Abstract
A crucial step in lysosomal biogenesis is catalyzed by “uncovering” enzyme (UCE), which removes a covering N-acetylglucosamine from the mannose 6-phosphate (Man-6-P) recognition marker on lysosomal hydrolases. This study shows that UCE resides in the trans-Golgi network (TGN) and cycles between the TGN and plasma membrane. The cytosolic domain of UCE contains two potential endocytosis motifs: 488YHPL and C-terminal 511NPFKD. YHPL is shown to be the more potent of the two in retrieval of UCE from the plasma membrane. A green-fluorescent protein-UCE transmembrane-cytosolic domain fusion protein colocalizes with TGN 46, as does endogenous UCE in HeLa cells, showing that the transmembrane and cytosolic domains determine intracellular location. These data imply that the Man-6-P recognition marker is formed in the TGN, the compartment where Man-6-P receptors bind cargo and are packaged into clathrin-coated vesicles.
INTRODUCTION
It is well established that in higher organisms newly synthesized acid hydrolases are targeted to lysosomes via the mannose 6-phosphate (Man-6-P) recognition system. The generation of the Man-6-P signal on the hydrolases involves a two-step reaction. In the first step, GlcNAc-P is added to C-6-hydroxyl groups of selected mannose residues by the enzyme UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase (phosphotransferase). Then “uncovering” enzyme (UCE), the colloquial name for the enzyme N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase, removes the covering GlcNAc residue (Hasilik et al. 1980; Tabas and Kornfeld, 1980; Varki and Kornfeld, 1980; Varki et al., 1983). The Man-6-P moiety exposed by UCE action is responsible for the specific, high-affinity binding of the acid hydrolases to one of the two Man-6-P receptors (MPRs) in the trans-Golgi network (TGN), which transport the hydrolases to endosomes and subsequently to lysosomes (Kornfeld, 1987; Hille-Rehfeld, 1995). Thus, these two enzymes play a crucial role in lysosomal biogenesis. Phosphotransferase first acts on acid hydrolases in the endoplasmic reticulum (ER)-Golgi intermediate compartment and continues to transfer GlcNAc-P residues in the early (cis) Golgi (Lazzarino and Gabel, 1987; Dittmer and von Figura, 1999). However, the site of action of UCE in the Golgi is unknown, despite the fact that the enzyme has been studied for almost 20 years (Varki and Kornfeld, 1981; Waheed et al., 1981; Varki et al., 1983; Kornfeld et al. 1998). Most recently pure bovine UCE was shown to be a tetramer composed of 68-kDa monomers that contain sialylated oligosaccharides, suggesting that UCE travels to the TGN where sialyltransferase is located during its intracellular itinerary (Kornfeld et al., 1998). Because a number of early Golgi constituents slowly migrate to later Golgi elements from which they are retrieved, the finding of sialylated oligosaccharides alone does not reveal the normal location of UCE. Recently a human cDNA-encoding UCE was isolated (Kornfeld et al., 1999). This established that UCE is a type I membrane-spanning glycoprotein of 515 amino acids with a single 27-residue transmembrane domain and a 41-residue cytoplasmic tail that contains both a tyrosine-based internalization motif 488YHPL and an NPFXD sequence that can bind to Eps15 and has been found to promote endocytosis in yeast (Tan et al., 1996). The presence of 488YHPL raised the possibility that UCE, like TGN 38 and TGN 46 (Reaves et al., 1993; Wong and Hong, 1993; Ponnambalam et al., 1994, 1996; Kain et al., 1998), may travel to the plasma membrane and be returned to the TGN via coated vesicles. In this study we provide direct evidence that UCE does, indeed, reside in the TGN and constitutively recycles to the plasma membrane. This indicates that, even though phosphotransferase acts in the ER-Golgi intermediate compartment and cis-Golgi compartments, the Man-6-P recognition signal is probably generated by UCE in the final compartment of the Golgi where the MPRs reside.
