric considerations. In radioembolization, millions of Y90 sources are infused into the arterial vasculature. In order to predict ultimate Y90 deposition, a simulation angiogram is performed 1-2 weeks prior to treatment using 20-100 micron-sized technetium-99m labeled macroaggregated albumin(Tc-MAA) particles. Planar/SPECT gamma-camera imaging are then used to measure hepatopulmonary shunting in order to determine the average radiation dose that will be delivered to tumor/non-tumor areas. There is variability in correlating between Tc-MAA and actual microsphere deposition(Spearman's correlation:0.45-0.82)(21). Furthermore, the resulting estimates reflect the average dose for a certain volume and not the actual dose as calculated for external or interstitial radiotherapy. Historically, activity measured with intra-operative probes did correlate with the actual dose of radiation delivered and with Tc-MAA planar scintigraphy(22). Although the threshold absorbed dose resulting in objective tumor response remains a point of debate and depends on tumor type, vascularity, prior systemic agents and use of radiosensitizers, tumor responses have been reported with doses as low as 40 Gy(23).
These limitations in dosimetry do not impede the clinical use of Y90. Tumor shrinkage occurs almost invariably after Y90 using the current methods for activity calculation(8, 13). Research concepts based on tumor/non-tumor dosimetry methods applied to Tc-MAA planar and/or SPECT imaging have been proposed and await external validation(24, 25). As is well-known with radiotherapy, it may take 3-6 months for the optimal response(size reduction) to manifest; consequently, median time to response is 6.6 months(size) and 1.2 months(necrosis)(3, 8, 26). Progression is often the result of new lesions(intra/extra-hepatic) or within the treated area, since microscopic nests of tumor cells are unlikely to have been affected by Y90 given their lack or arterialization(8). Reported median TTP ranges from 7.9-10.0 months(entire cohort) and from 11.8-15.5 months for patients with absent portal vein invasion(3, 27). However, given unpredictable tumor biology, early progression may be anticipated by baseline tumor characteristics(multinodularity, bilobar disease, vascular invasion, elevated alpha-fetoprotein)(28). Identifying early progressors is important, since the role of systemic agents may be essential in improving long-term outcomes.
The co-existence of HCC and cirrhosis affects Y90 outcomes in a manner similar to other treatments. While vascular changes in the cirrhotic liver(arterioportal/venous shunts) may result in higher chances of technical contraindications, reduced functional reserve (increasing the risk of liver failure) following radiation mandates the adoption of technical methods maximizing parenchymal sparing(29). Imprecise dosimetry models that plague most arterial treatments hinder dose-tolerance analyses. In a 3-dimensional liver model, absorbed dose was higher around the portal area than the central venules, potentially explaining the higher Y90 tolerance compared to external beam irradiation(30). These models assume microspheres are lodged in the distal arterial branches and uniformly scattered throughout the entire liver parenchyma without clustering. In contrast, microspheres can be found in portal and hepatic veins in normal liver and in fibrotic septa of cirrhotic livers, where they may form clusters and distribute heterogeneously. Hence, given these limitations, a precise dose-event relationship in liver tolerance remains elusive. Despite this, there is general agreement to limit parenchymal dose to <50 Gy(7).
Aside from isolated benign changes in liver function, a form of sinusoidal obstruction syndrome appearing 4-8 weeks after Y90 manifest as jaundice, mild ascites and a moderate increase in gamma-glutamyl transpeptidase/alkaline phosphatase has been described in non-cirrhotic patients as radioembolization-induced liver disease(REILD)(31). This syndrome may also appear in 0-33% of cirrhotic patients treated in a whole-liver fashion and in 8-15% of those in which only a partial volume is targeted(32). In the largest series published, grade 3 or higher bilirubin levels were observed within 3 months after therapy in 6-14%(3, 7). Although a causal relationship could only be confirmed in controlled clinical trials, it is very likely that the increased bilirubin levels reflect some kind of REILD. This is further supported by the fact that increased bilirubin is not associated with changes in synthetic liver function(decreased albumin, prothrombin activity)(7). Nonetheless, these findings underscore the acceptable safety profile of Y90 in HCC.
Furthermore, other more comprehensive definitions of liver decompensation in patients receiving Y90 may be considered. For instance, extending the recording of adverse events as potentially-related to 6 months will provide a conservative estimate(33). Although such extended definition of toxicity may inform prospective clinical trials on Y90, it also gives rise to overestimations of REILD, as deterioration of liver function and performance status may occur in intermediate-advanced HCC, independently from any anti-cancer treatment.
