Abstract
Background
Platelet activation results in the release and upregulation of mediators responsible for immune cell activation and recruitment, suggesting that platelets play an active role in immunity. Animal models and retrospective data have demonstrated benefit of antiplatelet therapy on inflammatory mediator expression and clinical outcomes. This study sought to characterize effects of clopidogrel on the incidence and severity of community-acquired pneumonia (CAP).
Methods
A retrospective cohort study was conducted of Kentucky Medicaid patients (2001-2005). The exposed cohort consisted of patients receiving at least six consecutive clopidogrel prescriptions; the non-exposed cohort was comprised of patients not prescribed clopidogrel. Primary endpoints included incidence of CAP and inpatient treatment. Secondary severity endpoints included mortality, intensive care unit admission, mechanical ventilation, sepsis, and acute respiratory distress syndrome/acute lung injury.
Results
CAP incidence was significantly greater in the exposed cohort (OR 3.39, 95% CI 3.27-3.51, p < 0.0001) that remained after adjustment (OR 1.48, 95% CI 1.41-1.55, p < 0.0001). Inpatient treatment was more common in the exposed cohort (OR 1.96, 95% CI 1.85-2.07, p < 0.0001), but no significant difference remained after adjustment. Trends favoring the exposed cohort were found for the secondary severity endpoints of mechanical ventilation (p = 0.07) and mortality (p = 0.10). Pooled analysis of published studies supports these findings.
Conclusions
While clopidogrel use may be associated with increased CAP incidence, clopidogrel does not appear to increase – and may reduce – its severity among inpatients. Because this study was retrospective and could not quantify all variables (e.g., aspirin use), these findings should be explored prospectively.
Introduction
While it is well established that platelets are integral to hemostasis, more recent evidence points to an important role for platelets in inflammation and immunity. Not only do platelets internalize microorganisms and bind and sequester pathogens, they can also alter properties of endothelial cells and leukocytes to stimulate immune responses; for example, through the production of neutrophil extracellular traps (NETs) that capture bacteria.(1-5) Platelet activation and sequestration in pulmonary tissue is a key feature in inflammatory or infectious states such as sepsis and acute respiratory distress syndrome (ARDS). Platelets may mediate acute lung injury (ALI) by recruiting neutrophils and releasing granule contents.(6) Thrombocytopenia in the setting of sepsis is a harbinger of worse outcomes in intensive care (ICU) settings, although the reason(s) for higher mortality in thrombocytopenic patients remains unknown. One means to establish the contribution of platelets in infectious and inflammatory states is by studying consequences of antiplatelet therapy.
Antiplatelet drugs, including aspirin and thienopyridines, are a cornerstone of the treatment and prevention of arterial thrombosis. The thienopyridines target the P2Y12 subclass of adenosine diphosphate (ADP) receptors and thereby blunt platelet activation and aggregation stimulated by ADP. Clopidogrel, the most widely used thienopyridine, may attenuate platelet expression of inflammatory and immune markers and the release of inflammatory cytokines. (2, 7-9), including IL-1α, 2, 6, 13, 10, TNFα, and TNFβ.(10) Use of clopidogrel has been coupled with reductions in C-reactive protein (CRP) levels and decreased expression of CD40, CD40L, and P-selectin, in a variety of disease states, including cardiovascular disease, cerebrovascular disease, diabetes, and renal transplantation.(8, 9, 11, 12) Clopidogrel may have incremental benefit on reduction of inflammation in addition to aspirin, possibly due to a greater degree of platelet inhibition; additionally, reduction of P-selectin expression appears to be unique to clopidogrel. (8) These data point to the ability of clopidogrel to reduce markers of inflammation and immune activation, which could potentially affect infection incidence or severity. In animal models, clopidogrel pretreatment decreases platelet-leukocyte interactions and reduces neutrophil production of reactive oxygen species.(2) Moreover, recent preclinical data demonstrated that clopidogrel may reduce immune activation in models of sepsis. (7, 13) In particular, a reduction of LPS-mediated thrombocytopenia, fibrin deposition in the lungs, and inflammatory mediator upregulation occurs in mice pretreated with clopidogrel.(7, 13, 14)
Clinical studies also suggest that antiplatelet agents may be associated with better outcomes in patients with pneumonia and critical illness.(13, 15) In 224 hospitalized CAP patients, those receiving antiplatelet agents (n=44) for at least six months had lower need for ICU care and reduced length of stay (LOS) as compared to unmatched controls. Another study in consecutive (n=615) ICU patients found that pretreatment with antiplatelet agents was associated with lower mortality in critically ill medical and surgical patients and less of a decline in platelet counts.(15) An analysis done in Olmsted County, MN, indicated that pre-hospitalization antiplatelet therapy in critically ill patients was associated with a reduced incidence of ALI and ARDS.(16) Additionally, a retrospective cohort study of intensive care unit admissions revealed an association between aspirin use and survival in systemic inflammatory response syndrome (SIRS) and sepsis.(17)
Together, these studies suggest that antiplatelet agents may modulate outcomes in settings of severe infection and sepsis. The goal of the present study was to determine if an association exists between clopidogrel and either the incidence or the severity of community-acquired pneumonia (CAP) in a large, community-based population.
