Abstract
Background:
The purpose of the present study was to determine the post-operative analgesic effects of preemptive intravenous (iv) paracetamol and the amount of reduction in tramadol (Contramal®) consumption.
Materials and Methods:
Following local research ethics committee approval, ASAI-II, 300 patients were assigned in a randomized manner into three groups: Group I (preemptive) received iv paracetamol 1 g/100 mL 10 min before skin inscision and 100 mL of saline solution at the end of the operation, Group II (post-operative) received 100 mL of saline solution 10 min before skin inscision and iv paracetamol 1 g/100 mL at the end of the operation and Group III (placebo) received 100 mL of saline solution 10 min before skin insicision and 100 mL of saline solution at the end of the operation as well. The time to first analgesic requirement use and 24 h total analgesic consumption were recorded. Visual analog scale (VAS) pain scores were obtained from all patients at 15, 30, min 1, 2, 4, 6, 8, 12 and 24 h after the end of the operation.
Results:
Time to first analgesic requirement was significantly longer in Group I and Group II, compared to Group III (P < 0.05). Time to first analgesic requirement was significantly longer in Group I compared to Group II (P < 0.05). Total analgesic consumption and postoperative VAS pain scores recorded were significantly lower in Group I and II, compared to Group III. Total analgesic consumption and postoperative VAS pain scores recorded were significantly lower in Group I compared to Group II (P < 0.05).
Conclusion:
In conclusion, preemptive iv paracetamol provided effective and reliable pain control after cholecystectomy surgeries and reduced post-operative pain scores, the need for and use of supplementary opioids and the time to first request of analgesics.
Keywords: Cholecystectomy, IV paracetamol, preemptive, tramadol
INTRODUCTION
Despite the high level of awareness about the importance of post-operative pain management, lots of investigations and studies results show an unacceptable high rate of the observed incidence of pain after surgery.[1,2] The results of inadequate pain control after surgery are significant in terms of both physically and physiological trauma and can result in long-term complications, as well as in the medium term. These complications can include hypoxemia, atelectasis, pneumonia, deep vein thrombosis, pulmonary embolism, psychological trauma, delay in improvement of bowel function, myocardial ischemia and infarction, urinary retention.[3,4]
Some of the concerns about the side effects of analgesics, such as nausea and respiratory depression, leads to implementation of inadequate doses of pain relievers and and also leads to unnecessary patient complaints.[5]
Paracetamol is a centrally acting drug, which inhibits prostaglandin synthesis and cyclooxygenase (COX) of nervous system. Paths based on spinal serotonergic mechanism of action of other central mechanisms may be involved in the acting mechanism of paracetamol.[6,7] In clinical practice, paracetamol does not cause the side effects that seems typically with other nonsteroid antiinflamatory drugs (NSAID), which are thought to occur due to inhibition of peripheral COX-1 (gastric toxicity, antiplatelet activity).[6]
The results of controlled clinical trials, the recommended therapeutic doses, is safe and well tolerated, supports that it has high safety profile similar as placebo. Paracetamol is considered to be a safe drug, and it does not have such as gastrointestinal problems or central side effects according to other NSAI drugs and opioids.[8]
Preemptive pain control is an issue dealt with in recent years. Here, regional or systemic analgesics are applied before the start of the surgical procedure, thus by preventing central sensitization of pain pathways it is intended to reduce the amount of analgesic and analgesic requirements.[9] By taking action of antinociceptive before the administration of nociceptive stimulus, ability to reduce the requirement of and amount of analgesia demonstrated experimentally. However, data from several clinical studies have shown differences and not support it at all times. Also, there are some people who indicates that analgesia is better in this method on the other hand some other those who have advanced that there is no difference.[10]
The purpose of this study is to determine post-operative opioid (Contramal®) to what extent the use of reduced and effect of time of the first analgesic requirement with applying preemptive iv paracetamol.
MATERIALS AND METHODS
Ethics council approval was obtained Reserach project Number 2006/117 (30/10/2006), along with a written informed consent from the patients. A total of 300 patients undergoing an elective laparoscopic cholecystectomy under general anesthesia were included into the prospective, randomized, planned study. Patients were allocated into three groups. Patients who had history of allergic reactions to NSAIDs, history of usage of paracetamol, opioids, or NSAIDs for a long time (3 months), chronic alcoholism, deficiency of liver and kidney, cardiovascular system illness, bleeding diathesis, cases in such a mental or cultural condition that they are unable to use the patient-controlled analge-sia device, gastric or duodenal ulser were excluded. By visiting the patients one day before the opera-tion, related information and training was given about the anesthesia method to be applied, usage of the visual analog scale (VAS). The VAS represents the simple verbal scale and evaluate pain according to the following scores: 0=no pain; 10=excruciating pain. Pharmacological premedication was not applied to patients in whom oral intake was cut off 8 h prior to the operation.