Review of seminal data and comparative effectiveness
Over that last 10 years, there has been significant scientific advancement in the field of Y90. Standardization of practice and assessment of indications has transformed radioembolization from a procedure relying on local expertise to a routine procedure yielding predictable results in properly trained centers. Early series were limited by sample size, with a 43 and 24-patient series describing outcomes in small cohorts(6, 8, 28). Since then, 7 well-controlled investigations establishing the safety and anti-tumoral effect of Y90 have been published; these will be presented temporally(Table 1).
Table 1. Summary of large series reporting on long-term outcome after Y90-radioembolization.
| Intermediate Stage | Branch PVT | Main PVT | Branch or Main PVT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Child | N | OS (95%CI) | N | OS (95%CI) | N | OS (95%CI) | N | OS (95%CI) |
| Hilgard 2010 (N=108) | A/B | 51 | 16.4 (12.1-n.c.) | 33 | 10 (6-n.c.) | ||||
| Salem 2010 (N=291) | A B |
48 35 |
17.3 (13.7-32.5) 13.5 (6.4-25.4) |
19 | 16.6 (8.8-24) | 16 | 7.7 (3.3-13.2) | 35 57 |
10.4 (7.2-16.6) 5.6 (4.5-6.7) |
| 27 | 6.5 (5-8.5) | 30 | 4.5 (2.9-6.6) | ||||||
| Sangro 2011 (N=325) * | A B |
82 5 |
18.4 (13.6-23.2) 3.6 (2.4-10.8) |
44 | 10.7 (8.3-17.1) | 32 | 9.7 (4.8-11.8) | 76 | 10.2 (7.7-11.8) |
| Mazzaferro 2012 (N=51) | A B |
15 2 |
18(13-38) -- |
23 6 |
17(13-21) 8(5-10) |
5 1 |
9 (4-n.c.) 5 |
||
OS: overall survival (months). n.c. not calculable.
Unpublished data for branch and main PVT cohorts provided by authors.
One of the common indications for Y90 that has emerged is HCC with portal venous thrombosis(PVT). Since Y90 is a microembolic procedure causing minimal occlusion of hepatic arteries, it may be safely used in the setting of PVT(34). This is a relevant clinical scenario, since PVT significantly increases the chances of extrahepatic spread(9). Given this interest, the first large series analysis was a phase 2 study by Kulik, analyzing Y90 in 108 HCC patients with (34%) and without PVT (66%). Partial response rate of 42.2%(size) and 70%(necrosis) were reported(34). Survival varied by location of PVT and presence of cirrhosis. This study was important given its multi-center nature, challenging preconceived notions that embolotherapy could not be applied in the setting of PVT(ischemic hepatitis). Since Y90 is microembolic, this study re-introduced the idea of embolotherapy in the context of vascular invasion(14). Recently, mature long-term outcomes for PVT patients treated with Y90 in the Sorafenib era were updated(35). It is unknown if treating patients with PVT has any effect on metastatic dissemination, regardless of the response in the tumor thrombus.
In 2010, a detailed review of the pathologic findings following Y90 treatment was presented by Riaz in patients bridged/downstaged to transplantation(26). The intent was to examine the anti-tumoral effect of Y90, a pathological proof of concept. This analysis demonstrated very high rate (89%) of complete pathologic necrosis(CPN) in smaller lesions(1-3 cm), and a promising rate of CPN in larger lesions(65%;3-5 cm)(independent pathology review). These data were compared to the CPN achieved in an identical pathology review of HCC following conventional TACE(36), confirming that Y90 could achieve better antitumoral effect(pathology) when compared with the standard of care(TACE), there by introducing a new tool to the armamentarium of downstaging strategies.
In 2010, the seminal experience from Northwestern University confirmed the positive outcomes of 291 patients with HCC treated with Y90. This was a prospective, 5-year cohort study not only investigating long-term outcomes, but also presented imaging follow-up, response rate(size and necrosis), TTP and survival stratified by Child-Pugh, United Network for Organ Sharing(UNOS) and Barcelona Clinic Liver Cancer(BCLC). Child-Pugh A(with/without PVT) and Child-Pugh B (without PVT) potentially benefitted from treatment. TTP was longer for Child-Pugh A and B without PVT(15.5 and 13 months, respectively) when compared with those with PVT(5.6, 5.9 months, respectively). As expected, survival was negatively affected by liver function(Child-Pugh A:17.2 months, Child-Pugh B:7.7 months; P =.002). TTP and overall survival varied by patient stage(3). Most importantly, this study was the first to outline, in a structured manner, expected response rate, TTP and survival by Child-Pugh, UNOS and BCLC. This granularity of detail in phase 2 has permitted hypothesis generation and statistical powering of Y90 studies.
