Abstract
Cholangiocarcinoma (CCA) is characterized by an abundant stromal reaction. Cancer-associated fibroblasts (CAF) are pivotal players in tumor growth and invasiveness and represent a potential therapeutic target. To understand the mechanisms leading to CAF recruitment in CCA, we studied: 1) the expression of epithelial-mesenchymal transition (EMT) in surgical CCA specimens and CCA cells; 2) the lineage tracking of an EGFP-expressing human male CCA cell line (EGI-1) after xenotransplantation into severe-combined-immunodeficient mice; 3) the expression of platelet-derived growth factors (PDGFs) and their receptors in vivo and in vitro; 4) the secretion of PDGFs by CCA cells; 5) the role of PDGF-D in fibroblast recruitment in vitro; 6) the downstream effectors of PDGF-D signaling. CCA cells expressed several EMT biomarkers but not α-SMA. Xenotransplanted CCA masses were surrounded and infiltrated by α-SMA-expressing CAF, which were negative for EGFP and the human Y-probe, but positive for the murine Y-probe. CCA cells were strongly immunoreactive for PDGF-A and -D, whilst CAF expressed PDGFRβ. PDGF-D, a PDGFRβ agonist, was exclusively secreted by cultured CCA cells. Fibroblast migration was potently induced by PDGF-D and CCA conditioned medium, and was significantly inhibited by PDGFRβ blockade with Imatinib and by silencing PDGF-D expression in CCA cells. In fibroblasts, PDGF-D activated the Rac1 and Cdc42 Rho GTPases and JNK. Selective inhibition of Rho GTPases (particularly Rac1) and of JNK strongly reduced PDGF-D-induced fibroblast migration.
Conclusion
CCA cells express several mesenchymal markers, but do not transdifferentiate into CAF. Instead, CCA cells recruit CAF by secreting PDGF-D, which stimulates fibroblast migration via PDGFRβ and Rho GTPase and JNK activation. Targeting tumor/stroma interactions with inhibitors of PDGF-D pathway may offer a novel therapeutic approach.
Keywords: tumor reactive stroma, epithelial/mesenchymal cross-talk, epithelial to mesenchymal transition, Rho GTPases, PDGFRβ
An extensive desmoplastic reaction is a distinctive feature of cholangiocarcinoma (CCA), a highly aggressive cancer originating from the biliary epithelium, characterized by strong invasiveness with limited opportunities of curative treatment(1). The “tumor reactive stroma” is the site of complex functional interactions between cancer cells and the host microenvironment, and it plays a pivotal role in tumor growth and invasiveness(2).
Cancer-associated fibroblasts (CAF) provide tumor cells with proliferative and anti-apoptotic signals that ultimately promote cancer growth. On one hand, cancer cells produce a range of signals able to instruct the stromal microenvironment to become permissive and supportive for tumor progression(3). On the other hand, CAF communicate with other cell types (endothelial cells, pericytes and inflammatory cells) inducing angiogenesis and remodeling of the extracellular matrix(3) ultimately favoring tumor invasiveness. In CCA, overexpression of pro-inflammmatory cytokines in the tumor stroma is associated with a more malignant tumor phenotype(4). Paracrine signals from CAF protect CCA cells from pro-apoptotic stimuli(5).
The origin of CAF is still uncertain(6). It has been proposed that CAF undergo an epithelial to mesenchymal transition (EMT) of carcinoma cells, during which cancer cells lose their epithelial properties and acquire a mesenchymal phenotype that consequently favors increased invasive and migratory capabilities. Alternatively, CAF may be recruited by cancer cells from resident fibroblasts(6) or from circulating mesenchymal progenitor cells of bone marrow origin(7).
Members of the platelet-derived growth factor (PDGF) family are of interest because of their ability to promote fibroblast and hepatic stellate cell (HSC) migration and proliferation. Furthermore, PDGF expression has been shown to correlate with cancer progression in colon carcinoma, and to protect CCA cells from apoptosis(5,7). The PDGF family encompasses five dimeric ligand isoforms, PDGF-AA, -BB, -AB, -CC, and –DD, which signal through two structurally related tyrosine kinase receptors, PDGFRα and PDGFRβ. While PDGFRα binds all PDGF isoforms except for PDGF-DD, the PDGFRβ has a preferential and high affinity for PDGF-BB and PDGF–DD. The possible role of PDGF-D in tumor development and progression is only starting to be recognized(8).
To better understand the mechanisms underlying the formation of tumor reactive stroma in CCA, we investigated whether CAF are generated from cancer cells or are recruited by cancer cells via a PDGF-dependent mechanism. Specifically, this study sought: a) the expression of several EMT markers in human CCA specimens and cells; b) the fate of human CCA cells xenotransplanted in severe combined immunodeficient (SCID) mice after transfection with enhanced green fluorescent protein (EGFP); c) the expression of PDGF ligands and receptors in CCA specimens and cells; d) the ability of cultured human CCA cells to secrete PDGF isoforms; e) the role of PDGF-D in tumor epithelial/mesenchymal cross-talk in vitro, and f) the intracellular signaling pathways involved.
MATERIALS AND METHODS
Tissue samples and cells
Fifteen formalin-fixed, paraffin-embedded samples of surgically resected CCA livers, obtained from archival tissue at Bergamo Hospital were considered for the immunohistochemical study (10 Intrahepatic and 5 Extrahepatic). For each patient, the matched peri-tumoral sample was available. We also studied three human CCA cell lines: EGI-1, TFK-1 (Deutsche Sammlung von Mikroorganismen und Zellkulturen, DSMZ, Germany), and HuCCT-1 (Health Science Research Resource Bank, HSRRB, Japan), as well as three primary CCA cell lines isolated from human liver samples derived from surgical resections for intrahepatic CCA at Treviso Regional Hospital (CCA1, CCA2, CCA3), as described(9). Human cholangiocytes isolated from liver explants with alcoholic cirrhosis served as controls (n=3). See supplemental method for further details on human fibroblast isolation. All specimens were reviewed to confirm the histopathological diagnosis of CCA. Informed consent and local regional ethical committee approval were obtained before tissue collection and cell preparations.
Immunophenotyping for EMT markers and PDGF family members of CCA specimens and cells
Expression of a panel of phenotypic EMT markers, including E-cadherin, β-catenin, S100A4, the transcription factors Twist and Snail1, collagen-specific receptor tyrosine kinase discoidin domain receptor tyrosine kinase 2 (DDR2), vimentin, α-smooth muscle actin (α-SMA) and laminin, and expression of the PDGF family members (PDGF-A,-B,-C,-D and the cognate receptors, PDGFRα and -β) were evaluated by immunohistochemistry and immunocytochemistry in tissue sections and cultured cells, respectively, and by Western blotting (WB)(Suppl. Table 1). Expression of the PDGF ligands and receptors was also studied in tissue sections by dual immunofluorescence with K7 and α-SMA as markers for neoplastic cholangiocytes and CAF, respectively. Secretion of PDGF-AA and -BB (Raybiotech, Milan), and -DD (USCNK, Milan) was quantified by ELISA in culture medium collected from CCA cells and controls. To study whether hypoxia was a stimulus for PDGF-D secretion in CCA cells, PDGF-D secretion was studied in cultured CCA cells treated with 2-oxoglutarate analogues dimethyloxaloylglycine (DMOG, 3mmol/L for 18h) to induce chemical hypoxia(10). See Supplemental Methods for further details.
Morphometric analysis
Methodological details are given in the online Supplemental materials.
Xenotransplantation of CCA cells in SCID mice
The well-characterized invasive capabilities of EGI-1 cells in the SCID mouse model meant these particular CCA cells were selected for the in vivo experiments(11). EGI-1 cells were transduced with a lentiviral vector encoding the firefly luciferase and the enhanced green fluorescent protein (EGFP) reporter genes to enable detection of liver engraftment by in vivo bioluminescence imaging and by immunofluorescence in tissue sections, respectively, as described(11,12). After transduction, luciferase/EGFP-expressing EGI-1 cells were transplanted by intraportal injection into 10 male SCID mice, 6 to 8 week-old (Charles River, Wilmington, MA). Further details are provided in the Supplemental Methods. After the development of liver metastases, mice were sacrificed and tissue samples spanning the liver parenchyma were collected to perform immunohistochemical analyses to assess: a) the presence of tumor reactive stroma accompanying CCA liver implants; b) involvement of EMT in the formation of tumor reactive stroma by EGFP co-expression and fluorescent in situ hybridization (FISH) using both human and murine Y probes(13); c) involvement of a PDGF-mediated cross-talk between CCA cells and CAF.
