Abstract
Background:
Plastination is a new method of preserving tissue samples for a long time. This study aimed to compare the new plastination technique with the conventional preservative method in glycerin for fetus skeleton tissues and young rats dyed by Alizarin red- Alcian blue double staining.
Materials and Methods:
In this study, 4 groups of 1-day, 3-day, 12-day and mature rats were selected and, after being anesthetized and slaughtered, their skin was completely removed. In Alizarin red- Alcian blue double staining method, first the samples were fixed in 95% ethanol and then their cartilages were dyed by 0.225% Alcian blue solution; after that, they were cleared in 1% KOH. Then, the bones were dyed in 0.003% Alizarin red solution and finally the tissue was decolorized in 95% ethanol. In each group, half of the samples were preserved by the conventional method in a glycerin container and the other half were plastinated.
Results:
In the present study, the samples preserved by plastination technique were dry, odorless, indecomposable and tangible. Quality of coloring had an inverse relationship with rats’ age. Transparency of the plastinated samples had also an inverse relationship with rats’ age. Therefore, skeletal tissue of younger rats had higher quality and transparency in both preservation methods (glycerin and plastination).
Conclusion:
This study showed that plastination technique was an appropriate method in comparison with glycerin preservation, which conserved skeletal tissue of fetus and young rats colored by Alizarin red- Alcian blue double staining. And the final result was that plastination technique can generate dry, odorless, indecomposable and tangible samples.
Keywords: Double staining, plastination, rat
INTRODUCTION
One of the best methods of learning about anatomy is by observing natural samples, the aim of which is to gain better understanding of structure of body, organs and limbs. In this regard, skeleton investigation has significant importance.
In the study of fetus and small mature samples, it is very important to prepare an intact anatomic skeleton sample with correct orientation. Previously, in order to prepare skeletons, small samples were buried in soil for several months and then the complete skeleton was prepared after assembling the bones; one of the problems of this method was that, when the sample was unearthed, the remaining parts were a large number of small bones, the joints of which were destroyed and also easily lost in the soil. Another technique was boiling in a solution, in which the joints were destroyed and the bone components were damaged.[1,2]
Alizarin red-Alcian blue double staining is a simple and effective yet inexpensive technique for observing and evaluating appearance and even details of fetus skeleton and small samples without using expensive techniques like X-ray.[3] The first skeleton staining was performed by Alen B. Dawson in 1926.[4] For the first time, Inouye (1976) reported Alizarin red-Alcian blue double staining on skeletal and cartilage tissue.[5] Structural appearance evaluation using this technique allows for better understating of the performance of anatomical system and even evaluation of body structure of different species.[6,7] The conventional method of preserving stained samples in glycerin containers had many disadvantages including problems in samples’ displacement, fragility and inconvenience for educational purposes. These samples are only observable and cannot be touched or rotated in different directions, which is considered a disadvantage from educational point of view.
Plastination is a new method of preserving tissue samples for a longer period of time, which was invented by a German professor Gounther Von Hagens in 1977. In this technique, by removing the interstitial water and replacing it by specific polymers, an odorless, touchable and dry sample is obtained, which is completely similar to the natural one and is suitable for educational purposes and biology museums.[8] This technique can resolve the above-mentioned disadvantages of the method of preserving stained samples in glycerin.
The purpose of this study was to apply volumetric and sheet plastination technique for preserving the samples dyed by Alizarin red-Alcian blue double staining method and making a comparison with the conventional method of preserving the dyed samples in glycerin containers. Finally, color quality, tissue transparency, consolidation and ease of applicability of the samples were compared with each other.
MATERIALS AND METHODS
Animals preparation
Two groups of rats at different ages were selected, which included mature rat, newborn rat, 3-day rat and 12-day rat. Rats were anesthetized and slaughtered by chloroform, and then the samples,’ skin and viscera were removed. To expose the skeleton and to examine the effect of staining, the muscular tissue on one side of the rat bodies was completely removed. One mature rat was selected to prepare stained and plastinated saggital sections. After being anesthetized by chloroform and dying, the mature rat was frozen at -35°C. Then, the sample was molded using a specific polyurethane resin and finally it was cut into sections with 4-mm thickness using a special band saw.[9]
Steps for alizarin red-Alcian blue double staining
Fixation
In order to avoid decomposition of the tissues over time, before performing other steps, the samples were kept in 95% ethanol for 15-20 days to make them fixated. After cutting by the special band saw, the sections of the mature rat were also kept in 95% ethanol to fixate completely.