In the last few years, European studies have also confirmed the safety/efficacy of Y90. Hilgard et al analyzed Y90 in 108 consecutive patients with advanced HCC(27). They observed complete and partial response by necrosis criteria in 3% and 37%, respectively, with stable disease in 53%. TTP was 10.0 months, with overall survival of 16.4 months. This was the first study validating the technical reproducibility of outcomes when compared to the 291-patient cohort. Also, clinical outcomes were similar, suggesting the consistent outcomes less dependent on local expertise as previously considered. Finally, these findings provided a more compelling case for randomized controlled trials with or without systemic agents in advanced HCC(37).
The largest study of Y90 in HCC was published by Sangro et al in 2011(7). This was a multi-center, retrospective cohort review of 325 patients. Median overall survival was 12.8 months(BCLC A:24.4 months, BCLC B:16.9 months, BCLC C:10.0 months). Independent prognostic factors on multivariate analysis included performance status, tumor burden, international normalized ratio >1.2 and extrahepatic disease. Important observations were gained from this study. Despite its retrospective nature, this was the first study with a significant number of participating groups with reproducible data between centers(>8), validating multicenter feasibility in technically involved procedures. Also, data were very comparable to glass microspheres, confirming that radiation appears to be the dominant mechanism of action. Finally, outcomes data were displayed stratified by BCLC, critical for the design of clinical trials using this staging strategy(38, 39).
BCLC guidelines suggest that TACE is the standard of care for patients with intermediate disease. While this is universally recognized by clinicians caring for the HCC patient, investigators have challenged this notion, identifying possible subgroups within intermediate stage and suggesting a role for Y90 in the same setting(Bolondi et al, Seminar Liver Disease 2013 in press). Given the difficulties in performing randomized TACE vs Y90 studies, a large comparative effectiveness study was published in 2011(2). This compared 122 TACE and 123 Y90 patients(toxicity, response, TTP, survival). The groups were well-balanced by Child-Pugh, UNOS and BCLC, with only older age in the Y90 cohort (P <0.001). Findings included fewer transaminase elevations, a strong trend for better response(Y90:49%, TACE:36%, P=0.052) and longer TTP with Y90 (Y90:13.3 months, TACE:8.4 months, P=0.046). However, no survival difference could be identified(Y90:20.5 mo, TACE:17.4 mo, P=0.232). Several important conclusions were drawn from this analysis. First, although there was no survival difference, radioembolization(outpatient procedure) was able to provide better disease control(longer TTP) with less toxicity than TACE(inpatient procedure). Second, although TTP has been suggested as a potential surrogate of survival, this study did not seem to provide compelling evidence in support of this contention. Finally, given similarity of long-term survival outcomes, the findings brought into question the feasibility of a head-to-head comparative study between Y90 and TACE, requiring 1000 patient sample in order to demonstrate equivalence. Given the advent of Sorafenib as the standard of care for patients progressing beyond intermediate disease, the feasibility of a statistically pure head-to-head(without crossover) comparison appears unlikely(38). Hence, most investigators have begun to recognize Y90 for more advanced BCLC B/early BCLC C disease, since the secondary benefits of Y90 including clinical toxicities, quality-of-life, days hospitalized and cost-effectiveness have been explored through feasibility studies.
Most recently in 2012, the Milan-INT group presented the first, prospective phase 2 study powered to investigate Y90 in 52 patients with intermediate/advanced HCC(33). Findings included a TTP of 11 months and survival of 15 months. Some patients were downstaged to resection despite advanced stage. Furthermore, survival of PVT patients did not differ from intermediate(non-PVT) patients. This study further validated the reproducibility of Y90 under controlled investigations and reconfirmed survival outcomes in patients with well-preserved liver function and vascular invasion. Given the apparent failure of the TACE+Sorafenib combination(SPACE study abstract, press release), the recent interest in combining Y90 with Sorafenib has been reconsidered and subsequently catalyzed the development of head-to-head/combination studies with Sorafenib in patients with PVT.