Methods
Study Sample
Prior to study initiation, Institutional Review Board approval was granted from the University of Kentucky (IRB approval 09-0705-X1b) and the Kentucky Cabinet for Health and Family Services (CHFS, approval CHFS-IRB-DMS-FY10-15).The study sample included all adult (≥ 18 years) Medicaid beneficiaries in Kentucky enrolled in Medicaid for ≥ 12 months between January 1, 2001, and December 31, 2005. This time frame defines a dataset with substantial clopidogrel use prior to implementation of the Medicare Part D prescription drug plan (January 1, 2006), where many began using supplemental drug plans. Data were collected from the Kentucky Medicaid claims database with assistance from the Institute for Pharmaceutical Outcomes and Policy (Lexington, KY). Study subjects, comorbidities, and severity data were obtained using the International Classification of Disease, Ninth Revision Clinical Modification (ICD-9-CM) codes and procedure codes (CPT-4) specific for each condition. Pharmacy claims data were used to determine medication use. Each patient was included only once; in patients with multiple CAP diagnoses, only the first was selected.
Patients were excluded if they had a history of HIV/AIDS, cystic fibrosis, leukemia, lymphoma, hematopoietic stem cell transplant, solid organ transplantation, or lung cancer. Patients with pharmacy claims for any of the following medications or medication classes were excluded: chemotherapy (except antimicrobials), mycophenolate mofetil, mycophenolate sodium, cyclosporine, sirolimus, tacrolimus, azathioprine, or ticlopidine.
Outcomes
The primary study outcomes were incidence and severity of pneumonia. Pneumonia was categorized as a hospitalization, emergency department, and provider office visit, with a primary or secondary diagnosis of pneumonia. Determination of pneumonia severity was based on the need for inpatient treatment, following the recommendations of the Infectious Diseases Society of America-American Thoracic Society (IDSA/ATS).(18) The IDSA/ATS guideline recommends the use of a severity or prognostic assessment tool such as the pneumonia severity index (PSI) or CURB-65 score to guide site of care decisions in CAP.(18) Because the components of these tools are either subjective or based on laboratory data unavailable in a billing database, the site of care (inpatient vs. outpatient treatment), was used as a surrogate for initial severity assessment.
Secondary severity measurements in inpatients with pneumonia were: the need for mechanical ventilation; diagnosis of sepsis, severe sepsis, or septic shock; diagnosis of ALI or ARDS; ICU admission; and mortality. Secondary severity outcomes were examined individually and as a composite.
Predictor Variables
The present study investigated whether exposure to clopidogrel therapy was associated with increased risk of CAP and/or increased severity of CAP among those diagnosed. The clopidogrel cohort included patients with ≥ 6 prescription claims for clopidogrel during the study period. The comparator cohort was defined as patients without a prescription claim for clopidogrel during the five-year study period.
All analyses were adjusted for known pneumonia risk factors including: age, gender, race, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), heart failure (HF), receipt of systemic corticosteroids, receipt of pneumococcal conjugate vaccine (PCV, Pneumovax®), stroke, diabetes mellitus, and dementia. (19)
Subgroup Analysis
To better establish a relationship between severity of CAP and clopidogrel treatment, the investigators chose a priori to analyze the effect of treatment with clopidogrel at the time of CAP diagnosis. In the subgroup of patients with CAP, those with active treatment (clopidogrel prescription claim within 40 days of pneumonia diagnosis) were compared to those who did not receive clopidogrel for the aforementioned severity endpoints.
Statistical Analyses
Continuous variables were compared using an independent-samples t-test, and dichotomous variables were compared using either a chi-square test for association or Fisher’s exact test, where appropriate. Binary logistic regression models were fit to estimate unadjusted and adjusted odds ratios comparing those receiving at least six consecutive clopidogrel prescriptions to those not receiving any clopidogrel prescriptions on CAP incidence and, among those affected by CAP, on several measures of CAP severity. Binary logistic regression models were fit to estimate unadjusted and adjusted odds ratios comparing CAP patients with active clopidogrel prescriptions to CAP patients not receiving any clopidogrel prescriptions on several measures of CAP severity. The factors used to adjust the odds ratios were age, gender, race, CAD, COPD, HF, systemic corticosteroid use in previous 30 days, receipt of PCV, stroke, diabetes mellitus, and dementia.(19) Version 9.2 of SAS (SAS Institute, Inc, Cary, NC) was used to carry out statistical analyses. Statistical significance was defined by a p-value of less than 0.05.