Staining the cartilage
0.0225% Alcian blue solution with 4:1 ratio of 95% ethanol and glacial acetic acid was prepared and the samples were kept in the solution for 3 days until the cartilage was completely stained. Then, the samples were taken out of the dye and were rinsed until stain sediments were removed from the tissue. The samples were then cleared in 1% KOH solution.
Staining the bone
Dye solution which included 0.003% Alizarin Red in 1% KOH was prepared and the samples were kept in the solution for 2 days. Then, the samples were taken out of the dye solution and were put in 95% ethanol for 30 min in order for the muscles to decolorize and for the bones to have sharp colors.
Glycerin preservation
The conventional method of preserving the samples in glycerin containers was used for the first group of rats. For this purpose, the samples were transferred to a solution containing 10 g KOH, 800 ml water and 200 ml glycerin. After 3 days, the samples were taken out of the solution and were put in a container with 1:1 ratio of glycerin and 95% ethanol for two days. Then, they were transferred to a container with pure glycerin.[10,11,12,13,14]
Plastination steps
To preserve the second group of samples, plastination technique which was modified and designed in plastination laboratory of Isfahan University of Medical Sciences was used.[15,16]
Dehydration
The dyed samples were transferred to acetone bath at −25°C and the acetone purity was measured using acetonometer every two days. After a week, the acetone inside the container was replaced with pure acetone and this process was repeated until complete dehydration, i.e., when the acetone percent remained constant.[17]
Force impregnation
In this step, the volumetric dehydrated samples were put in a container with S110 resin (prepared in plastination laboratory of Isfahan Medical School) and the sectional samples were also put in a container of UP87 resin (prepared in plastination laboratory of Isfahan Medical School) and the container was placed in the vacuum chamber up to the pressure of 5 MM Hg. After 10 days and replacing the resin by acetone, the samples were taken out of the vacuum chamber.[15,16,17]
Processing
After taking volumetric samples out of S110 resin bath, they were placed on a steel mesh so that the remaining resins came off; then, S111 hardening fluid was sprayed on the samples and the samples were kept at 35°C so that the resin was hardened.[15,16]
After taking the sectional samples out of the vacuum chamber, UP87 resin was used to mold them by glass molds, and after the resins were hardened, the molds were opened and the covered sections were taken out of the mold using a polymer coating with the thickness of 8 MM.[18]
RESULTS
The results of this study showed that the samples stained by Alizarin red-Alcian blue had good transparency so that the samples’ skeletons were completely visible. Coloring of the cartilage and bone was successfully performed and for the samples with lower age, cartilage and bone were properly stained. With regard to mature rats, Alizarin red staining was properly performed but they had a lower level of chromaticity compared with younger samples. Alcian blue staining of mature samples was properly performed in intervertebral disks and rib cartilages [Figure 1a-d]. After the staining process, putting the samples in acetone resulted in the muscles to turn to white; however, comparison of the half of the body with removed muscular tissue with the other half which had its muscular tissue demonstrated that color of cartilage and bone tissue remained unchanged [Figure 2a-d]. When the samples were placed inside the resin in the vacuum chamber, no color change was observed in cartilage and bone. Plastination of the volumetric stained samples was properly performed without any color change in cartilage and bone, and in young samples skeleton was fully visible and cartilage and bone were completely distinguishable. After plastinating the mature sample by silicon resin (S110), no color change was observed; however, the sample's muscular tissue turned opaque and white. Color of the bones and cartilages in the dissected half was less intense [Figure 3a-d]. After molding and final process, the sections which were placed in polyester resin (UP87), had high quality and translucent tissue so that spatial formation of the spinal cord, muscles, cartilage and bone could be easily revealed [Figure 4a-d]
Figure 1.
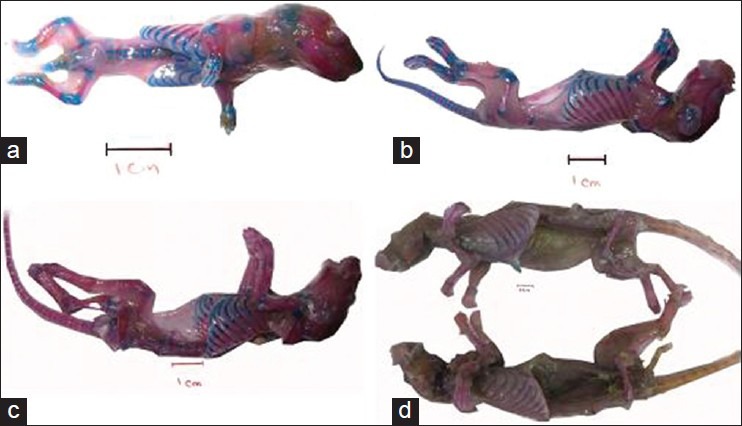
(a-d) Samples of 1-day, 3-day, 12-day and mature rats after double staining and preserving in glycerin
Figure 2.