Assessment of fibroblast proliferation and migration
To study the functional effects of PDGF in the cross-talk between cancer cells and fibroblasts, in human fibroblasts we assessed proliferation (MTS assay) and migration (Boyden chamber) following direct stimulation with rhPDGF-D (R&D Systems, Milan) at increasing doses (0.1, 1, 10, 100ng/ml), before and after administration of inhibitors of PDGFRβ, to determine a dose-response effect. PDGFRβ antagonism was achieved using the tyrosine kinase inhibitor Imatinib Mesylate (1µM for 24h, Cayman, Florence)(14). Furthermore, fibroblast migration induced by rhPDGF-D 100ng/ml was evaluated after selective inhibition of the small Rho GTPases, RhoA, Rac1 and Cdc42 and of c-Jun N-terminal kinase (JNK) (see below). As specific inhibitors, we used NSC23766 (75nM, Cayman, Florence) for Rac1(15), CASIN (5µM, Xcess Bioscience, San Diego, CA) for Cdc42(16), Y-27632 (10µM, Sigma, Milan) for RhoA/ROCK(17), and SP600125 (10µM, Sigma, Milan)(18). Proliferation and migration of human fibroblasts were also evaluated following stimulation with conditioned media from CCA cells (EGI-1, TFK-1, CCA1). Both experiments were run before and after Imatinib, and migration experiments also after siRNA for PDGF-D. SiRNA for PDGF-D was performed in EGI-1 cells using RNAiMax and StealthsiRNA (Invitrogen, Milan). See Supplemental Methods for details.
Assessment of the downstream effectors of PDGF-D signaling in fibroblasts after stimulation with rhPDGF-D
Human fibroblasts were exposed to increasing doses of rhPDGF-D (0.1,1,10,100ng/ml) for 24h. Among the potential effectors of PDGF-D signaling, we assessed the extracellular signal-regulated kinase 1/2 (ERK1/2) and JNK by WB of total cell lysates, and RhoA, Rac1 and Cdc42 by G-LISA. ERK1/2 (regulating cell proliferation) and the Rho GTPases (regulating cell migration) are downstream effectors of two major signaling pathways activated by PDGF-D, the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)/Akt, respectively(8). JNK is a MAPK family member induced in response to a number of growth factors. These signaling molecules were evaluated before and after Imatinib treatment. See Supplemental Methods for details.
Statistical analysis
Results are shown as mean of “x” experiments±standard deviation. Statistical comparisons were made using Student’s t-tests or the Wilcoxon-Mann-Whitney two-sample rank-sum test. In the Wilcoxon-Mann-Whitney two sample rank-sum test, the p-value was obtained from the exact permutation null distribution. The statistical analysis was performed using SPSS 16.0 software (SPSS, Bologna, Italy); p values <0.05 were considered as significant.
RESULTS
Partial expression of EMT phenotypic markers in resected human CCA samples (Table 1, Suppl. Figure 1)
Table 1.
Immunophenotypic characterization of EMT and PDGF signaling markers in 15 human CCA samples derived from surgical resection. The number of positive samples out of 15 are reported.
| MARKER | Cell type | N° of Positive Samples/15 |
|---|---|---|
| E-Cadherin* | CCA | 13 |
| β-Catenin* | CCA | 12 |
| Snail1 | CCA | 15 |
| TWIST | CCA | 13 |
| S100A4 | CCA | 10 |
| Vimentin | CCA | 9 |
| DDR2 | CCA | 5 |
| α-SMA | CCA | 0 |
| Laminin° | CCA | 10 |
| PDGF-A | CCA | 15 |
| CAF | 15 | |
| PDGF-B | CCA§ | 11 |
| CAF | 11 | |
| PDGF-C | CCA | 0 |
| CAF | 0 | |
| PDGF-D | CCA | 13 |
| CAF | 4 | |
| PDGFRα | CCA | 15 |
| CAF | 8 | |
| PDGFRβ | CCA | 0 |
| CAF | 15 |
positive samples when down-regulation or delocalization of membrane staining occurred
positive samples when uneven immunoreactivity along the bile duct profile occurred
only a weak expression (<30% of neoplastic bile ducts) in positive samples
The amount of tumor reactive stroma, measured as the percentage of α–SMA-positive area present within the boundaries of the neoplastic area, was homogeneously represented in all CCA samples (11.11±4.70%). As shown in Table 1 and Suppl. Fig.1, several phenotypic features of EMT were present in CCA bile ducts, but morphologic criteria supporting a complete transition towards a mesenchymal phenotype (co-expression of K7 and α-SMA) were never met. No EMT phenotype differences were observed between intrahepatic (n=10) and extrahepatic (n=5) CCA.
Lack of evidence of complete EMT in liver tumors derived from xenografted EGI-1 cells (Figure 1)
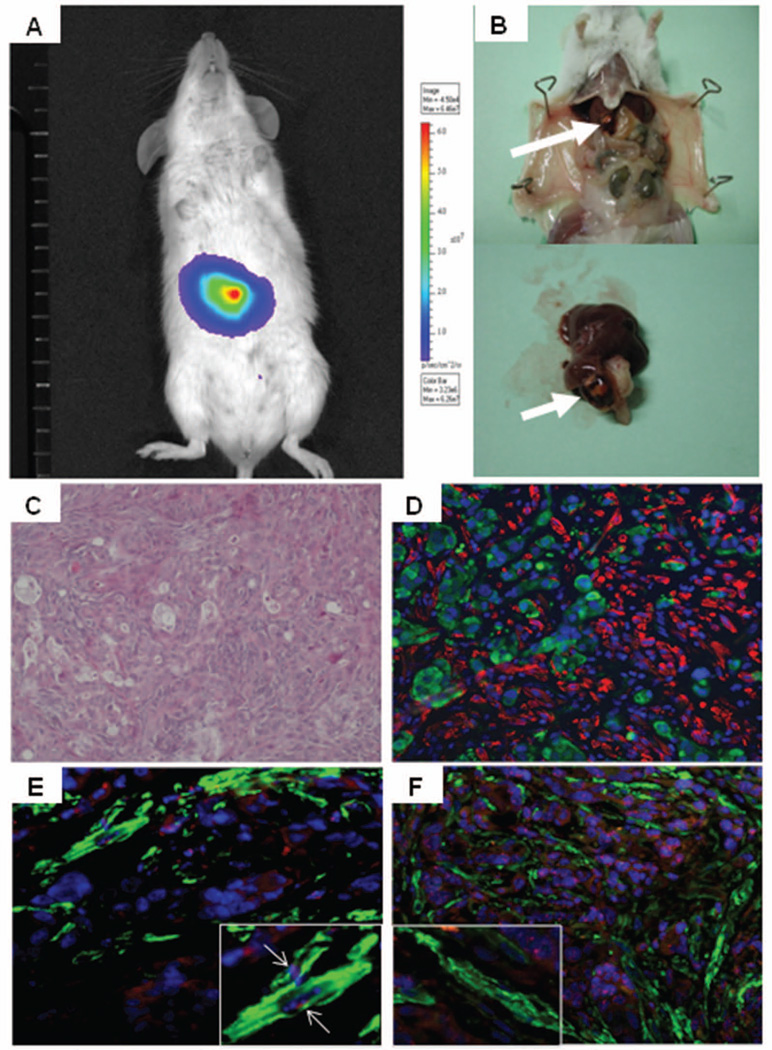
Figure 1. Bioluminescence imaging and histological assessment of EGI-1 cells after xenotransplantation into SCID mice.