Meta-Analyses
Our meta-analysis sought to examine the protective role of antiplatelet therapy in patients admitted to the intensive care unit with sepsis. Pre-specified outcomes of analyses were short term mortality, composite of acute lung injury and need for mechanical ventilation, and ICU and in-hospital length of stay. We searched MEDLINE (January 1980 to February 2011), the Cochrane databases (January 2011), EMBASE (January 1980 to February 2011), CINAHL (January 1982 to February 2011), the US Food and Drug Administration Web site (http://www.fda.gov), and BIOSIS Previews (January 1980 to February 2011) using the following database-appropriate MESH terms: sepsis, antiplatelet therapy, ICU, mortality, acute lung injury, and mechanical ventilation. Given the inherit differences in study designs, we conducted random-effects meta-analyses to obtain estimated odds ratios (ORs) for the pre-specified main clinical outcomes comparing anti-platelet treated patients to controls and reported them with their associated 95% confidence intervals (CIs). The estimated OR from separate studies was combined according to the DerSimonian and Laird method. (20, 21) The Review Manager software (RevMan version 5.1; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) was used for the analyses.
Results
Patient Characteristics
A total of 417,648 patients in the Kentucky Medicaid claims database met inclusion criteria. Of these, 391,503 patients (94%) had no prescription for clopidogrel and 14,947 (3.6%) had claims for six clopidogrel prescriptions in consecutive 30-day periods. Demographic and comorbidity data are presented in Table 1. Patients treated with clopidogrel tended to be older and were likely to be male, Caucasian, have asthma, CAD, COPD, diabetes mellitus, HF, dementia, experienced a stroke, and had received a PCV.
Table 1.
Baseline Patient Demographics
| No Clopidogrel Prescription (n=391503) |
≥ 6 Consecutive Clopidogrel Prescriptions (n=14947) |
p value | |
|---|---|---|---|
|
| |||
| Male Sex, n (%) | 128876 (32.9%) | 5226 (35.0%) | <0.0001 |
|
| |||
| Age, years (mean ± SD) | 44.4 ± 20.7 | 67.0 ± 14.7 | <0.0001 |
|
| |||
| Ethnicity, n (%) | |||
| Caucasian, n (%) | 328196 (83.8%) | 12786 (85.5%) | <0.0001 |
| African/American, n (%) | 41714 (10.6%) | 803 (5.4%) | <0.0001 |
| Hispanic, n (%) | 2255 (0.6%) | 39 (0.3%) | <0.0001 |
| Asian/Pac Island, n (%) | 1150 (0.3%) | 36 (0.2%) | 0.28 |
| Other, n (%) | 3283 (0.8%) | 237 (1.6%) | <0.0001 |
| Not specified, n (%) | 14905 (3.8%) | 1046 (7.0%) | <0.0001 |
|
| |||
| Asthma, n (%) | 42148 (10.8%) | 2537 (17.0%) | <0.0001 |
|
| |||
| CAD, n (%) | 30002 (7.7%) | 8840 (59.1%) | <0.0001 |
|
| |||
| COPD, n (%) | 48989 (12.5%) | 6077 (40.7%) | <0.0001 |
|
| |||
| Diabetes, n (%) | 59203 (15.1%) | 6860 (45.9%) | <0.0001 |
|
| |||
| Heart failure, n (%) | 6003 (1.5%) | 1563 (10.4%) | <0.0001 |
|
| |||
| Pneumovax, n (%) | 4397 (1.1%) | 378 (2.5%) | <0.0001 |
|
| |||
| Stroke, n (%) | 14847 (3.8%) | 4678 (31.3%) | <0.0001 |
|
| |||
| Dementia, n (%) | 20851(5.3%) | 3212 (21.5%) | <0.0001 |
Legend: CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; Pneumovax® (pneumococcal conjugate vaccine)
Primary Endpoints
The incidence of pneumonia was higher by unadjusted analysis in patients with at least six consecutive clopidogrel claims (5,166 patients; 34.6%) compared to patients with no clopidogrel claims (52,809 patients; 13.5%). The estimated OR comparing clopidogrel-treated patients to those with no clopidogrel claims was 3.39 (95% CI 3.27-3.51, p <0.0001). After adjustment for known pneumonia risk factors, the risk associated with clopidogrel use was diminished but still statistically significant (ORadj [estimated adjusted odds ratio] 1.48, 95% CI 1.41-1.55, p<0.0001). (Table 2)
Table 2.
