Abstract
Background:
Catamenial epilepsy is a kind of epilepsy, known in this name, when the periodicity of the exacerbation of the seizure is in association with menstural cycle. The present study examined the progesterone effectiveness as a complementary treatment in women with intractable catamenial epilepsy.
Materials and Methods:
The present study was conducted as a double-blind randomized controlled trial on 38 women with intractable catamenial epilepsy. Patients were assessed in two groups: The case group received in addition to AEDs, two (Mejestrol) 40 mg progesterone tablets in the second half of the cycle from 15th to 25th day. And the control group received in addition to AEDs, two placebo tablets daily. Age, BMI, epilepsy duration, types of the drugs used, progesterone level, and the number of the seizures in 3 months before and after the study were compared.
Results:
Based on the results of which there was no statistically significant difference in regard to age, BMI, epilepsy duration, types of the drugs used, progesterone level between the case and the control groups (P-value > 0.05). The number of the seizures after treatment has significantly decreased compared to before-treatment state. The degree of decreasing in the case group receiving the progesterone was higher than in the control group receiving the placebo. The difference, thus, is significant, based on statistical tests (P-value = 0.024).
Conclusion:
Based on the findings of this study using progesterone in women with intractable catamenial epilepsy has a significant effect on the degree of decreasing in the number of the seizures.
Keywords: Catamenial epilepsy, epilepsy, progesterone, seizures
INTRODUCTION
Epilepsy is a neurological chronic disorder characterized by unpredictable seizures.[1] Seizures do not occur accidentally and occur in clusters or consecutively in men and women developing the epilepsy. It can also occur periodically.[2,3,4] Epileptic females differ from epileptic males, as the patients with epilepsy are faced with social and physical problems associated with sex and it seems that the issue of sex in epilepsy is focused on in recent decades.[1]
Catamenial epilepsy is a kind of epilepsy, known in this name, when the periodicity of the exacerbation of the seizure is in association with menstural cycle.[5] In conducted studies, the prevalence of catamenial epilepsy in epileptic patients has been reported to be 31% to 60%.[6,7,8] It shows that of each three epileptic patients, one develops catamenial epilepsy. In the United States, it has been estimated that there are 1.3 million epileptic women, 400,000 of whom, approximately, have catamenial epilepsy, based on the stated prevalence of catamenial epilepsy.[9,10] Resistant to available drugs used for treatment, most often, catamenial epilepsy limits many of the ordinary activities, some of the seizures of which causing impairment of consciousness. There are some reasons showing the role of hormones in the pathophysiology and in the treatment of epilepsy.[11] Changing the neuronal migration and metabolism, reproductive hormones affect neuronal excitation by particular mechanisms.[12,13] Neuroactive properties of reproductive hormones and their periodic and cyclic secretion in the serum are important pathophysiological factors. Physiological secretion of endocrine in premenstrual cycles influences the occurrence of the seizure in ovulatory cycles and seizure frequency is in positive and statistically significant association with the estradiol/progesterone ratio.[14] The ratio is the highest during the days before ovulation and menstruation and the lowest during early- and mid-luteal phase. The exacerbation of the seizure during premenstruation is related to early deprivation of anti-epileptic effects of progesterone.[5,14] Estradiol has long been known to decrease seizure threshold, while progesterone and some of its metabolites decrease the seizure frequency in women; it is related to the anti-epileptic effects of progesterone.[15] Catamenial epilepsy can be due to progesterone deprivation and/or a relative increase in estradiol/progesterone ratio of the serum level. Early discontinuation of anti-epileptic effects of progesterone during premenstruation is accounted for as an important factor in the exacerbation of the seizures during menstruation.[16] Nearly half of the women with epilepsy are likely to develop some dysfunctions in reproductive hormones such as amenorrhea, oligomenorrhea and/or long and short intervals between menstrual cycles. These dysfunctions of menstrual cycles are recognized through the cycles with inadequate luteal phase in a way that the secretion of progesterone is abnormally low in the second half of the cycle.[15] Slight secretion of progesterone during the luteal phase is considered as a significant factor in the exacerbation of the seizures occurring in the second half of the cycle.[15,16,17,18] Followed by higher frequency of seizure, estradiol/progesterone ratio is abnormally high in the cycles with inadequate luteal phase.[17,18] Natural progesterone has been cited to be as the preferred treatment in the cycles with inadequate luteal phase.[19,20] Based on some obtained information, progesterone and some of its metabolites have anti-epileptic effects. Moreover, based on the findings of a study, complementary treatment of catamenial epilepsy (in 8 women in the complex and partial form) with natural progesterone has been positively effective.[21]
Since the researchers do not agree upon the issue of seizure treatment with progesterone as a complementary treatment and there are conflicting opinions about it and also because of the limits of the clinical trial in this respect, the present study, with the aim of investigating the degree of progesterone effectiveness as a complementary treatment in women with refractory seizure during catamenial period, was designed and carried out.