High correspondence between the bioluminescence signal in the liver (A) and the macroscopic detection of liver tumors at autopsy (arrow, B) after xenotransplantation of EGI-1 cells into SCID mice by intraportal injection. Before transplantation, EGI-1 cells were transduced with lentiviral vectors encoding the firefly luciferase gene. Mice were sacrificed once the bioluminescence signal intensity in the liver reached a value >105 p/sec/cm2/sr. Histological analysis of liver metastases showed that EGI-1 cells laid embedded in a rich stroma (H&E staining, C). By dual immunofluorescence for EGFP (green) and α-SMA (red), we showed that CAF were strictly adjacent to EGI-1-derived tumors, but coincident labeling between EGFP and α-SMA was never observed (D). In liver tumors formed by EGI-1 cells, FISH showed that α-SMA-positive CAF (green, E, F) co-expressed mouse (red, white arrow, E), but not human (red, F) Y-Chr, which was instead expressed by tumoral EGI-1 cells (F). High specificity of both Y-probes was confirmed in preliminary experiments. Original magnification: C-F, 200x, insets in E,F, 400x.
EGI-1 cells were xenotransplanted in SCID male mice after transduction with lentiviral vectors encoding firefly luciferase and EGFP, to detect the tumor engraftment in the liver in vivo. Nine out of ten xenotransplanted SCID mice developed a luminescent signal over the liver area, 30 to 150 days post-xenotransplantation. One animal died at day 55 before developing a detectable luciferase signal. Once the bioluminescence signal intensity in the liver reached a value >1×105 p/sec/cm2/sr, tumor-bearing mice were sacrificed at a median of 71 days after xenotransplantation (range 50–155). Fig.1A–B shows the correspondence between the bioluminescence signal and the macroscopic presence of liver tumors. Liver tumors were analyzed by dual immunofluorescence for EGFP (expressed by transplanted EGI-1 cells) and α-SMA (myofibroblast/CAF marker). Xenotransplanted cancer cells that underwent a complete EMT would be expected to co-express EGFP and α-SMA. EGFP-positive, EGI-1-derived tumors were found embedded in abundant stroma, rich in α-SMA-positive cells strictly adjacent to the tumor cells (Fig.1C–D). However, coincident labeling between EGFP and α-SMA was never observed (Fig.1D). In selected mice, a FISH analysis was performed using both the human and the mouse Y probes for their co-expression with CAF to confirm the above results. Preliminary studies in mouse (n=2) and human liver specimens (n=2), indicated that both Y probes were highly specific and do not cross-react between the two species. Consistent with the EGFP data, α-SMA-positive cells expressed the mouse but not the human Y probe, which was instead normally expressed by infiltrating EGI-1 cells (Fig.1E–F). These data demonstrate that CAF infiltrating liver metastases are not generated through an EMT of xenografted EGI-1 cells.
In human CCA samples, CCA cells express PDGF-A, -D and PDGFRα, whereas CAF express PDGFRβ (Table 1, Figure 2, Suppl. Figure 2)
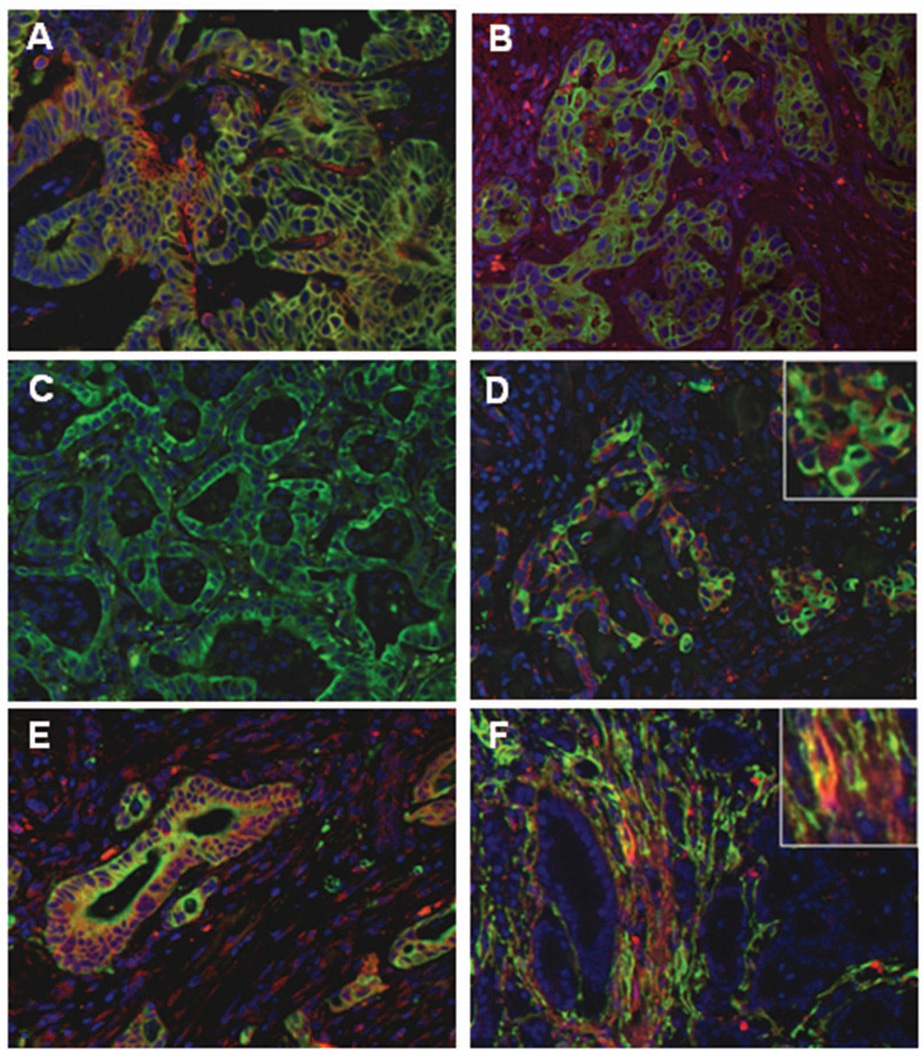
Figure 2. Expression of PDGF ligands and receptors in human CCA samples.
Neoplastic bile ducts (K7, green, A-E) were strongly positive for PDGF-A (red, A), and PDGF-D (red, D), weakly positive for PDGF-B (red, B), and negative for PDGF-C (red, C). In addition, neoplastic bile ducts expressed the PDGFRα, though extra-CCA PDGFRα staining was also found in some scattered surrounding cells, including CAF (red, E). On the other hand, CAF (α-SMA, green, F) were strongly decorated by PDGFRβ, which was negative in CCA cholangiocytes (red, F). Coincident staining appears in yellow. Original magnification: A-F, 200x, insets in D-F, 400x.
Immunohistochemical analysis of CCA specimens showed that neoplastic bile ducts were strongly positive (>70% of ducts) for PDGF-A (Fig.2A), PDGF–D (Fig.2D) and for PDGFRα (Fig.2E), but only weakly positive (<30%) for PDGF-B (Fig.2B), and negative for PDGF-C (Fig.2C) and PDGFRβ (Fig.2F). CAF, identified as α-SMA-positive cells localized in close vicinity to neoplastic ducts outside of vascular structures, were instead extensively positive for PDGFRβ (Fig.2F), whereas their expression of PDGFRα was patchy (Fig.2E). In extra-tumoral liver samples, bile ducts were consistently negative for PDGF ligands and receptors (not shown). This reciprocal expression of the members of the PDGF family between neoplastic bile ducts and CAF, suggests a role for PDGF-mediated cross-talk in CAF recruitment. In addition to CCA cells, immunofluorescence studies showed that PDGF-D was also expressed by a fraction of CD45-positive inflammatory cells, scattered within the tumor reactive stroma, whereas it was negative in endothelial cells (Suppl. Fig.2A,B). This finding indicates that inflammatory cells populating the stromal microenvironment behave as additional paracrine source of PDGF-D.
Cultured CCA cell lines secrete PDGF-A and PDGF-D (Suppl. Table 2, Suppl. Figures 3, 4 and Table 2)
Table 2.