CAP Incidence
| No Clopidogrel Prescription (n=391503) |
≥ 6 Consecutive Clopidogrel Prescriptions (n=14947) |
Estimated Odds Ratio (95% CI) p value |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| CAP†, n (%) | 52809 (13.5%) | 5166 (34.6%) | 3.39 (3.27-3.51) p<0.0001 |
1.48 (1.41-1.55) p<0.0001 |
Legend: CAP: community-acquired pneumonia
Of those with a diagnosis of pneumonia, 2,908 of 5,166 patients (56.3%) with at least six consecutive clopidogrel claims were more likely (OR 1.96; 95% CI 1.85-2.07, p <0.0001) to be treated as inpatients than the patients with no clopidogrel claims (20,974 of 52,809 patients; 39.7%). When adjusted for covariates, no statistically significant elevation in the probability of inpatient treatment was associated with clopidogrel treatment (ORadj = 1.00, 95% CI 0.94-1.07, p=0.88). (Table 3)
Table 3.
CAP Site of Care and Secondary Severity Indices
| No Clopidogrel Prescription |
≥ 6 Consecutive Clopidogrel Prescriptions |
Estimated Odds Ratio (95% CI) p value |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
|
Among those with
CAP |
n=52809 | n=5166 | ||
| Inpatient, n (%) | 20974 (39.7%) | 2908 (56.3%) | 1.96 (1.85-2.07) p<0.0001 |
1.00 (0.94-1.07) p=0.88 |
|
Among inpatients
with CAP |
n=20974 | n=2908 | ||
| ICU Admission, n (%) |
739 (3.5%) | 97 (3.3%) | 0.94 (0.76-1.17) p=0.61 |
1.06 (0.84-1.33) p=0.65 |
| Mechanical Ventilation, n (%) |
398 (1.9%) | 41(1.4%) | 0.74 (0.54-1.02) p=0.07 |
0.90 (0.64-1.28) p=0.57 |
| Sepsis, n (%) | 184 (0.9%) | 29 (1.0%) | 1.14 (0.77-1.68) p=0.52 |
1.21 (0.79-1.86) p=0.37 |
| ARDS/ALI, n (%) | 452 (2.2%) | 50 (1.7%) | 0.79 (0.59-1.07) p=0.13 |
1.01 (0.74-1.39) p=0.95 |
| Mortality, n (%) | 160 (0.8%) | 14 (0.5%) | 0.63 (0.36-1.09) p=0.10 |
0.71 (0.40-1.25) p=0.23 |
| Composite Severity*, n (%) |
1483 (7.1%) | 180 (6.2%) | 0.87 (0.74-1.02) p=0.09 |
1.00 (0.84-1.19) p=0.98 |
Legend: ALI: acute lung injury; ARDS: acute respiratory distress syndrome; CAP: community-acquired pneumonia; ICU: intensive care unit.
Among CAP inpatients, those with active clopidogrel prescriptions had a higher incidence of sepsis (OR 1.62, 95% CI 1.06-2.46, p = 0.02) than those with no clopidogrel prescriptions. A significant difference was retained upon adjustment for covariates (OR 1.73, 95% CI 1.10-2.73, p = 0.02), contributing to a near-significant trend on the composite severity endpoint after adjustment (OR 1.20, 95% CI 0.98-1.46, p = 0.08).
Secondary Severity Endpoints
By unadjusted analyses, there were trends towards reduced incidences of mechanical ventilation, mortality, and the composite severity endpoint among CAP inpatients treated with clopidogrel, with a numerical reduction in the incidence of ARDS/ALI (Table 3). Compared to CAP inpatients without clopidogrel prescriptions, CAP inpatients with at least six consecutive clopidogrel prescriptions had a lower incidence of mechanical ventilation (OR 0.74, 95% CI 0.54-1.02, p = 0.07); mortality (OR 0.63, 95% CI 0.36-1.09, p = 0.10); and the composite severity endpoint (OR 0.87, 95% CI 0.74-1.02, p = 0.09). These trends were not significant after adjustment for covariates. There was a 21% reduction in risk of ARDS/ALI in clopidogrel-treated patients (OR 0.79, 95% CI 0.58-1.07, p = 0.13) in unadjusted analysis only, although it did not reach statistical significance. With or without adjustment for covariates, there were no significant differences or trends thereto for ICU admission or sepsis endpoints.