All subjects were administered with 5 mL kg-1 Ringer's lactate or saline solution in 30 min before the induction of anesthesia, and the infusion was maintained at 8-10 mL kg-1 h-1 throughout the operation. After standard monitorization, baseline heart rate (HR), non-invasive blood pressure, peripheral oxygen saturation (SpO2), and respiratory rate (RR) values were recorded. General anesthesia was intravenously induced with 5 mg kg-1 thiopental and 1 μg kg-1 fentanyl, and the trachea was intubated with an endotracheal tube under muscle relaxation with 0.5 mg. kg-1 atracurium. Anesthesia was maintained with isoflurane (1.5 %) in 50 % nitrous oxide with oxygen.
Group I
These patients received iv paracetamol 1 g / 100 mL (Perfalgan®, Bristol-Myers Squibb Pharmaceuticals Ltd, UK, IV paracetamol) before operation for 10 min and they received iv saline 100 mL for 10 min after the end of operation
Group II
These patients received iv saline 100 mL before operation for 10 min and they received iv paracetamol 1 g/100 mL for 10 min after the end of operation
Group III
These patients received iv saline 100 mL before operation for 10 min and they received iv saline 100 mL for 10 min after the end of operation
Post-operative pain was assessed at recovery room after the 1st h of surgery and then at the patients rooms. VAS pain scores were recorded at 15th, 30th min and 1., 2., 4.,6.,8.,12.,18., and 24 h after the completion of surgery. In case of inadequate analgesia (VAS score greater than 4), patients of all groups received tramadol (Contramal® 100 mg/2 mL), IV 100 mg of starting dose and the same dose was repeated with a maximum dose of 400 mg daily.
Side effects, such as nausea, vomiting, respiratory depression, itching, rash, allergic reaction, stomach irrita-tion, diarrhea, and constipation, headache, drowsiness, dry mouth, sweating, hypotension (noted as mild, moderate, severe) were recorded.
Patient satisfaction assessed by the patient at the end of 24 h. 0=poor, 1=moderate, 2=good, 3=very good, 4=excellent.
Statical analysis was performed using SPSS 12.0 for windows (SPSS Institue, Chicago, IL). P values <0.05 were considered significant. Data are presented as mean values and standard deviation (mean±SD (min-max)). Demographic data, duration of anesthesia and surgery, the first analgesic time and total analgesic consumption between the groups were analyzed using ANOVA, followed by Bonferroni when significance was obtained. Pain scores and the number of analgesic consumption were analyzed with the Kruskal-Wallis test. Wilcoxon X test was used to compare post-operative VAS values to the preoperative VAS values. Patient satisfaction among groups was analyzed using χ2 test. Gender, ASA, the number of patients requiring suplemental analgesics and the incidence of side effects were analyzed with Fisher's test and χ2 test.
RESULTS
There were no significant differences between the groups with regard to demographic variables (age, gender, weight and height) and ASA physical status or the mean duration of anesthesia and surgery time [Table 1].
Table 1.
Demographic properties, and operation and anesthesia duration [Mean±SD, n]
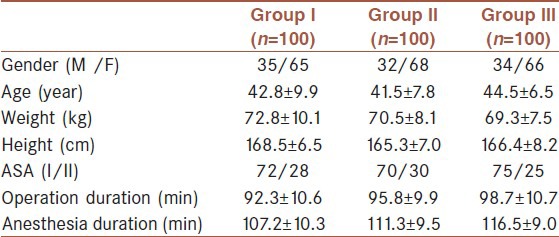
The changes in post-operative VAS pain scores are shown in Table 2. VAS pain scores recorded at 15, 30 min, 1, 6, 8, 12 and 18 h after the operation were higher in Group III, compared to Group I (P < 0.05). VAS pain scores recorded at 15, 30 min, 8 h, and 18 h after the operation were higher in Group III, compared to Group II (P < 0.05). VAS pain scores recorded at 30 min, 1 h after the operation were higher in Group II, compared to Group I (P < 0.05).
Table 2.