MATERIALS AND METHODS
Construction of Cytosolic Tail Mutants of Human UCE
Mutations were inserted into the human UCE expression vector as previously described (Kornfeld et al., 1999) with the use of the Quik Change Site-directed Mutagenesis kit (Stratagene, La Jolla, CA) and a pair of sense- and antisense-priming nucleotides encompassing the desired changes. For the Y488A mutant the sense strand primer 5′-CATGGGGACTATGCAGCCCACCCGCTGC AGG-3′ was used. For the H510 stop mutant the sense strand primer 5′-CAGCCAGGGGGC GCCTAGGACCCCTTCAAGGAC-3′ was used. To prepare the green fluorescent protein (GFP)-UCE transmembrane domain-cytosolic tail constructs, polymerase chain reaction (PCR) of the wild type and mutant UCE described above was carried out with the use of a sense strand primer including an in frame BglII restriction site at the 5′-end (GGGCGGGAGAGATCTCCTTTTTCACC) and an antisense strand primer including an MluI site at the 3′-end (5′ to 3′-GGGAAACAAGCTTACGCGTCGTGCCACC). The PCR products of ∼280 bp each (encoding E441through the stop condon at 510 or 516) were gel purified and sequenced in both directions with the use of the PCR primers. After restriction digestion with BglII and MluI, the fragments were each inserted downstream of the preprolactin signal sequence-GFP prepared as follows. The signal sequence of preprolactin was fused to the N terminus of the mGFP (Siemering et al., 1996) with the use of the standard PCR protocols with the overlap extension technique (Ho et al., 1989). An appropriate partial complementary pair of oligonucleotides was used as internal primers with a downstream primer that created an EcoRI restriction site at the 5′-end of the preprolactin sequence and an upstream primer that created an in frame BglII site at the 3′-end of the mGFP. The final PCR products were subcloned into pSFFVneo as described by Rohrer et al. (1995). All coding sequences created by PCR were verified by sequencing.
Selection of Stably Transfected L-Cells Expressing Mutant Human UCE
The human wild-type and mutant UCE constructs in the expression vector pcDNA3.1(−) were transfected into mouse L-cells, maintained in αMEM-10% heat-inactivated fetal bovine serum, with the use of Lipofectamine plus reagent (Life Technologies, Rockville, MD) as previously described for COS cells (Kornfeld et al., 1999). The transfecting DNA was removed on day 2, the cultures were allowed to grow until day 4 in nonselective medium and then transferred to medium containing 500 μg/ml G418, and resistant clones were selected as previously described (Rohrer et al., 1995). Clones were screened for expression by UCE activity assays and Western blotting as previously described (Kornfeld et al., 1999). Expressing clones were maintained in selective medium (500 μg/ml G418).
Measurement of Cell Surface UCE Activity on L-Cell Lines
L-cells and L-cells expressing wild-type, mutant Y488A, or mutant H510 Stop UCE were seeded into the wells of a 24-well tissue culture plate in triplicate and grown at 37°C in 5% CO2 in a moist incubator in either 1 ml of αMEM-10% heat-inactivated fetal bovine serum (L-cells) or the same medium plus 500 μg/ml G418 (all others). A set of empty wells contained only medium and served as blanks. When the cells reached ∼60% confluency the media were aspirated and the cells were washed two times with 0.5 ml of TBS, pH 7 (10 mM Tris-HCl–150 mM NaCl). The A set of wells then received 120 μl of UCE substrate (0.5 mM [3H]GlcNAc-P-Man αMe) in TBS, pH 7, to measure cell surface enzyme activity; the B set received 120 μl of substrate in 1% Triton X-100–TBS, pH 7, to measure total cellular enzyme activity; the C set received only 120 μl of TBS, pH 7, for subsequent assay for UCE activity that was secreted. After incubation at 37°C in the tissue culture incubator for 60 min, the 120 μl were removed from each C well into a tube containing UCE substrate and incubated for 60 min at 37°C. The 120 μl were removed from each A and B well into 3 ml of 2 mM Tris, pH 8, to stop the reaction. The separation of released [3H]GlcNAc from 3H-substrate was carried out as previously described (Mullis and Ketcham, 1992). Meanwhile, the cell layers remaining in wells A and C were each extracted in 150 μl of 0.1 N NaOH for 1 h at 4°C and used to measure the protein concentration by the Micro BCA assay (Pierce, Rockford, IL) standardized with bovine serum albumin. The secretion of UCE activity by the cells in the C wells during 60 min was assumed to have occurred linearly and thus represents twice the average amount that would have been present during the in situ assay in the A wells. Accordingly the secreted activity is calculated as ½C, and the activity on the cell surface is calculated as A − ½C. When the effect of methyl β-cyclodextrin on the cell surface expression of UCE was measured, cells were incubated for 2 h at 37°C in αMEM containing 10 mM methyl β-cyclodextrin, which was then aspirated before the cell layers were assayed for UCE in 10 mM methyl β-cyclodextrin in the presence or absence of Triton X-100 as described above.