Meta-Analyses
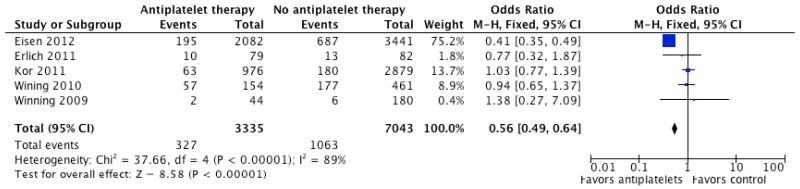
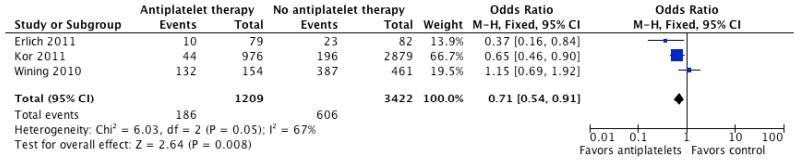
Based on the trend towards reduced incidence of ARDS/ALI in hospitalized CAP who had been previously prescribed clopidogrel, we performed a separate pooled analyses of observational studies in which the effect of anti-platelet therapy in pneumonia, critical illness, and/or acute lung injury was reported. As shown in Table 4, five studies were identified that reported outcomes in individuals hospitalized for pneumonia critical illness by anti-platelet therapy at presentation. Two of the studies limited the analysis to aspirin (acetyl salicylic acid) use only. In the pooled analysis, anti-platelet therapy was associated with significantly lower odds of short-term mortality (OR 0.56, 95% CI 0.49 to 0.64, p <0.00001; Figure 1). When lung injury and need for mechanical ventilation was reported, anti-platelet therapy was associated with a significant reduction in those outcome (OR 0.71, 95% CI 0.54 to 0.91, p =0.008; Figure 2). There was no statistically significant difference in ICU or hospital stay in patients who presented on anti-platelet therapy as compared to those who did not.
Table 4.
Effects of Active Clopidogrel Treatment in Patients with CAP
| No Clopidogrel Prescription (n= 52809) |
Active Clopidogrel Prescription (n=3053) |
Estimated Odds Ratio (95% CI) p value |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
|
Among those with
CAP |
n = 52809 | n = 3053 | ||
| Inpatient Treatment, n (%) |
20974 (39.7%) | 1771 (58.0%) | 2.10 (1.95-2.26) p<0.0001 |
1.05 (0.96-1.14) p=0.29 |
|
Among inpatients
with CAP |
n = 20974 | n = 1771 | ||
| ICU Admission, n (%) |
739 (3.5%) | 66 (3.7%) | 1.06 (0.82-1.37) p=0.66 |
1.17 (0.89-1.54) p=0.25 |
| Mechanical Ventilation, n (%) |
398 (1.9%) | 24 (1.4%) | 0.71 (0.47-1.08) p=0.11 |
0.87 (0.57-1.35) p=0.54 |
| Sepsis, n (%) | 184 (0.9%) | 25 (1.4%) | 1.62 (1.06-2.46) p=0.02 |
1.73(1.10-2.73) p=0.02 |
| ARDS/ALI, n (%) | 452 (2.2%) | 32 (1.8%) | 0.84 (0.58-1.2) p=0.33 |
1.07 (0.73-1.56) p=0.74 |
| Mortality, n (%) | 160 (0.8%) | 14 (0.8%) | 1.04 (0.60-1.80) p=0.90 |
1.11 (0.63-1.96) p=0.71 |
| Composite Severity*, n (%) |
1483 (7.1%) | 128 (7.2%) | 1.05 (0.87-1.26) p=0.63 |
1.20 (0.98-1.46) p=0.08 |
Legend: ALI: acute lung injury; ARDS: acute respiratory distress syndrome; CAP: community-acquired pneumonia; ICU: intensive care unit
Composite severity was used to designate any patient who had one of the above secondary severity indicators.
Figure 1.
Forest plot of unadjusted odds ratio (95% confidence intervals) for short-term mortality in patients on anti-platelet therapy as compared to controls in retrospective studies. A significant decrease in mortality is noted in patients on anti-platelet therapy with an odds ratio of 0.56 (CI; 0.49-0.64, P < 0.001).
Figure 2.
Forest plot of unadjusted odds ratio (95% confidence intervals) for lung injury and mechanical ventilation in patients on anti-platelet therapy as compared to controls in retrospective studies. A significant decrease in mortality is noted in patients on anti-platelet therapy with an odds ratio of 0.71 (CI; 0.54-0.91, P < 0.01).