Y90 Level of Evidence and Ongoing Controversies
There continues to be growing clinical interest in Y90 as a treatment modality for HCC. However, one of the ongoing controversies has been challenging the level of evidence with Y90(no RCTs) and a thorough discussion of what would be required for Y90 incorporation into treatment guidelines. While the European Society of Medical Oncology and the National Comprehensive Cancer Networks have recognized Y90 as a treatment option, the American and European Associations for the Study of the Liver have not(40-42). A recent discussion at the International Liver Cancer Association meeting in Berlin highlighted the challenges that have been encountered over several years when attempting to raise the level of evidence with Y90 to achieve the level necessary for hepatology-oriented guidelines. These are summarized herein.
It is acknowledged that for new therapies(including Y90) to become widely accepted, controlled research investigations are necessary. Other important factors include reproducibility and multicenter implementation. Furthermore, the economic feasibility of new approaches is important as is the proper framing of previously collected experiences. Given this background, historical details of Y90 should be provided.
Over one decade ago(1999), the Food and Drug Administration(FDA) approved, under a humanitarian basis, the use of implantable, radioactive microspheres for patients with unresectable HCC. To put this in context, this was prior to the publication and adoption of BCLC guidelines, the completion of the seminal studies establishing that TACE provided a survival benefit(2002), and the approval of Sorafenib(2008)(37, 43, 44). This regulatory mechanism was necessary since at the time, there were no approved agents for HCC(no comparator). In 2002, European approval for Y90 in liver neoplasia was also obtained. However, despite regulatory approvals, it was recognized that more controlled randomized studies would be necessary to gain worldwide acceptance. Hence, in 2006, an international, randomized phase 3 trial comparing Y90 with best supportive care in advanced HCC was initiated. During the protocol review and site selection phase, the positive findings of Sorafenib study were announced. The HCC landscape changed, with Sorafenib becoming the standard of care in advanced disease(37). The study was subsequently put on hold. However, given the compelling phase 2 evidence and safety profile in patients with PVT, the FDA expanded the label for Y90(2006) to include PVT(34). Therefore, in the strictest sense, the agent first approved for the treatment of advanced HCC(PVT) was Y90. This came 40 years after the first attempts in HCC using the same isotope(45). Despite these setbacks stemming from the ever-increasing complexity and dynamic research landscape of HCC, the evidence for Y90 has continued to grow.
Besides resulting in similar(if not better) survival in this population, Y90 is devoid of the significant side effects of Sorafenib. These toxicities lead to treatment discontinuation(44%) and dose reduction/withdrawal(64%) in post-marketing studies, denying patients the well-established benefit of Sorafenib(37, 46). Moreover, in the subset analysis of the pivotal phase III trial, the median overall survival among 108 patients with PVT receiving Sorafenib was 8.1 months and disease control rate(DCR) was 26.8%, while for patients with Child-Pugh A and PVT treated by Y90(Table 1), median overall survival ranges between 10-17 months with DCRs of 40-80%(47). Given this historical background, the limitations of comparing studies, as well as the need for higher level of evidence, several international, randomized, phase 3 studies have now been implemented(Table 2). SORAMIC and STOP-HCC both investigate Y90 when added to Sorafenib. SIRveNIB, SARAH and YES-p all compare Sorafenib to Y90. These trials further confirm the strong phase 2 signals resulting in advancement to phase 3 trials.
Table 2. Ongoing large scale randomized clinical trials with Y90-radioembolization in hepatocellular carcinoma.
| Acronym | NCT ID | Phase | Countries | N | Endpoint | Experimental Arm | Comparator | Estimated Completion Date | Status |
|---|---|---|---|---|---|---|---|---|---|
| PREMIERE | 00956930 | II | USA | 124 | TTP | Y90 | cTACE | Aug 2018 | Recruiting |
| SIRveNIB | 01135056 | III | Asia-Pacific | 360 | Survival | Y90 | Sorafenib | Jul 2015 | Recruiting |
| SARAH | 01482442 | III | France | 400 | Survival | Y90 | Sorafenib | Mar 2015 | Recruiting |
| STOP | 01556490 | III | USA-Europe | 400 | Survival | Y90 + Sorafenib | Sorafenib | Oct 2016 | Recruiting |
| SORAMIC | 01126645 | III | Europe | 375 | Survival | Y90 + Sorafenib | Sorafenib | Feb 2014 | Recruiting |
| YES-p | pending | III | Europe, Asia, USA | 328 | Survival | Y90 | Sorafenib | - | Initiated |
Clinical trials in BCLC B disease are more challenging given the long natural history of untreated disease, large sample sizes required to demonstrate survival differences, as well as the crossover that invariably occurs at progression(2). In fact, some have suggested that survival is not an appropriate endpoint when effective subsequent therapies exist(48). Difficulties with survival studies are further highlighted with the extremely long survival time(median:48 months) noted in hyperselected intermediate patients are treated with TACE(49). These observations further suggest BCLC B is a heterogeneous group that, with such prolonged survival times in select groups, limits the feasibility of randomized studies (TACE vs Y90). This heterogeneity was recently highlighted by an expert review panel(50). Despite this, PREMIERE is a randomized phase 2 trial comparing TACE/Y90 in intermediate disease(Table 2). Furthermore, through the use of clinical/molecular factors, comparable subgroups within heterogeneous intermediate stage will be studied in prospective RCTs using Y90 as the experimental arm. These will target tumor presentations in which amelioration of TACE results have already inferred, such as Child-Pugh B7, candidacy for transplantation after downstaging(up-to-7 concept, expanded UCSF) and preserved performance status(51-53).