Antibodies and Immunofluorescence Microscopy
Mouse monoclonal antibody (mAb) UC-1 to bovine UCE was prepared as previously described (Kornfeld et al., 1998). Rabbit antibody to human UCE was raised by injecting rabbits with an affinity-purified 6His-tagged fusion protein containing the carboxyl-terminal region of human UCE (amino acids 172–515) expressed in Escherichia coli BL21 cells from the pQE-32 QIA express vector as described by the manufacturer (Qiagen, Chatsworth, CA) The antisera were affinity purified with the use of a column of Ultralink (Pierce) to which the UCE antigen had been coupled. Rabbit anti-TGN 38 antiserum raised to a synthetic peptide corresponding to the C-terminal 21 amino acids of rat TGN 38 was kindly provided by Dr. George Banting (University of Bristol, UK). Sheep anti-human TGN 46 was from Serotec (Oxford, UK) and mAb GT2/36/118 specific for the human galactosyltransferase (Berger et al., 1986) was kindly provided by Dr. Erich G. Berger (University of Zurich, Switzerland).
Indirect immunofluorescence microscopy of MDBK cells was carried out on cells grown on glass coverslips in αMEM-10% fetal bovine serum, washed, fixed in 3.7% formaldehyde, permeabilized in 0.2% saponin, and treated with primary and secondary antibodies as described by Drake et al. (2000). The primary mouse mAb UC-1 was an ammonium sulfate fraction from tissue culture supernatant (containing ∼400 μg/ml mouse IgG) and was used at a dilution of 1:10; the rabbit anti-TGN 38 was diluted 1:500. The secondary antibodies were goat-anti-mouse Alexa 488 and goat anti-rabbit Alexa 594 conjugates (Molecular Probes, Eugene, OR) diluted 1:250.
Confocal immunofluorescence microscopy on GFP-UCE-transfected HeLa cells was carried out as described by Schweizer et al. (1988) except that the cells were grown on glass coverslips that were treated with nitric acid. Cells in Figure 6 were incubated for 30 min either in growth media supplied with a 1:1000 dilution of a concentrated brefeldin A (BFA) (Sigma, St. Louis, MO) solution (5 mg/ml in methanol) or in mock-treated growth medium. Formaldehyde-fixed and saponin-permeabilized cells were incubated with a 1:10 dilution of mAb GT2/36/118 against galactosyltransferase or a 1:200 dilution of sheep anti-human TGN 46 antibodies. The secondary antibodies were goat anti-mouse Alexa 568 and donkey anti-sheep Alexa 594 conjugates (Molecular Probes) diluted 1:1000. The coverslips were mounted on glass slides in Antifade (Molecular Probes) for viewing with an inverted Leica (Deerfield, IL) microscope equipped with the TCS confocal system and an Ar/Kr laser. Serial sections in the z-axis through the entire cells were taken, and the resulting stacks of images were analyzed with the use of the Imaris program (Bitplane AG, Zurich, Switzerland) and deconvoluted with the use of Huygens program (Mat & Met: Scientific Volume Imaging, Hilversum, The Netherlands,).
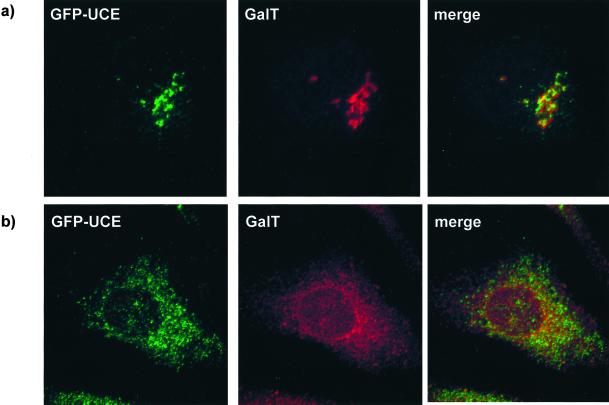
Figure 6.
GFP-UCE does not colocalize with galactosyltransferase (GalT). HeLa cells stably transfected with GFP-UCE wild type were labeled with anti-galactosyltransferase antibody after incubation for 30 min in the absence of BFA (a) or after 30 min of incubation with BFA (b). Left, GFP-UCE; middle, galactosyltransferase; right, merged image.