Discussion
Our retrospective, cohort study of the effects of clopidogrel on the incidence and severity of CAP in a large Medicaid database indicated that CAP was more common among patients taking clopidogrel. This association was largely attributable to differences in comorbidities and pneumonia risk factors between groups, and the magnitude of risk was reduced but not eliminated after adjustment for risk factors. Patients with clopidogrel prescriptions were more likely to be hospitalized for pneumonia, although this difference was not statistically significantly after adjustment for covariates. There were no significant differences in secondary severity indices, however, several near-significant trends in need for mechanical ventilation, risk of ARDS/ALI, and mortality favored clopidogrel treatment. These observations were supported by pooled analysis of studies reporting the association of anti-platelet therapy and outcomes in patients hospitalized for pneumonia, at risk for ALI, and/or critically ill. Pooled analyses revealed an association between anti-platelet therapy and reduction in (1), short-term mortality and (2), incidence of ALI and need for mechanical ventilation in this patient population. While significant, the reduction in mortality was mainly driven by the large study of Eisen et al., which may hamper the ability to generalize the results. Nonetheless, the results of the other studies and our overall analyses are consistent with the conclusions that antiplatelet therapy is not associated with increased harm and could, rather, be beneficial in patients admitted to the ICU. It is possible that these observations reflect a role for platelet activation in inflammation and immunity. Platelet activation causes the expression of cell surface receptors and release of molecules that can amplify the immune response. Specifically, expression of P-selectin increases platelet-leukocyte interactions, which associate with the severity of sepsis and septic organ dysfunction.(5, 22, 23) Platelet surface expression of CD40L and its subsequent binding to the CD40 receptor may also be involved in immune cell recruitment, chemokine production, IgM to IgG isotype switching, and dendritic cell maturation.(1, 24) Platelet α-granules contain chemokines and other soluble mediators of inflammation such as IL-1β, regulated on activation normal T cell expressed and secreted (RANTES) protein, macrophage inflammatory protein (MIP-1α), monocyte chemoattractant protein (MCP), thymus and activation-regulated chemokine (TARC), and antimicrobial defensins. (1, 5, 25, 26) Platelet-activating factor (PAF) is primarily expressed by platelets, and PAF receptor deficiency has been shown to improve the immune response to pneumococcal pneumonia.(27) Platelets also express toll-like receptors (TLRs), pathogen recognition receptors involved in activation of innate immunity, including TLR2 and TLR4 that recognize the common bacterial molecules peptidoglycan and lipopolysaccharide (LPS), respectively.(28-31) Interactions between platelet TLR4 and LPS may play a role in thrombocytopenia in sepsis and pulmonary fibrin deposition. Activated platelets, particularly in the context of LPS stimulation, trigger the release of extracellular DNA traps (NETs) from neutrophils. These DNA strands with proteolytic activity serve to capture and degrade microbes. Thus, immune-mediated effects of platelets may be important for the host defense response. At the same time, platelet activation may exacerbate acute lung injury by promoting the recruitment of neutrophils and release of pro-inflammatory mediators.(32)
Our findings that use of clopidogrel may be associated with increased risk of CAP and hospitalization as well as a trend towards reduced severity indices in hospitalized patients would be consistent with a biphasic role for platelets in lung infection and its sequelae. Platelets may be important in the initial clearance of pathogens. This is supported by the increased risk of sepsis seen among clopidogrel-treated patients. Once the infection progresses systemically, inhibition of platelet function may have beneficial consequences, especially in regards to mechanical ventilation, ALI, and ARDS. Further investigations can determine the magnitude of this effect.
Limitations to our cohort study include those inherent in its retrospective design, and approximation of clinical outcomes from a billing database. Coding may not accurately reflect endpoints and may affect quantification of the effects of clopidogrel treatment found in our study sample. The temporal relationship between clopidogrel treatment and pneumonia could only be determined in those patients with a CAP diagnosis, thereby limiting our ability to draw inferences by comparing patients with at least six consecutive clopidogrel prescriptions to patients with none. Although we used prescription claims in consecutive 30-day periods to approximate adherence, medication compliance could not be definitively determined. Due to over-the-counter use, aspirin therapy could not be monitored and its use or other medications that track with clopidogrel may confound results. We cannot distinguish a specific benefit of clopidogrel because many patients with prescriptions for clopidogrel would be expected to have one or more indications for lifetime aspirin or other medications for prevention of cardiovascular disease. Indeed, most patients in the clopidogrel group were likely receiving aspirin at the time of CAP diagnosis. It is also possible that the observed differences might have been diminished by unrecorded aspirin use in those without prescriptions for clopidogrel. Use of antiplatelet therapy may simply be a marker for the causative factor, for example, statin use. Finally, medical contact may be greater in patients who are receiving prescriptions for clopidogrel. If so, they may have more likely to be diagnosed with pneumonia and may have had a higher likelihood of being prescribed antibiotics early due to closer follow-up in the healthcare system. We were unable to monitor either access to healthcare or timing (and appropriateness) of initial antibiotic therapy, both of which could have confounded our results.
In summary, we report the first assessment of the impact of an antiplatelet drug on incident and severity of CAP. The trend towards reduced secondary pneumonia severity endpoints observed in our cohort analysis and the findings of the meta-analysis should stimulate future prospective investigation to determine if anti-platelet therapy can modulate the severity of outcomes related to pulmonary or systemic infection. Clinically, this may necessitate an evaluation of whether antiplatelet therapies are continued in critically ill patients. This area of research will continue to evolve in both clinical and translational arenas.