Clinical paradigm shift and novel applications with Y90
One of the pervasive observations with Y90 is that as an embolotherapy, it represents a major paradigm shift when compared with TACE. TACE often involves patient preparation with antibiotics, anti-emetics and narcotics. The patient is admitted for a period ranging from 1-5 days for post-embolization syndrome resulting from chemotherapy/arterial occlusive effects. Patients usually require 1-2 weeks for overall recovery depending on their baseline performance status. In contradistinction, while Y90 requires a planning angiogram to identify and delineate the vascular anatomy, Y90 treatment also involves same day discharge(23 hours in Europe), often without the need for antibiotics or pain management. Hence, for 2 therapies(TACE, Y90) that intuitively target the same population(intermediate disease), differences in technical/side-effect/out-patient profiles create challenges in patient enrollment during the informed consent process. These challenges were confirmed in a prospective phase 2 study comparing TACE/Y90 using quality-of-life metrics. The study demonstrated that despite enrolling more advanced patients(larger tumors, performance status 1-2) to Y90, Y90 outperformed TACE(small tumors, segmental injections)(54) by quality-of-life measures.
As clinical experience has been gained with this technology, several investigators have consistently made novel observations with Y90. Although these have not been tested in the multi-center setting, they are of clinical interest and worthy of brief description in this review article. The first novel concept relates to surgical intervention for HCC and is termed “radiation segmentectomy”, in reference to the ability of applying radiation doses to small sectors of liver tissue 1000× greater than achieved using external beam(18). Using this idea, small sectors of tumor-bearing liver, usually considered for ablation/resection but contraindicated because of location/co-morbidities/insufficient liver reserve, can be obliterated using Y90. The sector of liver resorbs with time and disappears on cross-sectional imaging(“segmentectomy”).
Expanding on segmentectomy, the second concept is termed “radiation lobectomy”, observed in patients with right lobe disease potentially amenable to curative resection but excluded because of small future liver remnant(55). Although the traditional method of inducing hypertrophy is portal vein embolization(PVE), hypertrophy rates are suboptimal in cirrhosis. In this patient population, treating the right lobe disease with Y90(as opposed to PVE) potentially accomplishes 3 important clinical tasks: 1) the tumor is treated while hypertrophy is being induced(PVE does not treat the HCC), 2) as the right lobe HCC regresses with concomitant right lobar atrophy, a more controlled diversion of portal venous flow ensues, with hypertrophy rates of over 40% in radiation-naïve future left lobe remnants and, 3) waiting 6-12 weeks for lobectomy to be manifest mandates a biologic test of time, identifying those patients that would best be served by resection(56, 57). Although the predictability and extent of segmental-lobar atrophy induced by Y90 is in need of adjustment, it is a fact that Y90 may combine anti-cancer and ablative effects on the target liver territory. That will add efficacy endpoints to be tested in patients rescued to surgical approaches not only in HCC but also in selected cases of liver metastases(Figure 1).
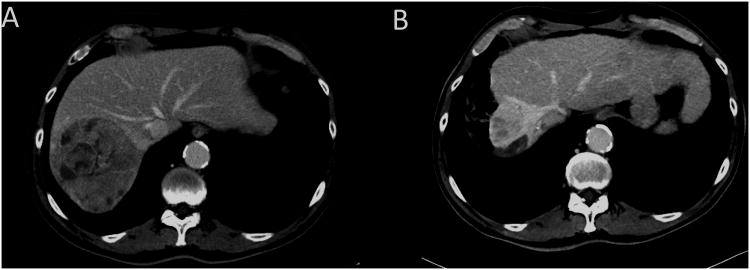
Figure 1. Right lobe radiohepatectomy demonstrating simultaneous Y90 treatment to the HCC, lobar atrophy and regeneration/hypertrophy of the remnant liver after one single session of Y90.