RESULTS
UCE Colocalizes with TGN 38 in MDBK Cells
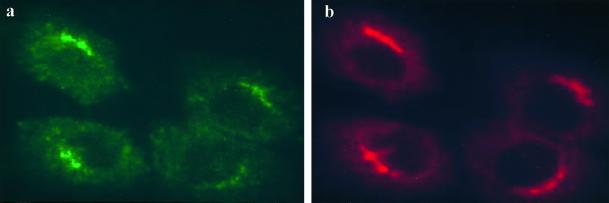
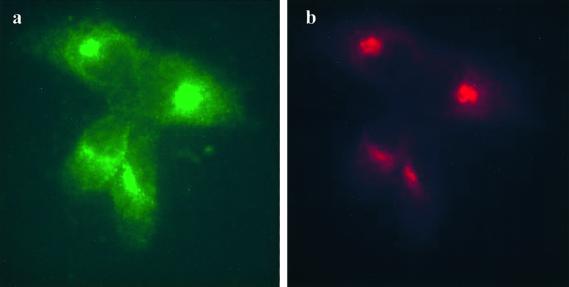
Indirect immunofluorescence microscopy was used to double label Madin-Darby bovine kidney (MDBK) cells with the mouse monoclonal anti-bovine UCE antibody UC-1 and a rabbit polyclonal antibody to TGN 38, a well characterized TGN marker. Figure 1 shows that the endogenous UCE (a, green) and TGN 38 (b, red) in MDBK cells colocalize in a juxtanuclear region consistent with UCE residing primarily in the TGN. Given this finding one would expect the glycoprotein enzyme to become sialylated by the sialyltransferases that reside in the Golgi and the TGN.
Figure 1.
UCE colocalizes with TGN38 in MDBK cells. Cells were fixed and permeabilized and double labeled with mAb UC-1 to bovine UCE (a, green) and rabbit antibody to TGN38 (b, red).
Human UCE Expressed in Mouse L-Cells Is Sialylated
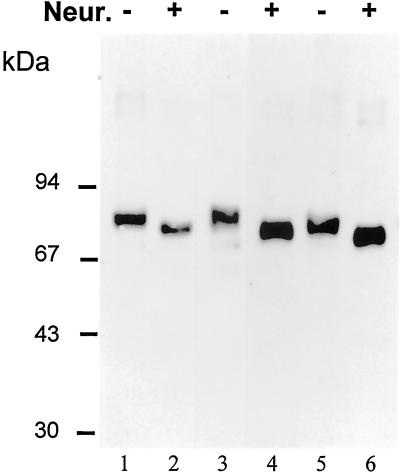
The cDNAs encoding human UCE and two mutants of the cytosolic tail that disrupt the tyrosine-based (Y488A) and NPF (H510stop) endocytosis motifs were stably expressed in mouse L-cells. The levels of enzyme activity expressed in the solubilized membrane extracts of these cells varied from 22- to 55-fold higher than the endogenous activity in the parental L-cells and 12- to 29-fold higher than the endogenous activity in human Hep G2 cells. When the membrane extracts of stably transfected cells were desialylated by treatment with neuraminidase, subjected to SDS-PAGE under reducing conditions, blotted, and probed with rabbit anti-human UCE antibody, the results shown in Figure 2 were obtained. Without treatment, the wild type (lane 1) and Y488A mutant (lane 3) have monomer UCE bands at ∼80 kDa, similarly to endogenous human UCE in Hep G2 cells (not shown). These bands shift to ∼75 kDa after neuraminidase treatment (lanes 2 and 4). In contrast, the H510 stop mutant has monomer bands at 76 kDa before (lane 5) and at 70 kDa after neuraminidase treatment (lane 6), reflecting its slightly smaller size because of the removal of its carboxyl-terminal peptide HNPFKD. These results show that the wild-type and mutant UCEs must have been exposed to sialyltransferase in the trans-Golgi-TGN of the L-cells. Furthermore, the discrete shifts observed (rather than smears) indicate that essentially every monomer of the enzyme has become sialylated and probably to the same extent. It is not possible to determine what extent of sialylation has occurred because removal of sialic acid from a glycoprotein not only alters its size but also its charge and can produce anomalous mobility changes on SDS-PAGE.
Figure 2.