Levels of PDGF-A, -B and -D secretion by established (EGI-1, TFK-1, HuCCT-1) and primary (CCA1, CCA2, CCA3) CCA cell lines and control cholangiocytes (ctrl) measured by ELISA (pg/ml).
| PDGF-A | PDGF-B | PDGF-D | |
|---|---|---|---|
|
EGI-1 (n=8) |
484.71±128.51 | N.D. | 420.52±120.23* |
|
TFK-1 (n=5) |
484.71±125.51 | 6.81±5.76 | 108.75±29.02* |
|
HuCCT-1 (n=5) |
50.72±84.66* | 4.23±2.82 | 96.89±24.94* |
|
CCA1 (n=5) |
556.30±160.51 | N.D. | 138.16±26.18* |
|
CCA2 (n=5) |
680±160.51 | 5.47±5.92 | 65.88±22.96* |
|
CCA3 (n=5) |
249.38±77.40* | 2.36±2.01 | 87.17±40.60* |
|
Ctrl (n=5) |
380±51 | N.D. | N.D. |
p<0.05 vs ctrl; N.D, not detectable.
The immunophenotype of cultured CCA cells assessed by immunocytochemistry, reproduced the expression pattern of the neoplastic bile ducts observed in CCA histological sections (Suppl. Table 2 and Suppl. Fig.3 and Fig.4). Expression of PDGF receptors was confirmed by WB. ELISA was used to assess the secretory functions of the different PDGF isoforms. PDGF-B secretion was undetectable in both CCA cells and controls; secretion of PDGF-A was similar between CCA cells and controls, whereas PDGF-D was variably secreted by CCA cells only (from 65.88pg/ml to 420.52pg/ml), and was undetectable in controls.
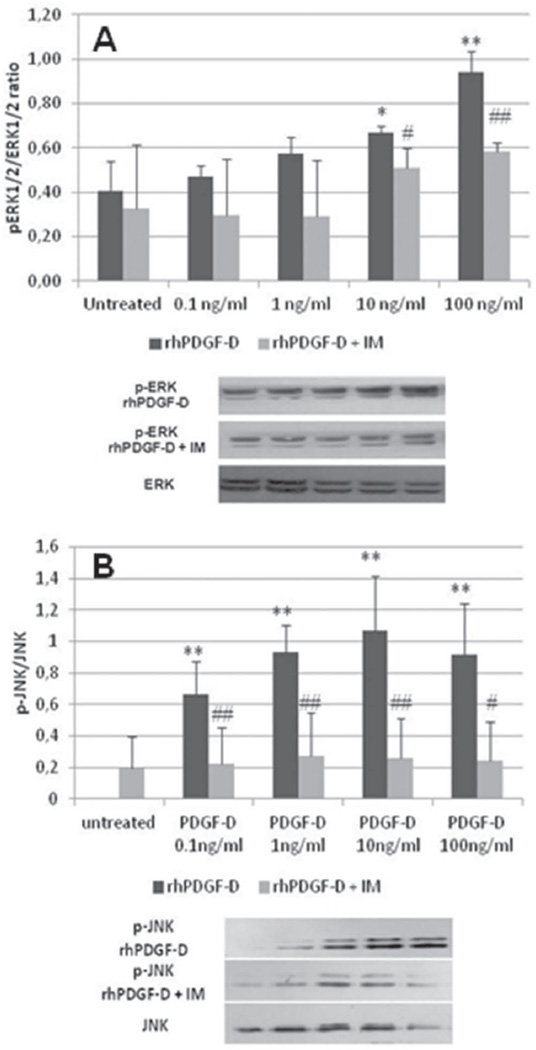
Figure 4. Activation of ERK1/2 and JNK in human fibroblasts stimulated with rhPDGF-D. A.
By WB, stimulation of human fibroblasts with increasing doses of rhPDGF-D resulted in a mild increase of p-ERK1/2/ERK1/2 which reached significance only at the highest doses (black columns, 0.1ng/ml, 0.47±0.05; 1ng/ml, 0.57±0.08; 10ng/ml, 0.67±0.03; 100ng/ml, 0.94±0.09 vs untreated, 0.40±0.14; n=5 experiments), and was inhibited after addition of Imatinib 1µM (gray columns, untreated, 0.33±0.29; 0.1ng/ml, 0.30±0.26; 1ng/ml, 0.29±0.25; 10ng/ml, 0.51±0.09; 100ng/ml, 0.58±0.04). B. In contrast with ERK1/2, JNK was activated since the lowest doses of rhPDGF-D (black columns, 0.1ng/ml, 0.66±0.21; 1ng/ml, 0.93±0.17; 10ng/ml, 1.07±0.34; 100ng/ml, 0.91±0.33 vs untreated, n.d.; n=4 experiments), an effect that was significantly reduced by Imatinib 1µM (gray columns, untreated, 0.27±0.20; 0.1ng/ml, 0.18±0.23; 1ng/ml, 0.32±0.27; 10ng/ml, 0.27±0.26; 100ng/ml, 0.36±0.25). *p<0.05 treated vs untreated; **p<0.01 treated vs untreated; #p<0.05 treated vs Imatinib 1µM; ##p<0.01 treated vs Imatinib 1µM. IM, Imatinib Mesylate. The columns of bands in the Western blots below are respective of each of the five conditions displayed in the graph.
PDGF-D secretion is further and significantly increased in cultured PDGF-D- secreting CCA cells following chemically-induced hypoxic stimulus (Suppl. Figure 5)
In CCA cells with high PDGF-D secretion (EGI-1, TFK-1, CCA1), PDGF-D secretion was also measured in conditions of chemical hypoxia, following treatment with DMOG. In all the three CCA cell lines, DMOG induced a further and significant increase in PDGF-D secretion (Suppl. Fig.5A), greater than 3 times, and associated with a significant up-regulation of HIF1α of the same degree (Suppl. Fig.5B).
PDGF-A, -D and PDGFRα are expressed by xenografted EGI-1 cells, while PDGFRβ is expressed by infiltrating CAF (Suppl. Figure 6)
Specimens from xenotransplanted CCA were analyzed by dual and triple immunofluorescence to assess the expression of PDGF ligands and receptors. PDGF-A (Suppl. Fig.6A) and -D (Suppl. Fig.6B) were expressed by EGI-1 cells, together with PDGFRα (Suppl. Fig.6C) but not PDGFRβ (Suppl. Fig.6D). Conversely, CAF localized in close vicinity to EGFP-positive cells were diffusely and intensely decorated by the anti-PDGFRβ antibody (Suppl. Fig.6D) but unevenly by anti-PDGFRα. These findings confirmed that the reciprocal expression of ligands and receptors between cholangiocytes and CAF observed in native CCA, is maintained in our experimental model of CCA.
Immunophenotyping of human fibroblasts
Phenotype of cultured fibroblasts isolated from alcoholic cirrhosis was similar to CAF isolated from CCA, characterized by the expression of vimentin and α-SMA, in conjunction with that of PDGFRβ (Suppl.Fig7A–C). See supplemental result section for further details.