MATERIALS AND METHODS
The present study is conducted as a double-blind randomized controlled trial in 18- to 45-year-old women with catamenial epilepsy between June 2011 and March 2012 in. It was approved, clinically and in respect to EEG, that these patients had either complex partial seizure (CPS), secondary generalized seizure (SGS), or primary generalized seizure (PGS). Moreover, the patients received full-dose anti-epileptic drugs. Other inclusion criteria were as follows. (1) Seizure patterns in participants had to be in the catamenial form and seizure in these patients had to get exacerbated during the premenstrual period (between the 25th day of the previous cycle and the second day of the next cycle) or the whole period of the luteal phase of the cycle (2nd to 10th days of the cycle). In other words, in the chart of seizures, on average and in three consecutive periods, seizure frequency of patients during the catamenial epilepsy period compared to that of patients during the remaining days of the cycle had to be doubled. (2) There had to be cycles with inadequate luteal phase in patients (it was determined by the progesterone level of lower than 5 mg/ml in mid luteal phase). (3) There had to be premenstrual exacerbation in the patients with normal progesterone in the mid luteal phase. (4) They had not to be pregnant and lactating (breastfeeding). (5) They had not to use major tranquilizers and antidepressants. (6) They had not to be with amenorrhea and abnormal uterine bleeding (AUB). (7) They had not to use oral contraceptive pills (OCP) during last 3 months before the study. (8) And finally, they were supposed to fill out the written informed consent forms. They were excluded from the study as well, because of immigration, inaccessibility, serious progesterone side effects such as Thromboemboli, DVT, severe bleeding in them and/or not consenting for further cooperation. The present study was investigated and approved in Isfahan University of Medical Sciences and after the participating patients were explained about and informed of the purposes of the study, written informed consent was obtained from them all.
The patients meeting the inclusion criteria were selected in a simple non-random manner and in the order of attending the hospital and they were randomly divided into two 19-member groups of case and control by the random-maker software “Random Allocation” after the written informed consent was obtained from them. The case group included 19 patients who, in addition to anti-epileptic drugs (AEDs), received two (mejestrol) 40 mg progesterone tablets manufactured by pharmaceutical company “Tehran Daroo” daily (twice a day), in the second half of the cycle from 15th to 25th day. Moreover, progesterone was discontinued from 25th day of the cycle in the patients to have their natural process of monthly bleeding. On the other side, the control group included 19 patients receiving, in addition to AEDs, two placebo tablets (manufactured in Isfahan University, faculty of pharmacy, formally as the same as progesterone tablets) daily in the same manner the patient of the case group received drugs.
Before drug use, data associated with patients’ age, weight, height, epilepsy duration, types of the drugs used, progesterone level in the mid luteal phase (third week of the cycle and/or 21th day of the cycle) and the number of the seizures in 3 recent months were evaluated. Then, the patients, after treatment and during 3 months of follow-up, were monthly visited and the number of the occurred seizures in them was registered. Finally, the above-mentioned variables were compared between the studied groups.