The human UCE expressed by stably transfected L-cells is sialylated. Membrane extracts of cells expressing human UCE wild type (lanes 1 and 2), Y488A mutant (lanes 3 and 4), or H510 stop mutant (lanes 5 and 6) were treated (+; lanes 2, 4, and 6) or not treated (−; lanes 1, 3, and 5) with Vibrio cholera neuraminidase (Neur.) for 80 min at 37°C. The reaction mixtures were boiled in SDS-PAGE sample buffer and subjected to reducing SDS-PAGE on a 7.5% gel. The gel was blotted to nitrocellulose, which was probed with affinity-purified rabbit anti-human UCE antibody (1:1000) and detected by enhanced chemiluminescence.
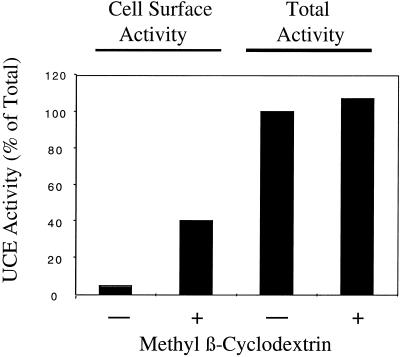
It thus appears that wild-type and mutant UCE, like other type I transmembrane glycoproteins, are core glycosylated as they enter the ER, traverse the Golgi apparatus where their oligosaccharides are processed to complex-type structures, and finally undergo addition of sialic acid in the TGN. Where else does UCE traffic? The presence of the internalization motif 488YHPL suggests that UCE may travel to the plasma membrane and be returned to the TGN via clathrin-coated vesicles, as is the case for TGN 38 (Reaves et al., 1993; Wong and Hong, 1993; Ponnambalam et al., 1994) and TGN 46 (Ponnambalam et al., 1996; Kain et al., 1998). The L-cells expressing wild-type and Y488A mutant UCE were examined by indirect immunofluorescence microscopy with the use of the rabbit anti-human UCE antibody. Wild-type UCE was visualized in the Golgi region of permeabilized L-cells, whereas Y488A UCE was seen on the cell surface of nonpermeabilized L-cells (data not shown). To quantitate the amount of UCE on the cell surface of intact cells, compared with total intracellular UCE, advantage was taken of the sensitivity of the enzymatic assay for UCE activity. Parental L-cells and L-cells expressing wild-type, Y488A, and H510 stop human UCE were grown in triplicate in the wells of a 24-well tissue culture plate. After the cell layers were washed, the A wells were used to measure cell surface enzyme activity on intact cells, the B wells were used to measure total cellular enzyme activity in the presence of Triton X-100, and the C wells were incubated without substrate and the supernatant was then assayed for secreted enzyme activity. Table 1 shows the average results of these enzyme assays from two separate experiments. After subtraction of the contribution from the parental L-cells, it can be seen that <1% of the total wild-type UCE is present on the plasma membrane, whereas 63% of the Y488A mutant UCE is present on the plasma membrane at steady state. The H510 stop mutant is intermediate with 7.1% of the enzyme activity at the cell surface. These results suggest that UCE normally traffics to the plasma membrane and is efficiently internalized via the 488YHPL motif in its cytosolic tail. The NPF endocytosis motif, absent in the H510 stop mutant, may play a minor role in endocytosis at the cell surface. We next examined whether inhibition of endocytosis at the plasma membrane would cause the wild-type UCE to accumulate at the cell surface. Methyl β-cyclodextrin, which extracts cholesterol from cell membranes (Kilsdonk et al. (1995) has been shown by Rodal et al. (1999) and Subtil et al. (1999) to inhibit clathrin-coated vesicle-mediated endocytosis of transferrin receptor. When the mouse L-cells expressing wild-type human UCE were preincubated with methyl β-cyclodextrin before assaying the distribution of UCE activity, the results shown in Figure 3 were obtained. Although the total UCE activity in cells was unchanged by methyl β-cyclodextrin treatment, the proportion of UCE activity at the cell surface rose to 40% of the total activity compared with 3% in untreated cells. This result indicates that inhibition of endocytosis at the plasma membrane has trapped UCE that has trafficked from the TGN to the plasma membrane. To answer the question whether the tyrosine-based internalization motif is used in normal cells to return plasma membrane UCE to the TGN, we turned again to MDBK cells.
Table 1.
Distribution of UCE activity in stably transfected L-cells
| Cell line | Total activity (nmol h−1 mg−1) | % Activity on cell surface | % Activity secreted |
|---|---|---|---|
| Parental L-cells | 5.2 | 1.6 | 1.2 |
| Wild-type UCEa | 47 | 0.84 | 0.55 |
| Y488A mutant UCEa | 77.5 | 63.4 | 3.9 |
| H510 stop mutant UCEa | 176 | 7.1 | 2.2 |
The results are averages of two separate experiments.