PDGF-D is responsible for CAF recruitment, increasing motility without affecting proliferation (Figure 3 and Suppl. Figures 8 and 9)
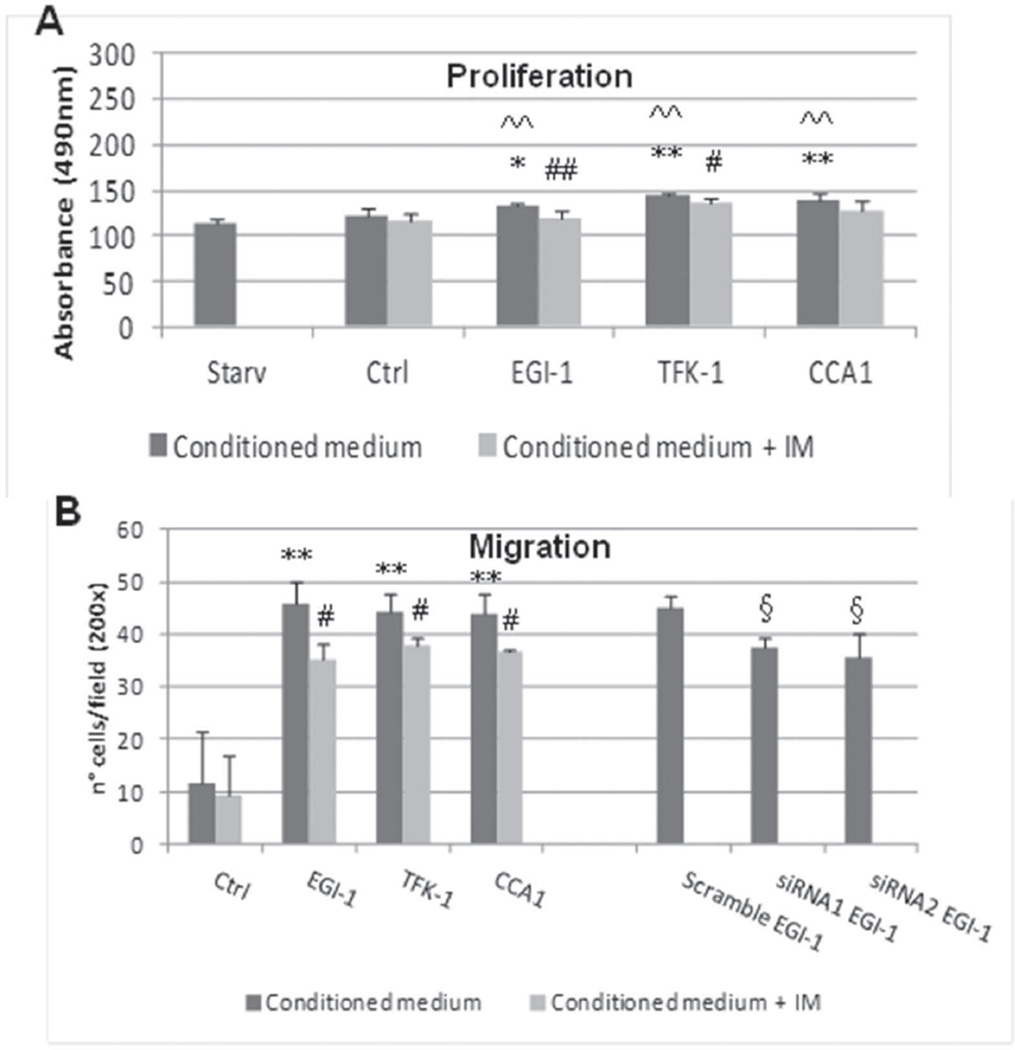
Figure 3. Proliferation (A) and migration (B) of human fibroblasts stimulated by conditioned media from CCA cholangiocytes.
Proliferation of human fibroblasts was evaluated by MTS assay and expressed as absorbance at 490nm (A), whilst recruitment was evaluated by Matrigel-coated transwell chambers and expressed as number of transwell-invaded nuclei (B). A. Conditioned media obtained from EGI-1, TFK-1, and CCA1 cholangiocytes induced only a slight proliferative response with respect to starved fibroblasts and to control cholangiocytes (ctrl) (black columns: EGI-1, 132.33±5.05; TFK-1, 144.33±2.94; CCA1, 140.33±7.09, vs control, 123.67±6.19 and starved, 114.33±6.15; n=6 experiments). This effect, although small, was significantly reduced following treatment of cultured cells with Imatinib Mesylate 1µM (A) (gray columns: EGI-1, 119.83±7.22; TFK-1, 135.33±6.31; CCA1, 129.17±10.17; control, 117.83±6.74; n=6 experiments). B. Conditioned media from the same cell lines induced a potent chemotactic response on human fibroblasts with respect to ctrl (black columns: EGI-1, 45.9±4.01; TFK-1, 44.17±3.59; CCA1, 43.82±3.99 vs control, 11.76±9.83; n=3 experiments), an effect significantly reduced by Imatinib Mesylate 1µM (gray columns: EGI-1, 35.08±3.33; TFK-1, 37.83±1.55; CCA1, 36.82±0.41, control, 9.16±7.62; n=3 experiments). A similar significant reduction in fibroblast invasion was achieved using conditioned media from EGI-1 cells treated with PDGF-D siRNA, as compared with EGI-1 scramble (siRNA1, 37.45±2.08; siRNA2, 35.45±4.70 vs EGI-1 scramble, 44.98±2.30; n=3 experiments). *p<0.05 treated vs control; **p<0.01 treated vs control; #p<0.05 treated vs IM; ##p<0.01 treated vs IM; ^^p<0.01 treated vs starv; §p<0.05 siRNA vs scramble. IM, Imatinib Mesylate.
EGI-1, TFK-1 and CCA1 were selected for experiments with human fibroblasts (8). Effects of conditioned media from CCA cells on fibroblast proliferation (MTS assay) and migration (Boyden chamber) were studied before and after Imatinib, a PDGFRβ antagonist. Effects of EGI-1 cells on fibroblast recruitment were also tested after siRNA for PDGF-D, resulting in a significant down-regulation of PDGF-D secretion (of about 35–40% as compared with scramble, p<0.01 with siRNA1, p<0.05 with siRNA2) (Suppl. Fig.8).
Conditioned media from CCA cells induce only a minor increase in fibroblast proliferation (Figure 3A)
As compared with starved fibroblasts, or with fibroblasts exposed to conditioned medium derived from control cholangiocytes, human fibroblasts showed only a mild increase in proliferative activity after exposure to conditioned media from the different CCA cells (from 7% to 15%, as compared to control cholangiocytes). PDGFRβ blockade induced a significant reduction in the rate of proliferating cells in fibroblasts stimulated by EGI-1 and TFK-1 (p<0.01 and p<0.05, respectively).
CCA cells secreting PDGF-D strongly stimulate fibroblast recruitment, an effect that is significantly reduced by PDGFRβ antagonism and by PDGF-D siRNA (Figure 3B)
As compared with control cholangiocytes, all conditioned media from the different CCA cells induced a potent migration of human fibroblasts (increase of about 73–74%), which reduced significantly following PDGFRβ blockade (p<0.05 for all CCA cells). Notably, in EGI-1 cells, both PDGF-D siRNA showed a significant reduction in fibroblast recruitment of an extent similar to PDGFRβ blocker (p<0.05 as compared with scramble).
Human fibroblasts exposed to rhPDGF-D showed a similar behavior. Effects of rhPDGF-D on migration were significantly reduced when fibroblasts were exposed to Imatinib. These results are detailed in the supplemental section and shown in Suppl. Fig.9.
The small Rho GTPases are activated in fibroblasts following stimulation with rhPDGF-D, and they are inhibited by Imatinib (Figure 4,Figure 5 and Suppl. Figure 10)
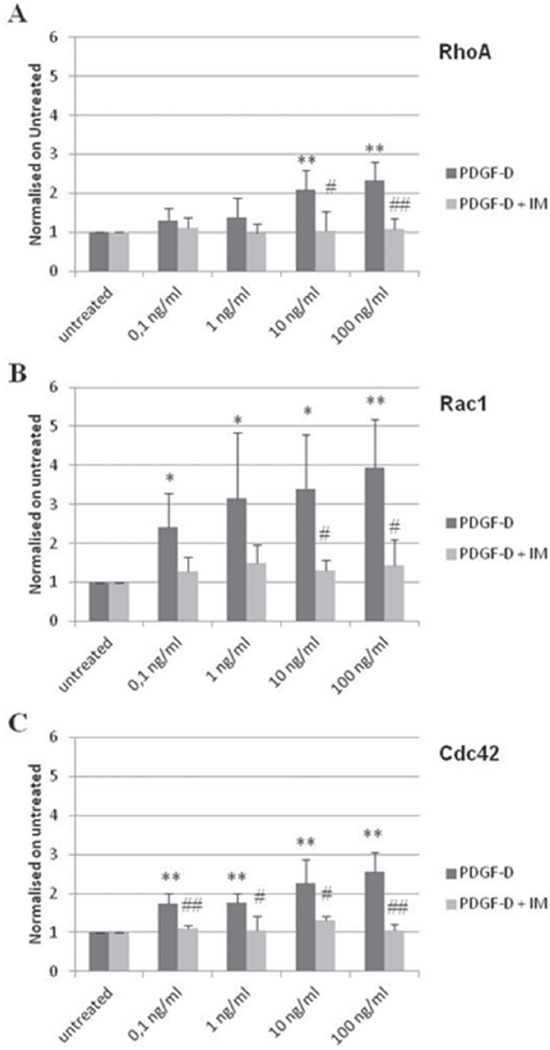
Figure 5. Dose-response activation of RhoA, Rac1 and Cdc42 in human fibroblasts stimulated with rhPDGF-D.