Sample size was calculated by comparison of means formula. Collected data were analyzed using SPSS-20 software. Studied variables were presented in terms of Mean ± SD and [IQR] Median. Considering the sample volume in the studied groups, the non-parametric Mann–Whitney U test was used to compare the age, weight, height, epilepsy duration, progesterone level, and the number of the seizures before and after drug use between groups. In addition, the process of changes in the number of the seizures before treatment, in proportion to after-treatment state, was compared between the patients of two groups by repeated measurements of ANOVA. The level of significance in all cases was considered to be lower than 0.05.
RESULTS
Figure 1 shows the flow chart of the study. Based on the intrusion criteria, totally 43 patients were investigated, 5 of whom didn't enter the study (3 due to not meeting the intrusion criteria and 2 because of not consenting to participate in the trial). Eventually, 38 patients were studied in two 19-member groups of case and control. During the treatment and follow-up, two patients of the case group were excluded from the study due to progesterone side effects (severe headache, nausea and vomiting). Ultimately, collected data associated with 17 patients of the case group were analyzed.
Figure 1.
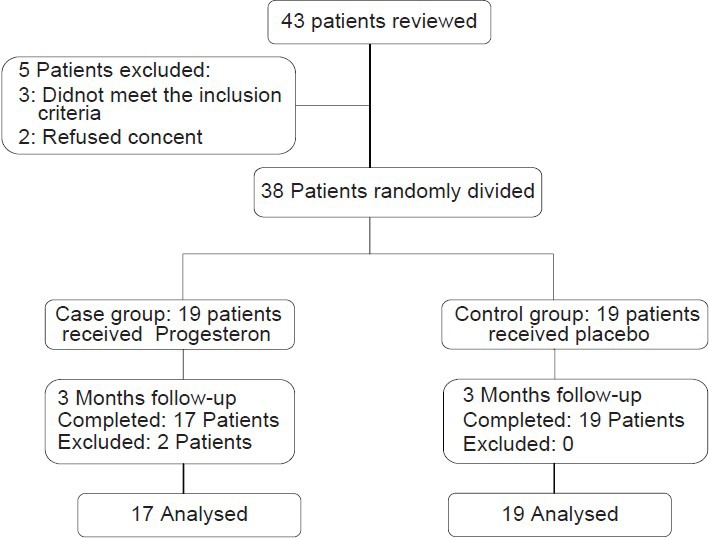
Patients who entered the study were divided into the study groups, followed up, and analyzed
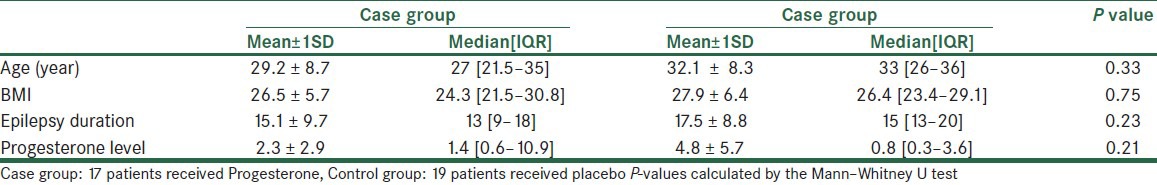
The mean age of the studied patients was 30.5 ± 8.5 years. Moreover, these patients had the mean BMI of 26.7 ± 6.1 kg, epilepsy duration of 16.3 ± 9.3 years, and the mean progesterone level of 3.7 ± 1.1 mg/ml. In Table 1, age, BMI, epilepsy duration, and the progesterone level of the patients are compared to one another between the studied groups, based on the results of which there was no statistically significant difference in regard to these variables between the case and the control groups (P-value > 0.05).
Table 1.
Age, BMI, epilepsy duration and the progesterone level in 36 women with intractable catamenial epilepsy
The mean number of the seizures of the patients during the last 3 months before the study was 7.8 ± 7.2, being higher in the control group than in the case group. No significant difference, however, was observed between two groups (P-value = 0.46). In addition, the mean number of the seizures of all patients during 3 months of follow-up was obtained which was 3.3 ± 3.8 and higher in the control group, as the same as the previous time. But the difference was statistically significant (P-value = 0.003, Figure 2).