The contribution of L-cells has been subtracted.
Figure 3.
The effect of methyl β-cyclodextrin on surface expression of UCE. L-cells expressing wild-type human UCE were treated (+) or not (−) with 10 mM methyl β-cyclodextrin for 2 h before assaying for UCE activity with or without methyl β-cyclodextrin, and with Triton X-100 (total) or without Triton X-100 (cell surface) as described in MATERIALS AND METHODS. The activity of UCE is expressed as the percentage of total activity in the non-methyl β-cyclodextrin treated and is the average of two separate experiments.
Cycling of UCE from Plasma Membrane to the TGN
To demonstrate that native UCE cycles between the TGN and the plasma membrane, mAb directed against the luminal domain of bovine UCE was incubated with MDBK cells for 2 h at 37°C. The cells were washed and then processed for immunofluorescence with the use of secondary anti-mouse antibody conjugated to Alexa Fluor 488 (green), as well as double-labeled with a primary rabbit antibody to TGN 38 detected with a secondary anti-rabbit antibody conjugated to Alexa 594 (red). As shown in Figure 4, the two fluorescent probes colocalize, indicating that UCE at the plasma membrane has carried the anti-UCE antibody (a, green) to the TGN where endogenous TGN 38 (b, red) resides. Cells incubated for 2 h at 4°C with anti-UCE showed no uptake of antibody. Measurement of MDBK UCE activity, both total and cell surface, as described in Table 1 showed that 1.4% of the total UCE was present at the plasma membrane at steady state. This experiment shows that the normal traffic of UCE includes its movement from the TGN to the plasma membrane, followed by its internalization and return to the TGN.
Figure 4.
UCE cycles from the plasma membrane to the TGN. MDBK cells were incubated with antibody UC-1 for 2 h at 37°C, fixed, permeabilized, and probed with anti-mouse antibody (a, green) and also double labeled with rabbit antibody to TGN38 (b, red).
Intracellular Location of GFP-UCE Fusion Proteins
To determine whether the large luminal domain of UCE was involved in the trafficking of UCE and to obtain finer immunofluorescence images, wild-type and mutant constructs of UCE containing GFP in place of the luminal domain were prepared and expressed in HeLa cells.
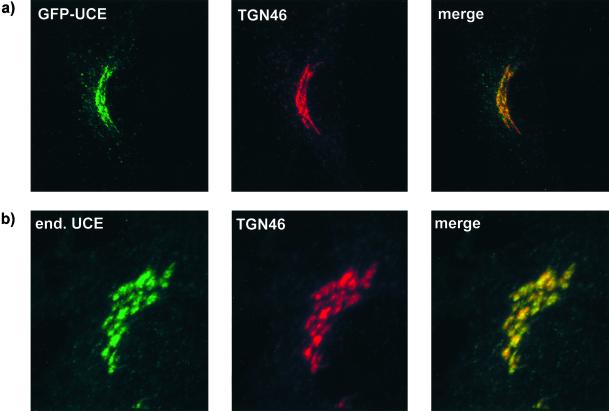
The structures of the various GFP-UCE TM-cytosolic tail constructs are shown in Figure 7. The construct containing the wild-type UCE TM-cytosolic tail was transfected into HeLa cells and a stably expressing clone (no. 9) was isolated. As shown in Figure 5, the GFP-UCE wild type in clone 9 cells as well as endogenous UCE in untransfected HeLa cells showed colocalization of UCE with endogenous TGN 46 in the trans-Golgi network. The colocalization of UCE and the GFP-UCE chimera indicates that the intracellular location of UCE is dictated by its transmembrane and cytosolic tail domains. To establish in which part of the Golgi apparatus the GFP-UCE resides, the experiment shown in Figure 6 was performed with stably transfected HeLa cells with the use of double labeling with an antibody to galactosyltransferase, a marker for the trans-Golgi cisternae. Figure 6a shows that the GFP-UCE and endogenous galactosyltransferase both appear in the Golgi region but, except for slight regions of overlap, do not colocalize. When BFA is added to the HeLa cells for 30 min (Figure 6b) the galactosyltransferase signal is dispersed into the reticular network of the ER as expected for a protein in the Golgi cisternae (Lippincott-Schwartz et al., 1991; Wood et al., 1991). In contrast the GFP-UCE disperses into a different, nonoverlapping network, characteristic of TGN fusion with the endosomal system. These results indicate that GFP-UCE resides in the TGN.
