Previews
Osteomyelitis is a debilitating bone infection often caused by the bacterial pathogen Staphylococcus aureus. In this issue, Cassat and colleagues (2013) develop a high-resolution micro-computed tomography (microCT) method to visualize bone remodeling during S. aureus infection and discover that the metalloprotease aureolysin plays a critical role in modulating osteomyelitis pathogenesis.
Osteomyelitis is a bacterial infection of the bone or bone marrow for an alarming number of patients that are admitted to hospitals (Lew and Waldvogel, 2004). Staphylococcus aureus is a prominent bacterial pathogen and the most frequent isolated causal agent of infection-induced osteomyelitis (Hatzenbuehler and Pulling, 2011). Treatment of osteomyelitis infections is challenging due a variety of factors, including the poor bioavailability of antibiotics in bone tissue, rising antibiotic resistance in bacterial pathogens, and the biofilm-like properties of the infection. In many cases, physicians are left with surgical debridement as the only option to remove the invading bacteria and achieve a sterile site. Where an infected prosthetic joint is involved, removal and replacement of the joint is often required (Zimmerli et al., 2004). Due to these challenges, osteomyelitis is devastating for many patients with painful long-term consequences and limited treatment options.
Improving the clinical outcomes of osteomyelitis infections would be a significant medical advance. Our current limited knowledge of disease progression and the availability of technologies to monitor these events is an unmet need that has hampered ongoing studies. In this issue of Cell Host & Microbe, Cassat et al. (2013) address these challenges by developing and utilizing a novel three-dimensional imaging modality, called high-resolution micro-computed tomography (microCT), that can visualize and quantify infectious damage to the bone. To perform microCT, a new model of murine osteomyelitis was developed and confirmed using a community-acquired methicillin-resistant S. aureus isolate of the USA300 type, an emerging cause of musculoskeletal infections (Kourbatova et al., 2005). In the murine model, a sterile 1 mm unicortical bone defect is created, serving as an S. aureus inoculation point to establish osteomyelitis. To monitor disease progression, microCT is performed to visualize cortical bone destruction and new bone formation, and computational algorithms translate these changes into a three-dimensional image. The end result is a visual reconstruction that captures a striking snapshot of bone remodeling as a result of S. aureus infection (see Figure 1). The developed imaging software provides informative volume outputs of bone loss and new bone growth that serve an important role in quantifying osteomyelitis-related damage. To verify the microCT findings, the authors (Cassat et al., 2013) performed histopathology on the murine model. Thus, the methods developed in this paper make it possible for the first time to monitor and quantify osteomyelitis disease using a powerful new imaging platform.
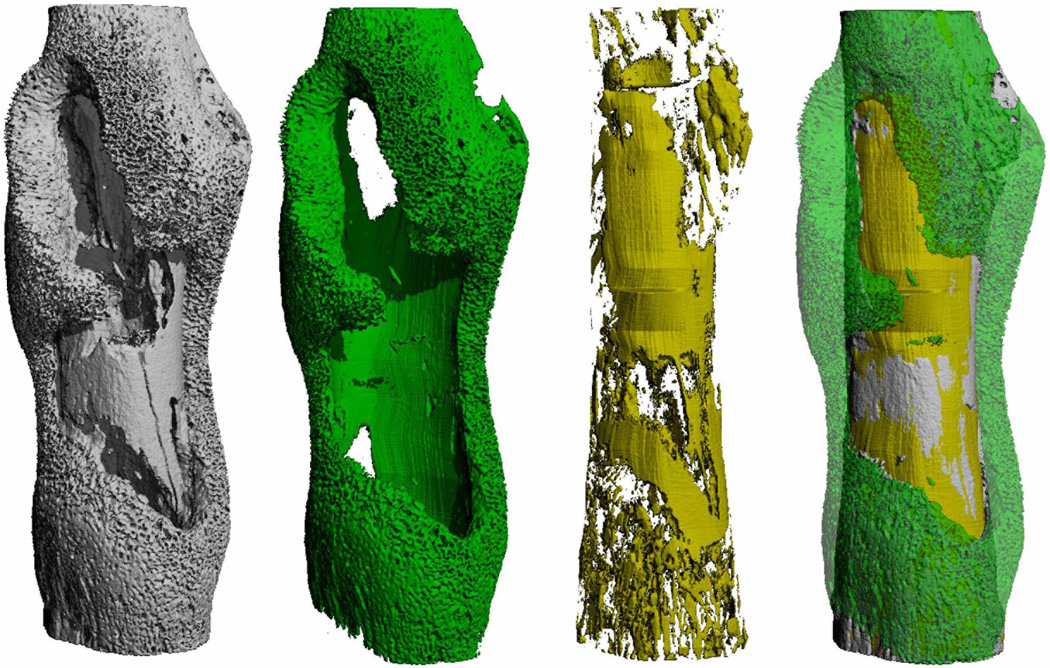
Figure 1. Imaging S. aureus-induced pathologic bone remodeling during osteomyelitis.