Post-operative VAS values [Mean±SD, (min-max)]
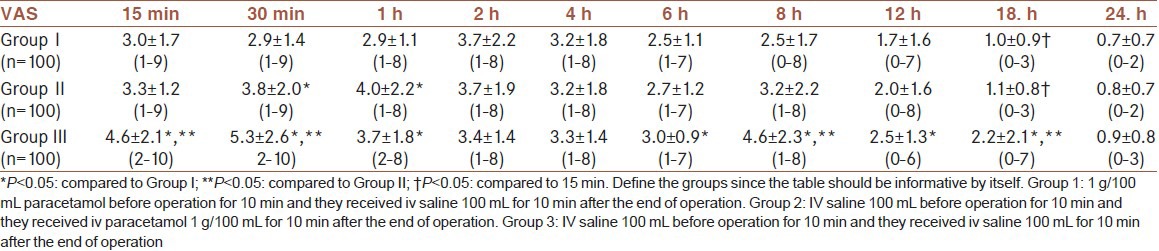
Time to first requested analgesic was significantly higher in Group I and II in comparison to the Group III (P < 0.001, P = 0.03, respectively) and it was higher in Group I in comparison to Group II (P = 0.08). Patients in Group I, experienced a longer interval (153.0 min) before taking post-operative analgesics compared with those patients in Group II (91.9 min) and Group III (33.7 min) (P < 0.05) [Table 3]. The cumulative consumed doses of opioid during the post-operative 24 h were significantly less in Group I (P < 0.001) and Group II (P < 0.001) when compared to Group III and less in Group I in comparison to Group II (P = 0.018) [Table 3]. Supplementary analgesics were used in 91% of the patients in Group III, 66% of the patients in Group II and 43% of the patients in Group I. The number of patients requiring supplemental analgesics at 0-6, 6-12 and 12-24 h was significantly lower in Group I and Group II compared to Group III (P < 0.05). The number of patients requiring supplemental analgesics at 0-6 and 6-12 h was significantly lower in Group I compared to Group II (P < 0.05) [Table 3]. Patient satisfaction was rated as ‘excellent” in 78 %, 56 % and 11 % of patients in Group I, Group II and Group III, respectively (P < 0.05) (χ2 = 121.949, P < 0.001). Rate of excellent in Group I was higher in comparison to Group II and III (χ2 = 13.03, P = 0.001; χ2 = 102.815, P < 0.001). Also this rate in Group II was higher when compared to Group III (χ2 = 58.729, P < 0.001) [Table 4].
Table 3.
First analgesic requirement time and post-operative analgesic requirements [Mean±SD, n].
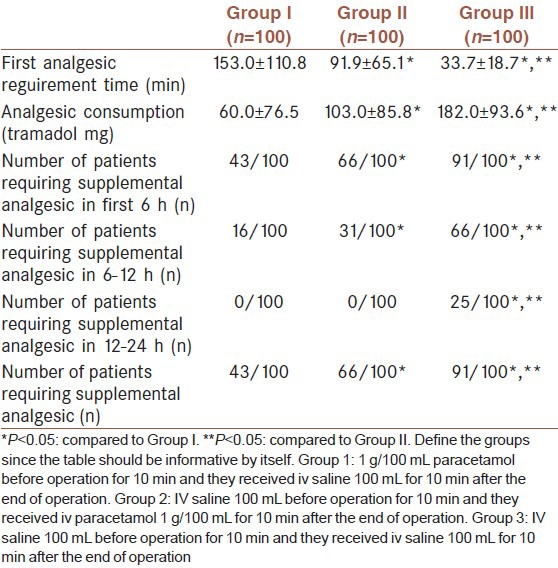
Table 4.
Patient satisfaction [n (%)]
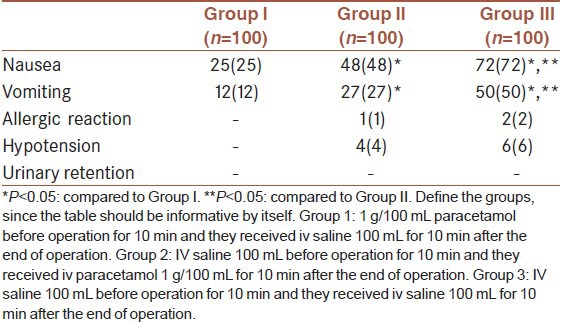
Post-operative side effects of the cases was shown in Table 5. Incidence of nausea and vomiting were higher in Group II and III when compared to Group I (P < 0.05) [Table 5]. Additionally, these side effects were higher in Group III in comparison to Group II. There were no significant differences among the groups in terms of other side effects. The most common side effect occured in all groups was nause. Nause was developed in 72, 48 and 25 patients in Group III, II and I, respectively. Vomitting was occured in 50, 27 and 12 patients in Group III, II and I, respectively (P < 0.05) [Table 5].