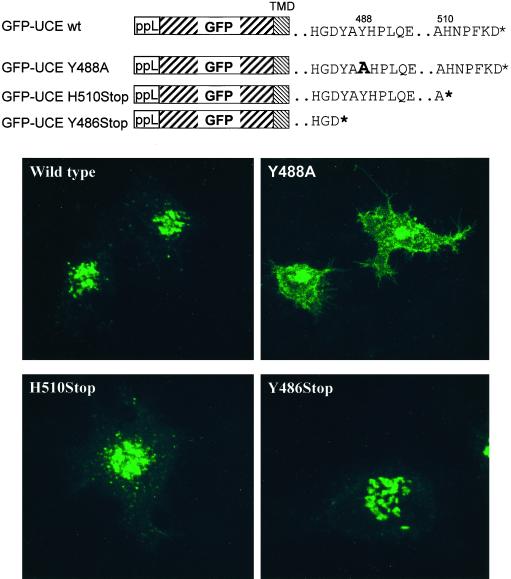
Figure 7.
The Y488A mutation in GFP-UCE inhibits its endocytosis from the plasma membrane. HeLa cells were transiently transfected with GFP-UCE TM-cytosolic tail constructs: wild type (wt), Y488A, H510Stop, or Y486Stop. The diagrams show the structures of these constructs: ppL is the preprolactin signal sequence followed by GFP, the transmembrane domain (TMD) of UCE, and the cytosolic tail of UCE as modified in the various mutants. The * indicates the end of the cytosolic tail.
Figure 5.
GFP-UCE and endogenous UCE both colocalize with TGN 46. (a) HeLa cells stably transfected with GFP-UCE-wild-type TM-cytosolic tail were fixed, permeabilized, and labeled with sheep anti-human TGN 46, followed by goat anti-sheep Alexa fluor 594. Left, GFP-UCE; middle, TGN 46; right, merged image. (b) Nontransfected HeLa cells were double labeled with rabbit anti-human UCE and sheep anti-human TGN 46, followed by goat anti-rabbit Alexa 488 and donkey anti-sheep Alexa 594, respectively. Left, UCE; middle, TGN 46; right, merged image.
To determine whether the internalization motifs in the cytosolic tail of the GFP-UCE altered its steady-state subcellular localization, the experiment shown in Figure 7 was carried out on HeLa cells transiently transfected with the wild-type and mutant constructs as depicted. GFP-UCE wild type is located in the TGN as before, the GFP-UCE Y488A is primarily located at the plasma membrane, and GFP-UCE H510stop is primarily in a TGN-like location. This indicates that the 488YHPL motif is required for endocytosis of GFP-UCE from the plasma membrane and return to the TGN, whereas deletion of the NPF motif has only a modest effect on this pathway. The intracellular distribution of the GFP-UCE TM-cytosolic tail construct faithfully duplicates the behavior of the intact UCE, further emphasizing the importance of the cytosolic YHPL motif in mediating return of UCE from the plasma membrane to the TGN. Interestingly, the mutant GFP-UCE Y486 stop in which the cytosolic tail was truncated before the 488YHPL endocytosis motif, was localized to the TGN. One might have expected this mutant to behave like the Y488A mutant, i.e., lacking a retrieval motif, to localize to the plasma membrane.
DISCUSSION
The findings presented in this study establish that the majority of UCE resides in the TGN at steady state. UCE colocalizes with TGN 38 in MDBK cells and TGN 46 in HeLa cells, whereas GFP-UCE does not colocalize with galactosyltransferase (a trans-Golgi marker) in HeLa cells. In addition, GFP-UCE redistributes into a distinct network after BFA treatment, differently from the redistribution of galactosyltransferase into the ER. These findings resolve a long-standing controversy over whether UCE is localized in the Golgi to the cis/middle or trans/TGN region based on various density gradient fractionation methods (Pohlmann et al., 1982; Deutscher et al., 1983; Goldberg and Kornfeld, 1983).