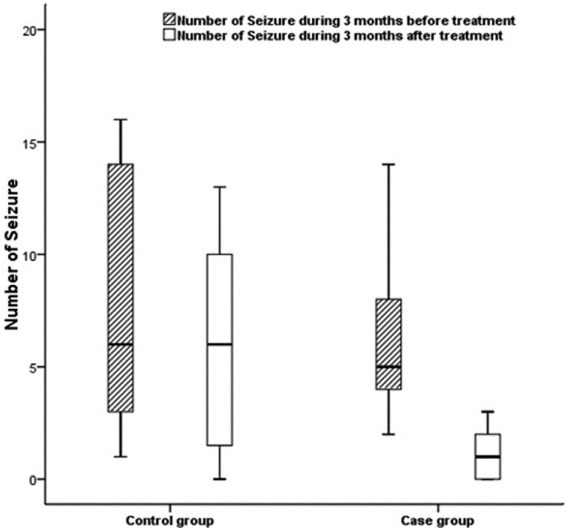
Number of the seizures within study groups during 3 months compared with 3 months of follow-up. In both groups after the study number of the seizures were decreased compared to before the study but decreased in the study group was statistically significant
Table 2 shows the comparison of the number of the seizures after treatment and before treatment between the case and the control groups. The number of the seizures after treatment has significantly decreased compared to before-treatment state. The degree of decreasing in the case group receiving the progesterone was higher than in the control group receiving the placebo. The difference, thus, is significant, based on statistical tests (P-value = 0.024).
Table 2.
Comparison of number of the seizures during 3 months before and after the study in 36 women with intractable catamenial epilepsy

DISCUSSION
Catamenial epilepsy is defined as a periodical increase in seizure during the menstrual period or other phases of the menstrual cycle[22] or as the occurrence of 75% of the seizures during a 10-day period of the menstrual cycle starting 4 days before menstruation.[23] Seizure can be considered as the main cause of the increase in menstrual disorders in these women, affecting steroid and menstrual cycle hormones in women with epilepsy.[24,25]
Some studies showed that the seizure frequency was decreased during the pregnancy period. It could probably mention the effect of sexual hormones (progesterone) on epilepsy especially high concentrations of them during pregnancy.[26] Acetate progesterone plays a vital role in catamenial epilepsy, anti-epileptic and anti-seizure properties of which are well-known in human beings and animals.[24] It is showed that catamenial seizure is in a direct association with the progesterone level.[6,27] In the mid-luteal phase, while progesterone level is increased, the number of the seizures decreases and when the progesterone level is decreased, the number of the seizures increases.[28,29] In patients with catamenial epilepsy, serum level of progesterone in the mid-luteal phase is significantly lower than in the control group.[6] There has already been no particular treatment for catamenial epilepsy and common anti-epileptic drugs are mostly used in controlling the catamenial epilepsy in women; however, given that the seizures are most often resistant to these drugs, more than one type of anti-epileptic drugs is used in these women, dependent on the seizure type.[30] Since progesterone has anti-epileptic effects, it can be useful for adjuvant treatment. Findings of the present study, with the aim of investigating the extent to which progesterone as a complementary treatment affects controlling of catamenial seizures in women with catamenial epilepsy, revealed that the number of the seizures in patients receiving Progesterone as an adjuvant treatment during 3 months of follow-up, compared to that of last 3 months before treatment, has decreased significantly. Although the number of the seizures was decreased in the control group after treatment, it has been a slight decrease, but not statistically significant. Additionally, the process of the decrease in the number of the seizures between two groups of case and control after treatment has been significant in proportion to that of before treatment.