S. aureus triggers profound alterations in bone remodeling during osteomyelitis (left image). Cassat et al. (2013) created imaging analysis algorithms to precisely quantify pathologic new bone formation (green) and cortical bone destruction (yellow), enabling comparison of the destructive capacity of different staphylococcal strains. Image provided courtesy of Dr. James Cassat, Vanderbilt University School of Medicine, USA.
To gain insight on S. aureus mechanisms of osteomyelitis pathogenesis, Cassat et al. (2013) employed the microCT imaging technology on USA300 and isogenic mutants in previously described key pathogenesis regulators. Due to the limited knowledge of contributing factors to this disease, a S. aureus strain lacking two global virulence regulators (Agr and Sae systems) was tested in the murine model. The double mutant showed significantly less cortical bone destruction and reduced new bone formation, indicating that secreted virulence factors were likely critical contributors to disease. To focus the subsequent studies, single mutants were tested, and a sae mutant caused less bone destruction and a significant decrease in S. aureus burden as compared to an agr mutant. To confirm these observations and delve deeper into mechanism, in vitro cell-culture assays of osteoblasts indicated that indeed the sae mutant displayed a less virulent phenotype. Further, proteomic approaches were used to investigate exoproteome changes and uncovered 49 proteins down-regulated and 31 proteins up-regulated in the sae mutant. Interestingly, the bacterial metalloprotease aureolysin was most enriched in abundance, a fact supported by previous regulatory studies on sae mutants.
Building on previous reports on how aureolysin modulates pathogenic capacity (Zielinska et al., 2011), Cassat et al. (2013) hypothesized that aureolysin functions as the primary switch responsible for inducing bone remodeling during infection by tailoring the S. aureus secreted virulence factor arsenal. Aureolysin is a calcium and zinc-dependent metalloenzyme and one of the four major extracellular proteases secreted by S. aureus strains. The enzyme self-activates upon secretion, preferentially cleaves on the amino-terminal side of branched-chain amino acids, and serves as the starting point of the proteolytic activation cascade. The authors (Cassat et al., 2013) compared exoproteome pools to identify proteins that were aureolysin labile. One group of proteins that stood out were the alpha-type phenol-soluble modulins (PSMα), which is a collection of four low MW peptide toxins with known functions in neutrophil killing and infection. Assessment of a S. aureus strain lacking PSMα1–4 indicated this mutant had pronounced defects in osteoblast killing as well as significant attenuation in bone destruction and new bone growth in the murine model. Follow-up studies relying on exogenous peptide addition demonstrated that chemically synthesized PSMα2 was the most cytotoxic to osteoblasts, whereas PSMα4 was relatively non-toxic. Pre-treatment of PSMα peptides with aureolysin greatly diminished their cytotoxic properties to osteoblasts, supporting previous studies that these peptides serve as aureolysin substrates (Gonzalez et al., 2012). Thus, by controlling the extracellular concentration of one enzyme, S. aureus is able to modulate the potency of its own secreted proteome and dictate the course of osteomyelitis and potentially other infections. While the authors focused on the contribution of PSMα peptides to osteomyelitis, proteomic analysis demonstrated numerous other factors are influenced by aureolysin levels, indicating that the exoproteome is a dynamic entity and aureolysin functions as a rheostat to modulate pathogenic capacity.