(a-d) Samples of 1-day, 3-days, 12-days and mature rats after dehydration step using acetone
Figure 3.
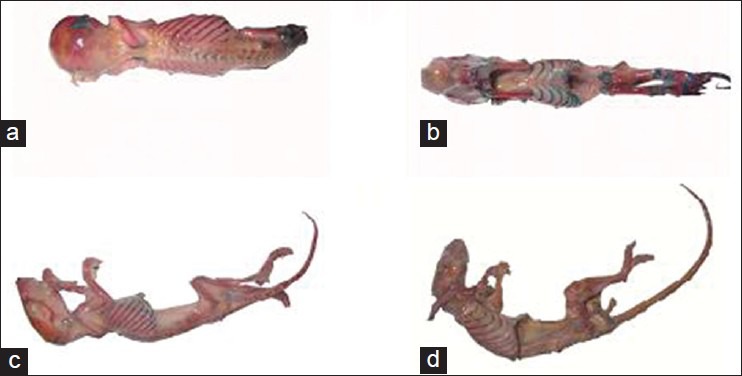
(a-d) Samples of 1-day, 2-days, 12-days and mature rats after plastination
Figure 4.
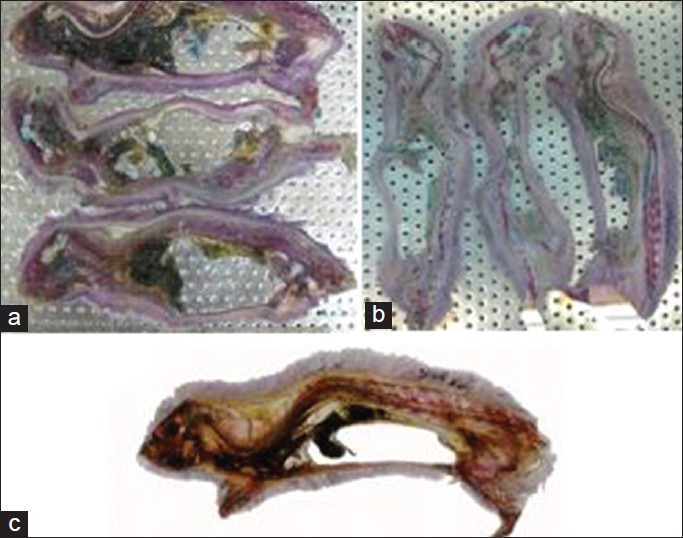
(a- c) Sections of mature rat (a) after double staining, (b) after dehydration with acetone, and (c) after plastination and molding
DISCUSSION
In this research, M. De Roberti's staining method was used with some corrections and modifications to obtain the most desirable results.[10] Finally, staining of the cartilage and bone was successfully preformed and staining quality in younger samples was better than that in the older ones. M. R. Haffajee used plastination method in order to preserve human fetus, which was stained by Alizarin red and prepared strong touchable samples for educational purposes.[19] The results of this study demonstrated that plastinated samples were more appropriate for teaching bone and cartilage anatomy and these samples had better applicability and strength and were easier for transportation compared with the samples, which were preserved in glycerin containers. Only for mature plastinated rats, these samples had less transparency compared to the samples preserved in glycerin and, in case of comparison with the plastinated samples in terms of transparency and skeleton visibility, 1-day rat had the most transparency and had the clearest skeleton among other volumetric samples. H. Steinke investigated a new method for tissue dying in sheet plastination to prevent from color change in dyed samples in plastination steps. The prepared sections had better contrast and distinction for educational purposes.[20] In this study, it was observed that for sections of the mature rat which was plastinated by UP87, plastination had no effect on double staining of the bone and cartilage. The tissue samples with great transparency, great differentiation between cartilage and bone tissues and chromaticity equal to glycerin preservation were prepared.
CONCLUSIONS
Finally, considering the obtained results in this study, it can be said that plastination technique is a better method for plastinating fetus and newborn samples colored by Alizarin red-Alcian blue double staining compared with glycerin preservation method and resolves the disadvantages of glycerin preservation method.