Acknowledgements
A. Kendall Gross, PharmD and Susan S. Smyth, MD, PhD are the guarantors of this manuscript. This work was partially supported by NIH grants HL080166 (SSS) and RR021954 (ZL, SSS). This material is the result of work supported with the resources and/or use of the facilities at the Lexington VA Medical Center. No relationships or financial associations exist with industry that might pose a conflict of interest in connection with this article.
The authors would like to acknowledge the contributions of Douglas Steinke, PhD, Jeffery Talbert, PhD, Darren Henderson, and Adam Lindstrom for their assistance with database administration and statistical analyses, and Susan Quick for editorial assistance.
This work was performed at the University of Kentucky.
List of abbreviations
- ADP
adenosine diphosphate
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- CAD
coronary artery disease
- CAP
community acquired pneumonia
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CURB65
confusion, uremia, respiratory rate, low BP, age 65 or greater
- HF
heart failure
- ICU
intensive care unit
- LPS
lipopolysaccharide
- OR
estimated odds ratio
- ORadj
estimated adjusted odds ratio
- PCV
pneumococcal conjugative vaccine
- PSI
pneumonia severity index
- TLR
toll-like receptors
Footnotes
Author contributions:
Kendall Gross: Study design, data collection and analysis, wrote manuscript
Steven P. Dunn: Study design and analysis, manuscript edits
David J. Feola: Data collection, study analysis, manuscript edits
Craig A. Martin: Data collection, study design and analysis, manuscript edits
Richard Charnigo: Data analysis, manuscript edits
Zhenyu Li: Study design and analysis, manuscript edits
Ahmed Abdel-Latif: Data collection and analysis, manuscript edits
Susan S. Smyth: Study design, data collection and analysis, wrote manuscript
No conflicts of interest.
Contributor Information
A. Kendall Gross, Pharmaceutical Services, UCSF Medical Center and Clinical Pharmacy, School of Pharmacy, University of California, San Francisco, San Francisco, CA kendall.gross@ucsfmedctr.org.
Steven P. Dunn, Pharmacy Services, University of Virginia Health System, Charlottesville, VA spdunn@virginia.edu.
David J. Feola, Pharmacy Practice and Science, College of Pharmacy, University of Kentucky, Lexington, KY djfeol2@email.uky.edu.
Craig A. Martin, Pharmacy Services, UK HealthCare and Pharmacy Practice and Science, College of Pharmacy, University of Kentucky, Lexington, KY craig.martin@uky.edu.
Richard Charnigo, Department of Biostatistics, College of Public Health, University of Kentucky, Lexington, KY rjcharn2@aol.com.
Zhenyu Li, Division of Cardiovascular Medicine, Gill Heart Institute, University of Kentucky, Lexington, KY zli226@email.uky.edu.
Ahmed Abdel-Latif, Lexington VA Medical Center and Division of Cardiovascular Medicine, Gill Heart Institute, University of Kentucky, Lexington, KY abdel-latif@uky.edu.
Susan S. Smyth, Lexington VA Medical Center and Division of Cardiovascular Medicine, Gill Heart Institute, University of Kentucky, Lexington, KY.
References
- 1.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 2.Evangelista V, Manarini S, Dell’Elba G, Martelli N, Napoleone E, Di Santo A, Savi P, Lorenzet R. Clopidogrel inhibits platelet-leukocyte adhesion and platelet dependent leukocyte activation. Thrombosis and Haemostasis. 2005;94:568–77. [PubMed] [Google Scholar]
- 3.Bayat B, Werth S, Sachs UJH, Newman DK, Newman PJ, Santoso S. Neutrophil Transmigration Mediated by the Neutrophil-Specific Antigen CD177 Is Influenced by the Endothelial S536N Dimorphism of Platelet Endothelial Cell Adhesion Molecule-1. J Immunol. 2010;184:3889–96. doi: 10.4049/jimmunol.0903136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese S, de la Motte C, Reyes BMR, Sans M, Levine AD, Fiocchi C. Cutting Edge: T Cells Trigger CD40-Dependent Platelet Activation and Granular RANTES Release: A Novel Pathway for Immune Response Amplification. J Immunol. 2004;172:2011–5. doi: 10.4049/jimmunol.172.4.2011. [DOI] [PubMed] [Google Scholar]
- 5.Diacovo T, Roth S, Buccola J, Bainton D, Springer T. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–57. [PubMed] [Google Scholar]
- 6.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or Adversary: Platelets in Lung Biology, Acute Injury, and Inflammation. American Journal of Respiratory Cell and Molecular Biology. 2009;40:123–34. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evangelista V, Dell’Elba G, Martelli N, Amore C, Pecce R, Piccoli A, Manarini S, Totani L. “Anti-inflammatory effects of clopidogrel in the mouse” Abstract presented at: Congress of the International Society on Thrombosis and Haemostasis; Geneva, Switzerland. 2007.Jul, [Google Scholar]
- 8.Klinkhardt U, Bauersachs R, Adams J, Graff J, Lindhoff-Last E, Harder S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease[ast] Clinical Pharmacology and Therapeutics. 2003;73:232–41. doi: 10.1067/mcp.2003.13. [DOI] [PubMed] [Google Scholar]