Going beyond this study, the development of the new microCT imaging platform has the potential to assess osteomyelitis with other pathogens or related bone infections. A number of other bacterial pathogens cause osteomyelitis, including Staphylococcus epidermidis, Streptococcus pyogenes, Acinetobacter baumanni, Pseudomonas aeruginosa, and Escherichia coli, and one could easily imagine applying this murine model and microCT approach to monitoring disease progression and follow-up mechanistic analysis with any of these pathogens. Additionally, as the author’s state (Cassat et al., 2013), the work could serve as means to test chemo- or immunotherapeutic approaches to osteomyelitis treatments, taking advantage of the imaging platform as a means of monitoring and quantifying bone damage and regrowth. There is also potential to apply the murine model and microCT approach to other related types of bone infections. S. aureus is a leading cause of prosthetic-joint infections (Kourbatova et al., 2005, Zimmerli et al., 2004), and the numbers of patients getting joint replacements is increasing. Infection rates following the initial replacement are usually low, only 1–2%, but increase substantially in revision surgery (Lentino, 2003). When an infection occurs, it can be a devastating complication having an associated high morbidity and substantial financial burden. Extending development of the microCT platform, it may be possible to assess disease progression in the presence of the implanted materials, allowing additional mechanisms of pathogenesis to be deciphered in this context. Going further, there is special consideration in orthopaedic surgery for the high rate of infections in osteoarticular allograft reconstructions. These reconstructions permit reestablishment of skeletal continuity and function after a wide resection of bone tumor, and allografts are increasingly used in salvage of difficult bone stock deficiencies following failed total joint replacements (Muscolo et al., 2005). However, allograft infection is a disastrous complication as it may lead to limb amputation (Mankin et al., 2005), and again S. aureus is one of the most common causes. A better understanding of the mechanism underlying these infections through further development of the murine model and microCT platform could greatly improve the outcomes of surgical reconstructions.
In recent years, many exciting advances in infection imaging have been pioneered by researchers that may eventually aid a physician’s assessment and treatment of certain diseases. The microCT imaging modality developed in this work is another impressive example that can be added to the expanding toolbox of approaches for monitoring disease course. Further development and translation of this technology has great potential to improve the disease prognosis for osteomyelitis patients by both facilitating the ability to track infection progression and aiding in the discovery and testing of innovative therapeutic interventions. Presently, this technology can be applied to enhance our knowledge of Staphylococcus aureus mechanisms of osteomyelitis and potentially extend to other bacterial pathogens as well. With the knowledge gained from imaging of this murine model, therapies that limit osteomyelitis-dependent bone destruction and enhance antimicrobial efficacy could eventually lead to improved patient outcomes for this devastating disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, Smeltzer MS, Torres VJ, Skaar EP. A secreted protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host & Microbe. 2013 doi: 10.1016/j.chom.2013.05.003. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DJ, Okumura CY, Hollands A, Kersten R, Akong-Moore K, Pence MA, Malone CL, Derieux J, Moore BS, Horswill AR, Dixon JE, Dorrestein PC, Nizet V. Novel phenol-soluble modulin derivatives in community-associated methicillin-resistant Staphylococcus aureus identified through imaging mass spectrometry. J Biol Chem. 2012;287:13889–13898. doi: 10.1074/jbc.M112.349860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84:1027–1033. [PubMed] [Google Scholar]
- Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am J Infect Control. 2005;33:385–391. doi: 10.1016/j.ajic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–1161. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J Bone Joint Surg Am. 2005;87:2449–2455. doi: 10.2106/JBJS.D.02170. [DOI] [PubMed] [Google Scholar]
- Zielinska AK, Beenken KE, Joo HS, Mrak LN, Griffin LM, Luong TT, Lee CY, Otto M, Shaw LN, Smeltzer MS. Defining the strain-dependent impact of the Staphylococcal accessory regulator (sarA) on the alpha-toxin phenotype of Staphylococcus aureus. J Bacteriol. 2011;193:2948–2958. doi: 10.1128/JB.01517-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]