Although, it is not officially known as a confirmed treatment for catamenial epilepsy, progesterone, due to its accessibility and great impact, has been considered as a valuable treatment for exacerbated catamenial seizure. Treatment with progesterone in these women, however, may cause hormonal effects such as vaginal bleeding and breast tenderness and in higher doses it may lead to CNS side effects including depression and asthenia.[22,24] In a study conducted by Herzog et al., progesterone is used as an adjuvant treatment in 25 women with catamenial epilepsy, in 72% of whom the number of the seizures during the first 3 months after treatment, has significantly decreased. Moreover, authors reported that there's a relationship between changes in the progesterone level and catamenial seizure.[5] It is also showed that natural progesterone has some effects on treating the catamenial and non-catamenial seizures in women.[31,32,33] In a study conducted in women with catamenial epilepsy, 100 to 200 mg of progesterone has been used as the adjuvant treatment during the 15th to 28th days of the menstruation and it was revealed that there has been a monthly decrease of 54% to 68% in the number of the seizures in 72% of the patients under a 3-month treatment.[32] Furthermore, 65% of these patients have used progesterone as the treatment beside the anti-epileptic drugs for 3 years, in whom a decrease of 62% to 74% has been reported in the number of seizures.[33] It has been showed in the studies that natural progesterone has a relative effect on catamenial seizures; however, it may lead to some side effects. Besides, the effect of synthetic progesterone on controlling of the catamenial seizures has been claimed to be without any side effects.[22] In the present study, 80 mg of acetate progesterone per day as the adjuvant treatment is showed to be significantly effective in comparison with placebo. These results are in line with the findings of the stated studies. It should be noted, however, that the prescribed dose of progesterone in the above-mentioned studies is higher than in the present study and there is no control group to which the variables be compared. In addition, it is probable that 65% of patients have continued the treatment for 3 years because of the satisfying results at the beginning of the study and the findings in this respect may not be generalizable to all of these patients.
Regarding the probability of the occurrence of the natural progesterone side effects in the patients of the present study, headache and nausea were observed in two of patients who received progesterone. Consequently, according to the study protocol and in order to prevent more severe side effects, treatment with progesterone was discontinued in these two patients and they were excluded from the study.
Considering the small number of case-control studies conducted on the investigation of the effect of the natural progesterone on controlling the catamenial seizures in women with catamenial epilepsy and according to undesirable severe side effects of using different doses of natural progesterone, more studies with higher sample volume and with different doses of progesterone are suggested to be done in these patients.
Overall, the results of the present study showed that using 80 mg of natural progesterone as an adjuvant treatment along with anti-epileptic drugs in controlling the catamenial seizures in women developing catamenial epilepsy has fallen effective compared to placebo usage. Based on these results, progesterone can be accounted for as the adjuvant treatment in these patients; however, conducting more studies in this respect sounds to be essential.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Tauboll E, Luef G. Gender issues in epilepsy-the science of why it is special. Seizure. 2008;17:99–100. doi: 10.1016/j.seizure.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Herzog AG. Catamenial epilepsy: Definition, prevalence pathophysiology and treatment. Seizure. 2008;17:151–9. doi: 10.1016/j.seizure.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Tauboll E, Lundervold A, Gjerstad L. Temporal distribution of seizures in epilepsy. Epilepsy Res. 1991;8:153–65. doi: 10.1016/0920-1211(91)90084-s. [DOI] [PubMed] [Google Scholar]
- 4.Fowler K, Massaro J, Harden C, Liporace J, Pennell P, Schomer D, et al. Distribution of seizure occurrence in women with epilepsy: Preliminary sata analysis in a prospective multicenter investigation. Epilepsia. 2006;47:1. [Google Scholar]