Table 5.
Incidence of side effects (%)
DISCUSSION
This study demonstrates that preemptive iv paracetamol produces significant opioid sparing effects compared to placebo in post-operative patients following cholecystectomies. It decreased 24 h total opioid consumption and increases the time to first analgesic use, thus its analgesic effect was not enough as a sole agent.
The results of controlled clinical studies demonstrated that the recommmended therapeutic doses of intravenous paracetamol is safe and well tolerated, with a profile that supports the high reliability similar to placebo. Paracetamol is considered to be a safe drug and it has no gastrointestinal and central nervous system side effects like opioids and NSAID (8).
It has been reported in numerous publications that oral paracetamol is effective and well tolerated agent in different surgical procedures.[5,8,11,12] However, oral paracetamol usage is limited after surgery. Patients undergoing surgical procedures require efficient and fast starting elimination of pain. In addition, the use of parenteral paracetamol has more rapid onset of action and has longer duration than oral paracetamol.[13]
Paracetamol intravenous 1 g has analgesic activity in moderate-severe post-operative pain similar those have shown ketorolac 30 mg,[14] diclofenac 75 mg,[15] morphine 10 mg.[16] In a double-blind placebo-controlled study, it has been shown that using the combination of intravenous paracetamol and ketopropefen reduce post-operative pain and cumulative opioid consumption in the first 48 h following surgery.[17,18,19] It also demonstrated that intravenous paracetamol has the effect on reducing the use of opioid. It reduces the need for the patient's total opioid by the rate of 24-46% and increases in patient satisfaction.[16,17,18,19,20]
Remy et al.[21] evaluated the effects of paracetamol on morphine consumption after major surgery in a meta-analysis and concluded that paracetamol reduces post-operative morphine usage.
In an other study, administrating of parasetamol per every 6 h 1g for 24 h to patients who have moderate pain after major orthopedic procedures provides rapid and effective analgesia, reduce the use of morphine and prolong the time of first analgesic request has been reported.[22]
Fijalkowska et al.[23] investigated the effectiveness of iv paracetamol to 92 patients scheduled for laparotomy or laparoscopy. Laparoscopy group, 16.3% of patients, the need for additional morphine, while the laparotomy group, 71.4% patients had additional morphine requirement. In conclusion, they reported that paracetamol reduces the need for opioid analgesics but in major surgeries a multimodal approach must be needed. Guner et al.[24] study that compared paracetamol and tramadol, they reported paracetamol and tramadol reduce opioid requirements after major abdominal surgeries, but alone could not provide adequate analgesia, therefore in major surgeries a multimodal approach have to be needed.
Also in our study in group II, we identified additional analgesic requirement in 66% patients while in group I, we identified additional analgesic requirement in 43% patients. We have identified that the implementation of preemptive iv paracetamol reduce the need for additional analgesics significantly. Also paracetamol reduces opioid requirements, we think that in major surgeries a multimodal analgesic approach would be more comfortable.
Toygar et al.[25] reported that application of paracetamol 1 g iv preoperatively and intraoperatively and every 6 h for 24 h for continued infusions of 1 g in patients scheduled for lumbar discectomy surgery may provide a better post-operative analgesia according to in the control group, will extend the time of the post-operative first morphine request, and reduce post-operative use of total morphine. Also, there is no preemptive analgesic effect of preoperative paracetamol application, but further studies may be required on the subject stated.
In recent years, the concept of preemptive analgesia on entering the practice of anesthesia in many experimental animal and clinical studies have been conducted.[26] Dahl et al.[27] by exploring the consequences of preemptive therapy in a meta-analysis of 80 studies, nonsteroidal anti-inflammatory drugs, intravenous opioids, ketamine, epidural, caudal, and spinal applications, and methods of infiltration of local anesthetic for preemptive and post-operative applications was concluded that there was no difference of providing analgesia. However, the same researchers stated that in the evaluation of preemptive analgesia is not only the timing, also duration, and the efficiency is important. Because of the studies do not contain these three factor it is emphasize that can not be obtain positive results from the studies and it is pointed need of further studies. Planning our work, we have endeavored to include all three factors.