This is a 70 year old male with HCV-related cirrhosis and good liver function (Child-Pugh A6, MELD 8) who presented with 2 right lobe HCCs (11 cm, 3 cm) associated with segmental portal vein invasion and AFP serum level of 3412 UI/mL.
A) Baseline CT Scan. The right hepatic lobe volume (segments 5-6-7-8) was 1325 cm3 while the remnant left lobe (segments 1-2-3-4) had a calculated volume of 434 cm3.
B) Six months after Y90 there is no evidence of active tumor, AFP is 14.3 UI/mL and near complete atrophy of the right lobe. The right lobe volume is now 152 cm3 while the left has hypertrophied to 1202 cm3. The patient was considered for resection but eventually maintained on strict imaging follow-up. He is recurrence-free 2 years after Y-90 radioembolization.
The third novel application involves the controversial(but routinely practiced) downstaging to liver transplantation. To date, 2 studies have demonstrated the ability of Y90 to downstage patients from UNOS T3 to T2(10). The first 35-patient series demonstrated a 56% downstaging rate(58). The second, a comparative effectiveness study in T3 patients, demonstrated better downstaging of Y90 when compared with TACE(58% vs 31%, P <0.05)(4). This is largely explained by the high anti-tumoral effect of Y90(necrosis/size criteria). In another comparative effectiveness analysis, a strong trend of improved response rate when compared with TACE was reported(Y90:49%, TACE:36%, P=0.052)(2). High response rates by necrosis/size criteria have consistently been reported, suggesting that Y90 represents another potential tool for downstaging(3, 7, 27, 33, 57)(Figure 2).
Figure 2. Salvage transplantation after Y90 downstaging.

This is a 58 y.o. patient with good performance status, Child B7 cirrhosis who received Y90 for a large infiltrating HCC with satellites and non-neoplastic portal vein thrombosis (i.e. beyond the up-to-7 and UCSF criteria, BCLC C). After 3 months, while there was near total tumor response with post-radiation right liver lobe atrophy and dense fibrosis, progressive deterioration of liver function occurred. The patient underwent liver transplantation because of tumor-unrelated Child-stage migration. The patient alive and well (no recurrence) 2 years after transplantation (30 months after Y90).
Finally, Y90 could represent an option to maintain select intermediate-advanced tumors within transplant possibility(bridging) when sustained tumor response exceeding 6 months has been observed, supported by “up-to-7” and UCSF expanded criteria. These options become feasible and transplant exceptions considered in light of competitive benefit with respect to more conventional indications for transplantation(52, 53)(Figure 2).
It is often stated that from a research perspective, Y90 is a technique that inherently competes with TACE in BCLC B since both are trans-arterial and involve the delivery of particulate “embolic” agents. However, this is not universally agreed upon by HCC experts. Rather, Y90 versatility translates into a potential role in many BCLC stages(59). Y90 in BCLC A is suggested in part by higher CPN compared to TACE and by the innovative concepts of segmentectomy/lobectomy(permitting resection) and downstaging(permitting transplantation)(18, 56, 57). For BCLC B, comparative studies are also complex, since inherent QOL differences, long natural history, as well as complications of crossover at progression, result in unachievable 1000-patient trial designs(2, 48, 54). Finally, in BCLC C, the dramatic effect on PVT(not seen with TACE) provides strong rationale for [combinations with/comparisons] to Sorafenib(33, 34, 60). Table 3 lists Y90 indications/contra-indications that are generally recommended by expert consensus.
Table 3.
| Indications |
|---|
| Conventional (based on retrospective and prospective cohort studies and case-control studies) |
| - Intermediate HCC: |
|
| - Advanced HCC: |
|
| Investigational (based on case-reports or single center uncontrolled studies) |
|
| Contra-indications |
| Relative |
|
| Absolute |
|
Conclusion
Radioembolization represents a promising treatment option challenging current paradigm of HCC treatment. Multiple centers have provided compelling data that suggest: a) high anti-tumoral effect, b) downstaging to transplantation, c) clinical application in PVT, d) TACE survival equivalence using an outpatient schedule/improved quality-of-life, e) conversion of surgically inoperable patients(small liver remnant) to potential cure with resection. Although clinical development has been challenging, the next few years will yield important information as results from the randomized phase 3 trials further define the role of Y90 in treatment algorithms.