Human fibroblasts were stimulated for 1min with increasing doses of rhPDGF-D (0.1, 1, 10, 100ng/ml) to assess a dose-response effect. Levels of activation are expressed as normalization on untreated cells. A linear dose-dependent increase was observed for Rac1 (B, black columns, 0.1ng/ml, 2.41±0.86; 1ng/ml, 3.14±1.70; 10 ng/ml, 3.39±1.40; 100ng/ml, 3.93±1.24), and for Cdc42 (C, black columns, 0.1ng/ml, 1.73±0.28; 1ng/ml, 1.77±0.22; 10 ng/ml, 2.26±0.61; 100ng/ml, 2.56±0.49) that was significant from the lowest dose. In contrast, activation of RhoA was observed only at the highest doses (A, black columns, 0.1ng/ml, 1.29±0.32; 1ng/ml, 1.39±0.49; 10 ng/ml, 2.08±0.50; 100ng/ml, 2.32±0.47). Imatinib 1µM blunted the activating effects of rhPDGF-D in all cases (gray columns, RhoA, 0.1ng/ml, 1.10±0.28; 1ng/ml, 0.97±0.25; 10 ng/ml, 1.02±0.51; 100ng/ml, 1.08±0.28. Rac1, 0.1ng/ml, 1.28±0.37; 1ng/ml, 1.49±0.48; 10 ng/ml, 1.30±0.26; 100ng/ml, 1.43±0.66. Cdc42, 0.1ng/ml, 1.11±0.07; 1ng/ml, 1.04±0.37; 10 ng/ml, 1.31±0.11; 100ng/ml, 1.05±0.16) (n=4 experiments). *p<0.05 treated vs untreated; **p<0.01 treated vs untreated; #p<0.05 treated vs Imatinib 1µM; ##p<0.01 treated vs Imatinib 1µM. IM, Imatinib Mesylate.
To study the signaling pathway activated by PDGFRβ in response to PDGF-D, we stimulated human fibroblasts with rhPDGF-D at increasing doses (0.1,1,10,100ng/ml), and then modulation of p-ERK1/2 and p-JNK expression (by WB) and activation of RhoA, Rac1 and Cdc42 (by G-LISA) were evaluated in the presence/absence of Imatinib treatment. To determine the kinetics of activation of RhoA, Rac1 and Cdc42, preliminary G-LISA experiments were run at 1,10,20,30 and 60min following stimulation with rhPDGF-D 100ng/ml. PDGF-D induced a significant increase of p-ERK1/2 only at the highest doses (p<0.05 at 10 and 100ng/ml), those able to stimulate also fibroblast proliferation, and this effect was abrogated by Imatinib (Fig.4A). In contrast, increase of p-JNK was significant starting from the lowest doses of rhPDGF-D (0.1ng/ml, p<0.01), and it was abolished by Imatinib (p<0.01) (Fig.4B).
A strong PDGF-D-dependent modulation of Rho GTPases was also documented. Time-course studies (Suppl. Fig.10) showed that PDGF-D induced a strong and early activation of Rac1 (nearly 5-fold increase) at 1min, followed by a rapid return to basal values (Suppl. Fig.10B). RhoA kinetics also showed an early, but smaller increase (2-fold), and then fluctuated (Suppl. Fig.10A). In contrast with Rac1 and RhoA, Cdc42 remained persistently activated up to 60min (nearly 4-fold increase, Suppl. Fig.10C).
We next performed a dose-response curve with increasing doses of rhPDGF-D (Fig.5). Rac1 and Cdc42 activity showed a clear dose-dependent linear increase, that was significant from the lowest dose (Fig.5B–C), while RhoA was activated only at the highest doses (Fig.5A). In all cases, GTPase activation was inhibited by Imatinib (p<0.05, Fig.5A–C). These data strongly suggest that PDGF-D secreted by CCA cells, by interacting with PDGFRβ expressed by mesenchymal cells, induces migratory effects resulting in CAF recruitment through activation of Rho GTPases, in particular Rac1 and Cdc42.
Treatment with small GTPases and JNK inhibitors abrogated PDGF-D-induced fibroblast migration (Figure 6)
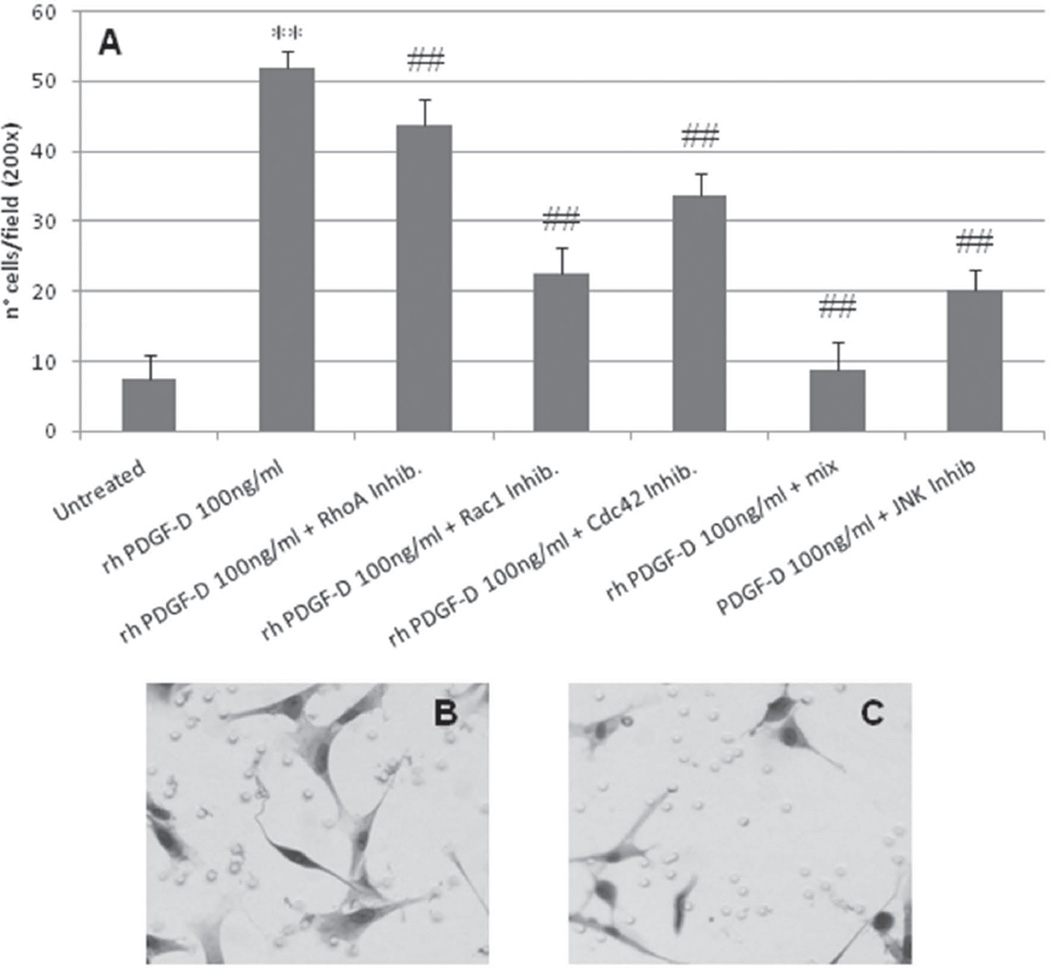
Figure 6. Effects of RhoA, Rac1, Cdc42 and JNK inhibitors on migration of human fibroblasts stimulated by rhPDGF-D.
Human fibroblasts treated with rhPDGF-D 100ng/ml showed a significant reduction in migration after treatment with chemical inhibitors of small GTPases. A. Treatment with Y-27632 10µM (RhoA inhibitor), NSC23766 75nM (Rac1 inhibitor), CASIN 5µm (Cdc42 inhibitor), and SP600125 10µM (JNK inhibitor) induced a significant reduction in migration of different degrees (43.79±3.75 for RhoA antagonism, 22.53±3.81 for Rac1 antagonism, 33.78±3.15 for Cdc42 antagonism, and 20.09±3.08 for JNK antagonism vs 52.01±2.21 for PDGF-D treatment, p<0.01 in all cases). Combined treatment with the three inhibitors of small GTPases (mix) completely abolished the PDGF-D-stimulated fibroblast migration (8.78±3.97 vs untreated, 7.52±3.42, p=n.s.) (n=4 experiments). It is worth noting the spindle shaped morphology of fibroblasts induced by PDGF-D (B) is lost following treatment with NSC23766 (C). **p<0.01 PDGF-D treated vs untreated; ##p<0.01 PDGF-D treated vs inhibitors. Mix, Y-27632 10µM + NSC23766 75nM + CASIN 5µm.