- 5.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–8. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla RH. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–26. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- 7.Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–4. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- 8.Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arg Neuropsiguiatr. 2005;63:751–6. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan PW, Norwitz ER, Ben Menachem E, Pennell PB, Druzin M, Robinson JN, et al. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 2007;11:283–91. doi: 10.1016/j.yebeh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Pennel PB. Antiepileptic drugs during pregnancy: What is known and which AEDs seem to be safest? Epilepsia. 2008;49(Suppl 9):43–55. doi: 10.1111/j.1528-1167.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzog AG. Hormonal therapies: Progesterone. Neurotherapeutics. 2009;6:383–91. doi: 10.1016/j.nurt.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SS. Estradiol administration increases neuronal responses to excitatory amino acid as a long- term effect. Brain Res. 1989;503:354–7. doi: 10.1016/0006-8993(89)91691-0. [DOI] [PubMed] [Google Scholar]
- 13.Herzog AG. Progesteron in seizure therapy [reply to letter] Neurology. 1987;37:1433. doi: 10.1212/wnl.37.8.1433. [DOI] [PubMed] [Google Scholar]
- 14.Backstom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. ActaNeurolScand. 1976;54:321–47. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 15.Herzog AG. Reproductive endocrine considerations and hormonal therapy for women with epilepsy. Epilepsia. 1991;32:27–S33. doi: 10.1111/j.1528-1157.1991.tb05889.x. [DOI] [PubMed] [Google Scholar]
- 16.Laidlaw J. Catamenial epilepsy. Lancet. 1956;271:1235–7. doi: 10.1016/s0140-6736(56)90003-4. [DOI] [PubMed] [Google Scholar]
- 17.Backstom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. ActaNeurolScand. 1976;54:321–47. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 18.Mattson RH, Kamer JA, Caldwell BV, Cramer JA. Seizure frequency and the menstrual cycle: A clinical study [abstract] Epilepsia. 1981;22:242. [Google Scholar]
- 19.Herzog AG, Seibel MM, Schomer DL, Vaitukaitis JL, Geschwind N. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch Neurol. 1986;34:341–346. doi: 10.1001/archneur.1986.00520040029014. [DOI] [PubMed] [Google Scholar]
- 20.Jones GS. The luteal phase defect. Fertil Steril. 1976;27:351–3546. doi: 10.1016/s0015-0282(16)41769-3. [DOI] [PubMed] [Google Scholar]
- 21.Herzog AG. Intermittent progesterone therapy and frequency of complex partial seizures in women with menstrual disorders. Neurology. 1986;36:1067–610. doi: 10.1212/wnl.36.12.1607. [DOI] [PubMed] [Google Scholar]
- 22.Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. 1993;34:827–31. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 24.Herzog AG. Disorders of reproduction in patients with epilepsy: Primary neurological mechanisms. Seizure. 2008;17:101–10. doi: 10.1016/j.seizure.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Morrell MJ, Montouris GD. Reproductive disturbances in patients with epilepsy. Cleve Clin J Med. 2004;71:S19–24. doi: 10.3949/ccjm.71.suppl_2.s19. [DOI] [PubMed] [Google Scholar]
- 26.Najafi MR, Sonbolestan F, Sonbolestan SA, Zare M, Mehvari J, Meshkati SN. The course and outcome of pregnancy and neonatal situation in epileptic women. Adv Biomed Res. 2012;1:4. doi: 10.4103/2277-9175.94426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, et al. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–9. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–6. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- 29.Herzog AG, Friedman MN, Freund S, Pascual-Leone A. Transcranial magnetic stimulation evidence of a potential role for progesterone in the modulation of premenstrual corticocortical inhibition in a woman with catamenial seizure exacerbation. Epilepsy Behav. 2001;2:367–9. doi: 10.1006/ebeh.2001.0232. [DOI] [PubMed] [Google Scholar]
- 30.Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: Implications for diagnosis and treatment. Epilepsy Behav. 2008;13:12–24. doi: 10.1016/j.yebeh.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzog AG. Intermittent progesterone therapy of partial complex seizures in women with menstrual disorders. Neurology. 1986;36:1607–10. doi: 10.1212/wnl.36.12.1607. [DOI] [PubMed] [Google Scholar]
- 32.Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1600–62. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 33.Herzog AG. Progesterone therapy in women with epilepsy: A 3-year follow-up. Neurology. 1999;52:1917–8. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]