Acknowledgments
Role of Funding: There was no funding provided for this study. RS is funded in part by NIH grant CA126809. VM is funded by the Italian Association for Cancer Research (AIRC).
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CI
95% confidence interval
- DCR
disease control rate
- EASL
European Association for the Study of the Liver
- HCC
Hepatocellular Carcinoma
- IA
Intra-arterial
- MRI
Magnetic Resonance Imaging
- PREMIERE
Prospective randomized trial of radioembolization and chemoembolization in hepatocellular carcinoma
- PVT
Portal venous thrombosis
- SARAH
Sorafenib Versus radioembolization in Advanced Hepatocellular Carcinoma
- SHARP
Sorafenib HCC Assessment Randomized Protocol Trial
- SIRveNIB
Phase III Multicenter Open-label Randomized Trial of Selective Internal Radiation Therapy versus Sorafenib in Locally Advanced Hepatocellular Carcinoma
- SORAMIC
Evaluation of Sorafenib in combination with local micro-therapy guided by Gd-EOB-DTPA enhanced MRI in patients with inoperable hepatocellular carcinoma
- STOP-HCC
Phase III Clinical Trial of Intra-arterial TheraSphere in the Treatment of Patients with Unresectable Hepatocellular Carcinoma
- TACE
Trans-arterial chemoembolization
- TTP
time-to-progression
- UNOS
United Network for Organ Sharing
- 90Y
Yttrium-90 radioembolization
- YES-p
a prospective randomized clinical trial of 90Y radioembolization vs Sorafenib for the treatment of advanced HCC with portal vein thrombosis
Footnotes
Conflict of Interest: RS and VM are advisors to Nordion. RS and BS are advisors to Sirtex Medical.
References
- 1.Parkin DM, B F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2011;140:497–507 e492. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 5.Riaz A, Miller FH, Kulik LM, Nikolaidis P, Yaghmai V, Lewandowski RJ, Mulcahy MF, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062–1069. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, Sergie Z, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 7.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 8.Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, Panizo A, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792–800. doi: 10.1016/j.ijrobp.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 9.Senthilnathan S, Memon K, Lewandowski RJ, Kulik L, Mulcahy MF, Riaz A, Miller FH, et al. Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: analyzing patterns of progression in 285 patients. Hepatology. 2012;55:1432–1442. doi: 10.1002/hep.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25:664–671. doi: 10.1111/j.1440-1746.2009.06126.x. [DOI] [PubMed] [Google Scholar]
- 12.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, Soulen MC, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Lewandowski RJ, Bui JT, Omary R, Hunter RD, Kulik L, Mulcahy M, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–529. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 15.Sangro B, Salem R, Kennedy A, Coldwell D, Wasan H. Radioembolization for hepatocellular carcinoma: a review of the evidence and treatment recommendations. Am J Clin Oncol. 2011;34:422–431. doi: 10.1097/COC.0b013e3181df0a50. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy AS, Kleinstreuer C, Basciano CA, Dezarn WA. Computer modeling of yttrium-90-microsphere transport in the hepatic arterial tree to improve clinical outcomes. Int J Radiat Oncol Biol Phys. 2010;76:631–637. doi: 10.1016/j.ijrobp.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–171. doi: 10.1016/j.ijrobp.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 19.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, Camphausen K. Cancer. What does radiotherapy do to endothelial cells? Science. 2001;293:227–228. doi: 10.1126/science.1062892. [DOI] [PubMed] [Google Scholar]
- 21.Knesaurek K, Machac J, Muzinic M, DaCosta M, Zhang Z, Heiba S. Quantitative comparison of yttrium-90 (90Y)-microspheres and technetium-99m (99mTc)-macroaggregated albumin SPECT images for planning 90Y therapy of liver cancer. Technol Cancer Res Treat. 2010;9:253–262. doi: 10.1177/153303461000900304. [DOI] [PubMed] [Google Scholar]
- 22.Lau WY, Leung TW, Ho S, Chan M, Leung NW, Lin J, Metreweli C, et al. Diagnostic pharmaco-scintigraphy with hepatic intra-arterial technetium-99m macroaggregated albumin in the determination of tumour to non-tumour uptake ratio in hepatocellular carcinoma. Br J Radiol. 1994;67:136–139. doi: 10.1259/0007-1285-67-794-136. [DOI] [PubMed] [Google Scholar]
- 23.Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TW, Liu CS, et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82:401–407. doi: 10.1016/j.ijrobp.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Garin E, Lenoir L, Rolland Y, Edeline J, Mesbah H, Laffont S, Poree P, et al. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53:255–263. doi: 10.2967/jnumed.111.094235. [DOI] [PubMed] [Google Scholar]
- 25.Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, Sposito C, et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: the experience of the National Tumor Institute of Milan. Q J Nucl Med Mol Imaging. 2011;55:168–197. [PubMed] [Google Scholar]