Flowchart.
The flowchart of the study
In our study, it is identified that the use of preemptive iv paracetamol reduce opioid requirements, prolong the duration to first need of analgesia and provide a significant reduction in post-operative pain scores.
In studies, it is showed that paracetamol did not make gastric irritation, erosion or bleeding whereas very rarely trombocytopenia, leukopenia, neutropenia, simple skin rash or hypersensitivity reactions from urticaria to anaphylactic shock have been reported.[28] Also in our study, these kinds of side-effects have not been encountered, patients who have peptic ulcus and have risk of bleeding have not been included in our study because of the current risk of thrombocytopenia.
The most frequent side effects are nausea and vomiting. However, the type of surgery, anesthetics, hypotension and the supplemental agents used should all be considered.[29] In our placebo controlled study, surgery type and general anesthesia applied was standard and there was no difference between the groups in peroperative hemodynamic values. Visceral and pelvic pains are frequent causes of post-operative nausea and vomiting. Studies reported the improvement of nausea after treatment of pain.[30,31,32] Dejonckheere et al.[33] reported an increased incidence of nausea with tramadol compared to propacetamol (injectable prodrug of acetaminophen). In our study, the incidence of nausea was 72% in Group III, 48% in Group II and 25% in Group I, and the incidence of vomiting was 50%, 27% and 12%, respectively. Six patients in Group III, 4 patients in Group II had hypotension, whereas none of the patients in Group I had hypotension. The reason for high incidence of nausea and vomiting in Group III and Group II may be due to higher consumption of opioids in the post-operative period, hypotension and pain.
In conclusion, this study demonstrated the early postoperative analgesic effect of 1 g of paracetamol in patients undergoing cholecystectomy. Because of providing decreased opioid consumption with lower side effects, paracetamol can be safely used in post-operative pain management.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kuhn S, Cooke K, Collins M, Jones JM, Mucklow JC. Perceptions of pain relief after surgery. BMJ. 1990;300:1687–90. doi: 10.1136/bmj.300.6741.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puig MM, Montes A, Marrugat J. Management of postoperative pain in Spain. Acta Anaesthesiol Scand. 2001;45:465–70. doi: 10.1034/j.1399-6576.2001.045004465.x. [DOI] [PubMed] [Google Scholar]
- 3.Report of the Working Party on Pain after Surgery. London: The Royal College of Surgeons of England, the College of anaesthetists; 1990. Commission on the Provision of Surgical Services. [Google Scholar]
- 4.Bonica JJ. Postoperative pain. In: Bonica JJ, editor. The management of pain. Philadelphia: Lee and Febiger; 1990. pp. 460–80. [Google Scholar]
- 5.Charlton JE. Treatment of postoperative pain. In: Giamberardino MA, editor. Seattle: IASP Press; 2002. pp. 351–5. [Google Scholar]
- 6.Bonnefont J, Courade JP, Alloui A, Eschalier A. Antinociceptive mechanism of action of paracetamol. Drugs. 2003;63:1–4. [PubMed] [Google Scholar]
- 7.Pini LA, Vitale G, Ottani A, Sandrini M. Naloxone-reversible antinociception by paracetamol in the rat. J Pharmacol Exp Ther. 1997;280:934–40. [PubMed] [Google Scholar]
- 8.Arslan M, Çiçek R, Celep B, Yılmaz H, Üstün-Kalender H. Comparison of the analgesic effects of intravenous paracetamol and lornoxicam in postoperative pain following thyroidectomies. Agri. 2011;23:160–6. doi: 10.5505/agri.2011.82788. [DOI] [PubMed] [Google Scholar]
- 9.Grape S, Tramèr MR. Do we need preemptive analgesia for the treatment of postoperative pain? Best Pract Res Clin Anaesthesiol. 2007;21:51–63. doi: 10.1016/j.bpa.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Kayhan Z. Klinik Anestezi: Logos Publishing; 2005. Postoperative pain and control; pp. 643–8. [Google Scholar]
- 11.Prescott LF. Paracetamol (Acetaminophen): A Critical Bibliographic Review. London: Taylor and Francis; 1996. Pharmacological actions and therapeutic use of paracetamol; pp. 197–539. [Google Scholar]