- 9.Hermann A, Rauch BH, Braun M, Schrör K, Weber A-A. Platelet CD40 ligand (CD40L) – subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001;12:74–82. doi: 10.1080/09537100020031207. [DOI] [PubMed] [Google Scholar]
- 10.Antonino MJ, Mahla E, Bliden KP, Tantry US, Gurbel PA. Effect of Long-Term Clopidogrel Treatment on Platelet Function and Inflammation in Patients Undergoing Coronary Arterial Stenting. The American Journal of Cardiology. 2009;103:1546–50. doi: 10.1016/j.amjcard.2009.01.367. [DOI] [PubMed] [Google Scholar]
- 11.Graff J, Harder S, Wahl O, Scheuermann E-H, Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: Effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers[ast] Clin Pharmacol Ther. 2005;78:468–76. doi: 10.1016/j.clpt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Steinhubl SR, Badimon JJ, Bhatt DL, Herbert J-M, Lüscher TF. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vascular Medicine. 2007;12:113–22. doi: 10.1177/1358863X07077462. [DOI] [PubMed] [Google Scholar]
- 13.Winning J, Reichel J, Eisenhut Y, Hamacher J, Kohl M, Deigner HP, Claus RA, Bauer M, Lösche W. Anti-platelet drugs and outcome in severe infection: Clinical impact and underlying mechanisms. Platelets. 2009;20:50–7. doi: 10.1080/09537100802503368. [DOI] [PubMed] [Google Scholar]
- 14.Aslam R, Speck ER, Kim M, Crow AR, Bang KWA, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-{alpha} production in vivo. Blood. 2006;107:637–41. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 15.Winning J, Neumann J, Kohl M, Claus RA, Reinhart K, Bauer M, Losche W. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit. Critical Care Medicine. 2010;38:32–7. doi: 10.1097/CCM.0b013e3181b4275c. [DOI] [PubMed] [Google Scholar]
- 16.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization Antiplatelet Therapy Is Associated With a Reduced Incidence of Acute Lung Injury. Chest. 2011;139:289–95. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neal HR, Jr., Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–50. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandell Lionel A, Wunderink Richard G, Anzueto A, Bartlett John G, Campbell G D, Dean Nathan C, Dowell Scott F, File J, Thomas M, Musher Daniel M, Niederman Michael S, Torres A, Whitney Cynthia G. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Communityâ€Acquired Pneumonia in Adults. Clinical Infectious Diseases. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson Michael L, Neuzil Kathleen M, Thompson William W, Shay David K, Yu O, Hanson Christi A, Jackson Lisa A. The Burden of Community-Acquired Pneumonia in Seniors: Results of a Population-Based Study. Clinical Infectious Diseases. 2004;39:1642–50. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Losche W. Platelet and Leukocyte Activation Correlate with the Severity of Septic Organ Dysfunction. Shock. 2002;17:263–8. doi: 10.1097/00024382-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland H, K W. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. European Journal of Clinical Investigation. 1995;25:843–51. doi: 10.1111/j.1365-2362.1995.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 24.Pitchford SC. Novel uses for anti-platelet agents as anti-inflammatory drugs. British Journal of Pharmacology. 2007;152:987–1002. doi: 10.1038/sj.bjp.0707364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Hundelshausen P, Weber KSC, Huo Y, Proudfoot AEI, Nelson PJ, Ley K, Weber C. RANTES Deposition by Platelets Triggers Monocyte Arrest on Inflamed and Atherosclerotic Endothelium. Circulation. 2001;103:1772–7. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. Journal of Cell Biology. 2001;154:485–90. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rijneveld AW, Weijer S, Florquin S, Speelman P, Shimizu T, Ishii S, van der Poll T. Improved Host Defense against Pneumococcal Pneumonia in Platelet-Activating Factor Receptor-Deficient Mice. Journal of Infectious Diseases. 2004;189:711–6. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential Roles of TLR2 and TLR4 in Recognition of Gram-Negative and Gram-Positive Bacterial Cell Wall Components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 29.Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K-i, Kawashima S, Yokoyama M. Expression of Toll-like receptors on human platelets. Thrombosis Research. 2004;113:379–85. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Andonegui G, Kerfoot SM, McNagny K, Ebbert KVJ, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–23. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide Stimulates Platelet Secretion and Potentiates Platelet Aggregation via TLR4/MyD88 and the cGMP-Dependent Protein Kinase Pathway. The Journal of Immunology. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynn MR. Instrument reliability and validity: how much needs to be published? Heart Lung. 1989;18:421–3. [PubMed] [Google Scholar]