- 26.Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, Abecassis M, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49:1185–1193. doi: 10.1002/hep.22747. [DOI] [PubMed] [Google Scholar]
- 27.Hilgard P, Hamami M, Fouly AE, Scherag A, Muller S, Ertle J, Heusner T, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 28.Inarrairaegui M, Martinez-Cuesta A, Rodriguez M, Bilbao JI, Arbizu J, Benito A, Alegre F, et al. Analysis of Prognostic Factors After Yttrium-90 Radioembolization of Advanced Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Furuse J, Ishii H, Nagase M, Kawashima M, Ogino T, Yoshino M. Adverse hepatic events caused by radiotherapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2005;20:1512–1518. doi: 10.1111/j.1440-1746.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 30.Gulec SA, Sztejnberg ML, Siegel JA, Jevremovic T, Stabin M. Hepatic structural dosimetry in (90)Y microsphere treatment: a Monte Carlo modeling approach based on lobular microanatomy. J Nucl Med. 2010;51:301–310. doi: 10.2967/jnumed.109.069278. [DOI] [PubMed] [Google Scholar]
- 31.Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, Chopitea A, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 32.Gil-Alzugaray B, Chopitea A, Inarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, Benito A, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2012 doi: 10.1002/hep.26191. [DOI] [PubMed] [Google Scholar]
- 33.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, et al. Yttrium(90) radioembolization for intermediate-advanced hepatocarcinoma: A phase II study. Hepatology. 2012 doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 34.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 35.Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, Riaz A, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: Impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riaz A, Lewandowski RJ, Kulik L, Ryu RK, Mulcahy MF, Baker T, Gates V, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with chemoembolization. Cardiovasc Intervent Radiol. 2010;33:1143–1152. doi: 10.1007/s00270-009-9766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 38.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 39.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 44.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RTP, Fan ST, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 45.Ariel IM. Treatment of Inoperable Primary Pancreatic and Liver Cancer by the Intra-Arterial Administration of Radioactive Isotopes (Y90 Radiating Microspheres) Ann Surg. 1965;162:267–278. doi: 10.1097/00000658-196508000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, Ferrari D, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol. 2012 doi: 10.1093/annonc/mds343. [DOI] [PubMed] [Google Scholar]
- 47.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Buyse M, Sargent DJ, Saad ED. Survival is not a good outcome for randomized trials with effective subsequent therapies. J Clin Oncol. 2011;29:4719–4720. doi: 10.1200/JCO.2011.38.4206. author reply 4720-4711. [DOI] [PubMed] [Google Scholar]
- 49.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, et al. Heterogeneity of Patients with Intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a Subclassification to Facilitate Treatment Decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 51.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 53.Yao FY, Hirose R, LaBerge JM, Davern TJ, 3rd, Bass NM, Kerlan RK, Jr, Merriman R, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 54.Gilbertsen P, Coffey S, Gonda E, Karp J, Marshall K, Memon K, Riaz A, et al. Abstract No 182: Quality of life assessment of patients treated with Yttrium-90 or transarterial chemoembolization: A comparative study using the fact-hep. Journal of vascular and interventional radiology: JVIR. 2011;22:S79. [Google Scholar]
- 55.Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–790. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- 56.Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, Ryu RK, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 57.Inarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D'Avola D, Herrero JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38:594–601. doi: 10.1016/j.ejso.2012.02.189. [DOI] [PubMed] [Google Scholar]
- 58.Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, Hunter RD, et al. Yttrium-90 microspheres (TheraSphere(R)) treatment of unresectable hepatocellular carcinoma: Downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 59.D'Avola D, Iñarrairaegui M, Pardo F, Rotellar F, Marti P, Bilbao J, Martinez-Cuesta A, et al. Prognosis of Hepatocellular Carcinoma in Relation to Treatment Across BCLC Stages. Annals of Surgical Oncology. 2011;18:1964–1971. doi: 10.1245/s10434-011-1551-4. [DOI] [PubMed] [Google Scholar]
- 60.Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz A, Ryu RK, Baker TB, Ibrahim SM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]