ACKNOWLEDGMENTS
This study was financially supported, Department of Anatomical Sciences and Molecular Biology, School of Medicine, Isfahan University of and Medical Science, Isfahan, Iran, We thanks Dr Mehran Vatanchian for his supervision in this research. All the expenses for this study were financed by Iranian center excellence of Anatomical Sciences Education.
Footnotes
Source of Support: School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared
REFERENCES
- 1.Leena Pramod K, Vaswani VR, Bind hu S. Museum preservation of skeleton of fetus and small vertebrates. Rec Res Sci Tech. 2011;3:54–8. [Google Scholar]
- 2.Culling CFA. Handbook of histopathological techniques (including museum technique) 2nd Ed. London: Butterworths; 1963. Museum technique; pp. 523–38. [Google Scholar]
- 3.Cortes Delgado N, Perez Torres J, Mario Hoyos J. Staining procedure of cartilage and skeleton in adult bats and rodents. Int J Morphol. 2009;27:1163–7. [Google Scholar]
- 4.Alden BD. A note on the staining of the skeleton of cleared specimens with Alizarin red. Stain Technol. 1962;1:123–4. [Google Scholar]
- 5.Inouye M. Differential staining of cartilage and bone in fetal mouse skeleton by Alcian blue and Alizarin red. Congenit Anom (Kyoto) 1976;16:171–3. [Google Scholar]
- 6.Erdodon D, Kadiodlu D, Peker T. Visualization of the fetal skeletal system by double staining with alizarin red and alcian blue. Gazi Medical Journal. 1995;6:55–8. [Google Scholar]
- 7.Thompsett DH. Anatomical Techniques. 2nd ed. Chapter 35. Edinburgh: E. & S. Livingstone; 1970. Cleared anatomical specimens; pp. 248–58. [Google Scholar]
- 8.von Hagens G, Tiederman K, Kriz W. The current potential of plastination. Anat Embryol (Berl) 1987;175:411–21. doi: 10.1007/BF00309677. [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Liu J, Yu S, Sui H. A new polyester technique for sheet plastination. J Int Soc Plastination. 2006;21:7–10. [Google Scholar]
- 10.De Roberti EM. Alcian blue – alizarin red skeletal staining. 2003. [Last updated on 2011 Jan 22]. Available from: http://www.hhmi.ucla.edu/derobertis/-Protocols- Mouse Protocols .
- 11.Dingerkus G, Uhler LD. Enzyme clearing of alcian blue stained whole small vertebrates’ dor demonstration of cartilage. Stain Technol. 1997;52:229–32. doi: 10.3109/10520297709116780. [DOI] [PubMed] [Google Scholar]
- 12.Potthof T. Clearing and staining techniques. In: Moser HD, editor. Ontogeny and systematic of fishes. Vol. 1. KS, USA: Special publication-American society of Ichthyologists and Herpetologist, Lawrence; 1984. pp. 35–7. [Google Scholar]
- 13.Wang Y, Xiao R, Yong F, Karim Bo, Lacovelli AJ, Cai J. Abnormalities in cartilage and bone development in the Apert syndrome FGFR2+S252W mouse. Development. 2005;132:3537–48. doi: 10.1242/dev.01914. [DOI] [PubMed] [Google Scholar]
- 14.Ranjbar R, Vatanchian M, Mohammadian B, Mayahi M. Chronological study of chicken embryo wing and leg skeleton development by alizarin red- alcian blue double staining technique. J Iranian Biology. 1385;19:164–79. [Google Scholar]
- 15.Rabiei AA. Isfahan: Isfahan University of Medical Science; 2003. Fabrication of polymer production of the whole human body with mass plastination method [PHD Thesis] [Google Scholar]
- 16.Rabiei AA, Asadi MH, Esfandiari E, Taghipour M, Bahadoran H, Setayesh M. Prepration of flexible plastinated sheets of human brain by P87 polyester. J Isfahan Med Sch. 2011;28:1961–6. [Google Scholar]
- 17.Von Hagen G. Preservation by plastination. J Int Soc Plastination. 1994;7:5–19. [Google Scholar]
- 18.Henry RW, Lotorre R. Polyester plastination of biological tissue. J Int Soc Plastination. 2007;22:59–68. [Google Scholar]
- 19.Haffajee MR. Plastination of a cleared fetus to show vascularization of ossifying bone. J Int Soc Plastination. 1996;10:26–7. [Google Scholar]
- 20.Steinke H, Rabi S, Saito T. Staining body slices before and after plastination. Eur J Anat. 2008;12:51–5. [Google Scholar]