- 12.Breivik H. Postoperative pain:towards optimal pharmacological and epidural analgesia. In: Giamberardino MA, editor. Seattle: IASP Press; 2002. pp. 337–49. [Google Scholar]
- 13.Jarde O, Boccard E. Parenteral versus oral route increases paracetamol eficiency. Clin Drug Invest. 1997;14:474–7. [Google Scholar]
- 14.Lee SY, Lee WH, Lee EH, Han KC, Ko YK. He effects of paracetamol, ketorolac, and paracetamol plus morphine on paincontrol after thyroidectomy. Korean J Pain. 2010;23:124–30. doi: 10.3344/kjp.2010.23.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akarsu S, Sahin S, Kara C, Akdemir N, Degerli S. A comparison between parenteral paracetamol and diclofenac for acute postoperetive pain treatment in patients after caeserean section. J Turk Soc Obstet Gynecol. 2010;7:262–6. [Google Scholar]
- 16.Craig M, Jevons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumaticlimb pain in the emergency department. Emerg Med J. 2012;29:37–9. doi: 10.1136/emj.2010.104687. [DOI] [PubMed] [Google Scholar]
- 17.Van Aken H, Thys L, Veekman L, Buerkle H. Assessing analgesia in single and repeated administration of propacetamol for postoperative pain: Comparison with morphine after dental surgery. Anesth Analg. 2004;98:159–65. doi: 10.1213/01.ANE.0000093312.72011.59. [DOI] [PubMed] [Google Scholar]
- 18.Delbos A, Boccard E. The morphine-sparing effect of propacetamol in orthopedic postoperative pain. J Pain Symptom Manage. 1995;10:279–86. doi: 10.1016/0885-3924(95)00004-I. [DOI] [PubMed] [Google Scholar]
- 19.Peduto VA, Ballabio M, Stefanini S. Efficacy of propacetamol in the treatment of postoperative pain Morphine-sparing effect in orthopedic surgery. Italian Collaborative Group on Propacetamol. Acta Anaesthesiol Scand. 1998;42:293–8. doi: 10.1111/j.1399-6576.1998.tb04919.x. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Palazon J, Tortosa JA, Martinez-Lage JF, Perez-Flores D. Intravenous administration of propacetamol reduces morphine consumption after spinal fusion surgery. Anesth Analg. 2001;92:1473–6. doi: 10.1097/00000539-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: Meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–13. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 22.Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–31. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Fijalkowska A, Trela-Stachurska K, Rechberger T. Efficacy of intravenous paracetamol for early postoperative analgesia after gynaecological surgery. Anaesth Int Therapy. 2006;38:66–3. [Google Scholar]
- 24.Güner AD, Baydar M, Gökçınar D, Süsleyen C, Karabeyoğlu I, Göğüş N. A comparison between paracetamol and tramadol for postoperative pain treatment in patients undergoing midline abdominal incision. New J Med. 2007;24:160–3. [Google Scholar]
- 25.Toygar P, Akaya T, Ozkan D, Ozel O, Uslu E, Gumus H. Does iv parocetamol hove preemptive analgesic effect on lumber disc surgeries? Agri. 2008;20:14–9. [PubMed] [Google Scholar]
- 26.Aida S, Baba H, Yamakura T, Taga K, Fukura S. The effectiveness of preemptive analgesia varies according to the type of surgery. Anesth Analg. 1999;89:711–6. doi: 10.1097/00000539-199909000-00034. [DOI] [PubMed] [Google Scholar]
- 27.Dahl J, Moiniche S. Pre-emptive analgesia. Br Med Bull. 2004;71:13–27. doi: 10.1093/bmb/ldh030. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefont J. Mechanism of antinociceptive effect of paracetamol. Drugs. 2003;63:1–4. [PubMed] [Google Scholar]
- 29.Watcha MF, White PF. Postoperative nausea and vomiting: İts etiology, treatment and prevention. Anesthesiology. 1992;77:162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Andrews PL. Physiology of nausea and vomiting. Br J Anaesth. 1992;69:2–19. doi: 10.1093/bja/69.supplement_1.2s. [DOI] [PubMed] [Google Scholar]
- 31.Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth. 1992;69:24–32. doi: 10.1093/bja/69.supplement_1.24s. [DOI] [PubMed] [Google Scholar]
- 32.Keeny GN. Risk factors for postoperative nausea and vomiting. Anaesthesia. 1994;49:6–10. doi: 10.1111/j.1365-2044.1994.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 33.Dejonckheere M, Desjeux L, Deneu S, Ewalenko P. Intravenous tramadol compared to propacetamol for postoperative analgesia following thyroidectomy. Acta Anaesth Belg. 2001;52:29–33. [PubMed] [Google Scholar]