To further confirm this hypothesis, we next tested the effects of selective inhibitors of RhoA/ROCK (Y-27632), Rac1 (NSC23766), Cdc42 (CASIN) and JNK (SP600125) on fibroblast migration stimulated by PDGF-D. All inhibitors induced a significant reduction in fibroblast migration, of about 15% for Y-27632, 35% for CASIN, and up to 60% for NSC23766 and SP600125 (p<0.001 in all cases, Figure 6A). Notably, the combined treatment with all the small GTPases inhibitors (mix) completely abrogated the migratory effects of PDGF-D, thereby indicating a synergic effect of Rho GTPases (Fig.6A). In addition, when Rac-1 was inhibited, PDGF-D-stimulated fibroblasts showed relevant morphological changes, characterized by the loss of the spindle-shape morphology and by the presence of short surface protrusions, consistent with a motile-halting phenotype (Fig.6B–C).
DISCUSSION
The incidence of CCA is increasing in Western countries, and it accounts for 10–20% of deaths from primary hepatobiliary malignancies. CCA is characterized by the presence of an abundant tumor reactive stroma, a feature common to other aggressive malignancies of ductal origin, such as pancreatic and breast carcinomas.
The tumor reactive stroma is the microanatomical site of multiple functional interactions between cancer cells and several kinds of host cells, and thus it behaves as an important determinant of cancer invasiveness. CAF, the main cellular component of the tumoral stroma, produce tumoral matrix and release a variety of growth factors and chemokines, which modulate tumor cell survival, migration and invasion(4). For example it has been shown that CAF-derived PDGF protects CCA cells from death induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in a Hedgehog signaling dependent-manner(5). CAF are also an important source of matrix metalloproteinases, cathepsins and plasminogen activators that enable cancer cells to escape from the primary site of growth(19). Some authors propose that factors originating from the stroma (TGF-β, HGF) signal to cancer cells to undergo an EMT and to become endowed with functional properties that favor the metastatic process, such as the ability to detach from the neoplastic cluster and to migrate to and invade lymphatic or blood vessels(20). The role of EMT in liver diseases and tumors remains unclear and controversial(21).
In this study, CCA cells expressed several phenotypic features, known to correlate with increased motility and invasiveness, including down-regulation of E-cadherin and β-catenin and up-regulation of Snail1, Twist and S100A4. However, there was no evidence of EMT. This conclusion is based on the lack of co-expression of K7 and α-SMA in CCA tissue sections, and on the lack of coincidence between CCA cholangiocyte lineage markers (EGFP and human Y-Chr) and an activated myofibroblast marker (α-SMA) after intraportal injection of the highly invasive EGI-1 cells into SCID mice. As shown in Figure 1, the EGFP-positive CCA cholangiocytes expressed the human Y-probe but did not express α-SMA, whereas α-SMA-positive CAF expressed the murine Y-probe rather than the human Y-probe. After xenotransplantation, in spite of the immunotolerant environment, an abundant stroma formed around the CCA cholangiocytes suggesting a direct effect of factors secreted by the tumoral cells.
Several factors can regulate epithelial-mesenchymal cross-talk, including Hedgehog, Wnt and PDGF. We present immunohistochemical and in vitro evidences suggesting that PDGF secreted by tumoral cells plays a key role on migratory properties of CAF.
We demonstrate that PDGF-D is secreted by neoplastic but not by control cholangiocytes. PDGF-D is one of the players responsible for the increased migration of fibroblasts when exposed to CCA conditioned medium. In contrast with the other members of the PDGF family, PDGF-D binds only to the PDGFRβ(22). Mechanisms leading to the up-regulation of PDGF-D in neoplastic cholangiocytes are uncertain. However, our data suggest that hypoxia may behave as a critical inducer of PDGF-D secretion, as shown by the potent stimulation exerted on CCA cells by DMOG, an agent preventing HIF-1α degradation. This effect is in line with the typical hypovascularization featuring CCA. Our immonofluorescence studies show that a subset of inflammatory cells may represent an additional source of PDGF-D released in the tumor microenvironment, albeit their PDGF-D expression is less relevant than CCA cells.
The importance of PDGF-D in cancer biology is just beginning to be understood(23,24). Our findings strongly suggest that PDGF-D plays a major role in promoting CAF recruitment in CCA. In fact, siRNA of PDGF-D significantly impaired the ability of CCA cholangiocytes to promote fibroblast migration. In addition, rhPDGF-D induced a clear dose-dependent effect on fibroblast migration, whereas the effect on proliferation was milder and evident only at the highest dosages. PDGFRα, which binds all isoforms except for PDGF-D, may theoretically contribute to CAF recruitment in CCA, since PDGFRα was also expressed by CAF, and EGI-1 cells were able to secrete PDGF-A. However, administration of conditioned medium from control cholangiocytes that contains amounts of PDGF-A comparable to those produced by CCA cells, exerted only a weak effect on fibroblast transwell migration. Interestingly, whereas PDGFRα signaling plays a pivotal role in embryonic development and in fibrosis in non-hepatic conditions, PDGFRβ seems to be more relevant in activating HSC(25) and in stimulating the production of pro-fibrogenic growth factors and ECM components by liver myofibroblasts.
By interacting with its cognate receptor PDGFRβ, PDGF-D can activate several signaling cascades to regulate cell survival, cell growth, cell differentiation, cell invasion, and angiogenesis(8). Because MAPK and PI3K/Akt are two major signal transduction pathways known to be activated by PDGF-D(8), we studied ERK1/2, JNK and the small Rho GTPases as downstream effectors respectively of MAPK and PI3K/Akt, able to control cell proliferation (ERK1/2)(10) and migration (JNK and Rho GTPases)(18,26). The ability of PDGF-B to induce cytoskeletal remodeling via Rac1 and JNK has recently been reported in NIH3T3 cells(26,27), but the effects of PDGF-D on these molecular effectors are hitherto largely unknown. Our findings show that exposure of fibroblasts even to low doses of PDGF-D strongly activates Rho GTPases and JNK, while expression levels of pERK increased only at the highest doses. These results strongly correlate with the different functional effects on fibroblast migration and proliferation of PDGF-D, as shown in Fig.3–Fig.5 and Supplemental Fig. 9. By regulating the cytoskeleton and adhesion dynamics, the Rho GTPases are key drivers of cell migration. The time course study of Rho GTPase activation further enforces the role of PDGF-D as a fundamental mediator of CAF recruitment. Rac1 and Cdc42 are two of the members of the family that are most activated by PDGF-D, however they show a different kinetics of activation. Rac1, which induces the assembly of actin-rich surface protrusions (lamellipodia) enabling the start of the mesenchymal cell movement (“random” migration)(27), shows a brisk but transient activation by PDGF-D. In contrast, Cdc42, which promotes the formation of actin-rich, finger-like membrane extensions (filopodia) regulating chemotaxis(28), shows a significantly sustained activation. These data indicate that by activating Rac1 and Cdc42 with different time-dependent patterns, PDGF-D may potentially regulate distinct steps of CAF recruitment, including chemotaxis towards tumoral cells, a critical function in the generation of the tumor stroma. The capability of the small GTPases to orchestrate fibroblast recruitment driven by PDGF-D is confirmed by the observation that fibroblast transwell migration elicited by PDGF-D was completely inhibited by a mix of selective inhibitors (Y-27632, NSC23766 and CASIN). While the regulation of cell motility by the Rho GTPases has been well documented in cancer cells(29), their involvement as fundamental molecular determinants of the tumor stromal reaction has not been reported yet. In addition to Rho GTPases, fibroblast migration in response to PDGF-D is also modulated by JNK, as previously shown in murine HSC and portal myofibroblasts(30). Notably, our data show that specific JNK inhibition halts fibroblast migration of an extent similar to Rac1, likely indicating that both pathways act in concert to orchestrate the PDGF-D-mediated paracrine fibroblast recruitment by CCA cells.
In addition to Rho GTPase and JNK inhibitors, we found that tyrosine kinase inhibitors were also highly effective in halting fibroblast migration and proliferation induced by PDGF-D. The potential clinical usefulness of tyrosine kinase inhibitors in CCA has recently been outlined by Andersen(4), particularly in those patients where overexpression of inflammatory functions in the microenvironment is a critical signature related to a worse prognosis. Data in this study show that selective blockade of PDGFRβ with Imatinib Mesylate, a tyrosine kinase inhibitor already in clinical use for other indications, significantly reduces fibroblast recruitment by CCA cholangiocytes in Boyden chambers. The therapeutic relevance of specifically targeting PDGFRβ in CCA is a topic of growing interest(5). Recently, the ability of PDGFRβ inhibitors to interfere with CAF-to-CCA paracrine signaling mediated by PDGF-BB has been reported. In fact, PDGFRβ promotes Hedgehog survival signaling in CCA cholangiocytes through protection from TRAIL cytotoxicity(5). Our study further extends the role of PDGFRβ molecular targeting in CCA, as it can prevent CAF recruitment induced by CCA cholangiocyte-derived PDGF-D. Notably, overexpression of PDGFRβ in the stromal compartment of CCA was related to the most significant “network connectivity” with the tumoral compartment(4). Pharmacological targeting of tumor/stroma interactions using PDGF inhibitors may represent a novel molecularly targeted therapeutic approach in CCA(31,32).
Supplementary Material
Acknowledgments
the authors wish to thank Dr. Scott Swenson (Section of Digestive Disease, Yale University School of Medicine) for assistance with FISH experiments. The support of Fondazione S. Martino, Bergamo is gratefully acknowledged.
Financial support:
Associazione Scientifica Gastroenterologica di Treviso (ASGET, “associazione di promozione sociale senza scopo di lucro”) to LF and IF. Telethon (grant #GGP09189) and Fondazione Amici Dell’ Epatologia (FADE) to LF. Progetto di Ricerca Ateneo 2008 (grant #CPDA083217) and 2011 (grant #CPD113799/11) to LF and MC. NIH Grant DK34989: Silvio O. Conte Digestive Diseases Research Core Centers – 5P30DK034989” and a grant from “PSC partners for a care” to MS.
Glossary
Abbreviations
- α-SMA
α-smooth muscle actin
- CAF
cancer associated fibroblast
- CCA
cholangiocarcinoma
- DAPI
4’,6-diamidino-2-phenylindole
- DDR2
discoidin domain receptor tyrosine kinase 2
- DMOG
2-oxoglutarate analogues dimethyloxaloylglycine
- EGFP
enhanced green fluorescent protein
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- FISH
fluorescent in situ hybridization
- HGF
hepatocyte growth factor
- HIF
hypoxia inducible factor
- HSC
hepatic stellate cells
- i.p.
intra-peritoneum
- JNK
c-Jun N-terminal kinase, N.D, not detectable
- MAPK
mitogen-activated protein kinase
- PDGF
platelet-derived growth factor
- PI3K/Akt
phosphatidylinositol 3-kinase
- SCID
severe combined immunodeficiency
- TGF-β
transforming growth factor-β
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- Y chr
Y chromosome
REFERENCES
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumor stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–2088. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;8:1461–1462. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- 7.Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol. 2006;169:2054–2065. doi: 10.2353/ajpath.2006.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Ahmad A, Li Y, Kong D, Azmi AS, Banerjee S, et al. Emerging roles of PDGF-D signaling pathway in tumor development and progression. Biochim Biophys Acta. 2010;1806:122–130. doi: 10.1016/j.bbcan.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirlì C, Melero S, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 10.Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, et al. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138:360–371. doi: 10.1053/j.gastro.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabris L, Cadamuro M, Moserle L, Dziura J, Cong X, Sambado L, et al. Nuclear expression of S100A4 calcium binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology. 2011;54:890–899. doi: 10.1002/hep.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indraccolo S, Habeler W, Tisato V, Stievano L, Piovan E, Tosello V, et al. Gene transfer in ovarian cancer cells: a comparison between retroviral and lentiviral vectors. Cancer Res. 2002;62:6099–6107. [PubMed] [Google Scholar]
- 13.Donnelly DS, Zelterman D, Sharkis S, Krause DS. Functional activity of murine CD34+ and CD34- hematopoietic stem cell populations. Exp Hematol. 1999;27:788–796. doi: 10.1016/s0301-472x(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 14.Abouantoun TJ, Macdonald TJ. Imatinib blocks migration and invasion of medulloblastoma cells by concurrently inhibiting activation of platelet-derived growth factor receptor and transactivation of epidermal growth factor receptor. Mol Cancer Ther. 2009;8:1137–1147. doi: 10.1158/1535-7163.MCT-08-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Rao Q, Wang M, Wei H, Xing H, Liu H, et al. Overexpression of Rac1 in leukemia patients and its role in leukemia cell migration and growth. Biochem Biophys Res Commun. 2009;386:769–774. doi: 10.1016/j.bbrc.2009.06.125. [DOI] [PubMed] [Google Scholar]
- 16.Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanzardo S, Curcio C, Forni G, Antón IM. A role for WASP Interacting Protein, WIP, in fibroblast adhesion, spreading and migration. Int J Biochem Cell Biol. 2007;39:262–274. doi: 10.1016/j.biocel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida K, Matsuzaki K, Mori S, Tahashi Y, Yamagata H, Furukawa F, et al. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol. 2005;166:1029–1039. doi: 10.1016/s0002-9440(10)62324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Kisseleva T, Brenner DA. Is it the end of the line for the EMT? Hepatology. 2011;53:1433–1435. doi: 10.1002/hep.24312. [DOI] [PubMed] [Google Scholar]
- 22.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- 23.Ustach CV, Taube ME, Hurst NJ, Jr, Bhagat S, Bonfil RD, Cher ML, et al. A potential oncogenic activity of platelet-derived growth factor D in prostate cancer progression. Cancer Res. 2004;64:1722–1729. doi: 10.1158/0008-5472.can-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Tong R, Cochran DM, Jain RK. Blocking platelet-derived growth factor-D/platelet-derived growth factor receptor beta signaling inhibits human renal cell carcinoma progression in an orthotopic mouse model. Cancer Res. 2005;65:5711–5719. doi: 10.1158/0008-5472.CAN-04-4313. [DOI] [PubMed] [Google Scholar]
- 25.Breitkopf K, Roeyen C, Sawitza I, Wickert L, Floege J, Gressner AM. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors alpha and beta in activated rat hepatic stellate cells. Cytokine. 2005;31:349–357. doi: 10.1016/j.cyto.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Okada A, Yaguchi T, Kanno T, Gotoh A, Nakano T, Nishizaki T. PDGF-D/PDGF-ββ receptor-regulated chemotaxis of malignant mesothelioma cells. Cell Physiol Biochem. 2012;29:241–250. doi: 10.1159/000337605. [DOI] [PubMed] [Google Scholar]
- 27.Miyata M, Rikitake Y, Takahashi M, Nagamatsu Y, Yamauchi Y, Ogita H, et al. Regulation by afadin of cyclical activation and inactivation of Rap1, Rac1, and RhoA small G proteins at leading edges of moving NIH3T3 cells. J Biol Chem. 2009;284:24595–24609. doi: 10.1074/jbc.M109.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 29.Jiang P, Enomoto A, Takahashi M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett. 2009;284:122–130. doi: 10.1016/j.canlet.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064–1074. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology. 2011;53:695–704. doi: 10.1002/hep.24145. [DOI] [PubMed] [Google Scholar]
- 32.Chiorean MV, Guicciardi ME, Yoon JH, Bronk SF, Kaufmanns SH, Gores GJ. Imatinib mesylate induced apoptosis in human cholangiocarcinoma cells. Liver Int. 2004;24:687–695. doi: 10.1111/j.1478-3231.2004.0984.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.