Although the majority of UCE is localized in the TGN, we found that a small but measurable amount (0.8–1.6%) of the enzyme is present at the cell surface at steady state. This cell surface enzyme recycles back to the TGN as shown by the ability of MDBK cells to internalize anti-UCE antibody from the cell surface and deliver it to the TGN. The YHPL motif in the cytosolic tail of UCE is critical for internalization since 63% of the enzyme activity of the Y488A mutant was found on the plasma membrane. Similarly the majority of the GFP-UCE construct with the Y488A mutation was localized to the cell surface. When L-cells expressing wild-type human UCE were treated with methyl β-cyclodextrin to inhibit endocytosis, 40% of the UCE accumulated at the cell surface, confirming that the enzyme cycles from the TGN to the plasma membrane. The UCE in which the NPFKD motif was deleted from the C terminus (H510stop) showed some increased presence at the cell surface (7%), suggesting a slower rate of return to the TGN. The lack of any obvious distortion of the distribution of the GFP-UCE construct with the same mutation in HeLa cells suggests that the role of the NPFKD motif in endocytosis may be secondary.
The presence of the majority of UCE in the TGN suggests that the enzyme acts on its lysosomal hydrolase substrates in the TGN rather than in earlier parts of the Golgi. Kinetic studies of the biosynthesis of phosphorylated oligosaccharides in cells pulse labeled with 2-[3H]mannose and chased for various times showed that oligosaccharides bearing phosphodiesters (GlcNAc-P-Man) were made early and subsequently replaced by those bearing phosphomonoesters (Man 6-P), that is “uncovered” species (Goldberg and Kornfeld, 1981). However this approach did not reveal the compartment where uncovering of the diesters occurred. More recently two studies were published that support the idea that phosphomonoester formation occurs in a compartment beyond the BFA block (i.e., in the TGN). Radons et al. (1990) studied the oligosaccharides on the lysosomal enzyme cathepsin D and Sampath et al. (1992) studied the total cellular phosphorylated oligosaccharides in cells treated with BFA. Both groups found that in vivo the presence of BFA caused a diminished rate of oligosaccharide phosphorylation but an almost complete inhibition of phosphodiester uncovering. This result is compatible with the presence of phosphotransferase activity in the ER-Golgi intermediate compartment and the cis-Golgi (Lazzarino and Gabel, 1987; Dittmer and von Figura, 1999) and the presence of UCE in the TGN.
It may be advantageous to delay uncovering until the substrates arrive at the TGN to ensure that random phosphatase action in the secretory pathway cannot remove the phosphate groups exposed by UCE and render the lysosomal hydrolases unable to bind the MPRs. Furthermore, hydrolases in the TGN that fail to bind to a MPR because their phosphorylated oligosaccharides are not converted to monoesters could be included in Golgi exocytic vesicles along with UCE and travel to the plasma membrane. During this transit UCE could complete the uncovering of their phosphorylated oligosaccharides, and once released at the plasma membrane these lysosomal hydrolases could be recaptured by MPRs at the cell surface and carried to lysosomes. In fact it is known that 5–10% of hydrolases produced by fibroblasts traffic to lysosomes via secretion-recapture, although the basis for this alternative routing has not been established (Vladutiu and Rattazzi, 1979). This scenario also provides a rationale for the recycling pathway of UCE between the TGN and plasma membrane. Chapman and Munro (1994) have speculated that the endoprotease furin, which also cycles between the TGN and the plasma membrane, could continue to degrade its substrates in exocytic vesicles leaving the Golgi.
Both furin and TGN 38/TGN 46, like UCE, undergo endocytosis from the plasma membrane that is dependant on the presence of a tyrosine-based motif in their cytosolic domains (Reaves et al., 1993; Wong and Hong, 1993; Ponnambalam et al., 1994, 1996; Kain et al., 1998; Molloy et al., 1999). In the cases of TGN 38 and TGN 46 this motif is YQRL; in furin it is YKGL. These signals, like the YHPL of UCE, fit the YXXφ motif (where φ is a bulky hydrophobic residue) present in many receptors that are internalized in clathrin-coated vesicles at the plasma membrane because their YXXφ sequence is able to bind to the μ subunit of the plasma membrane adaptor complex AP2 (Ohno et al., 1995). The cytosolic domains of internalized membrane proteins contain other sorting signals in addition to a tyrosine-based signal that play roles in their subsequent intracellular trafficking pathway. Mallet and Maxfield (1999) have provided evidence that chimeric forms of furin and TGN 38 are transported from the plasma membrane to the TGN by distinct endosomal pathways. It may be that UCE and TGN 46 also traffic through different endosomal pathways in HeLa cells on their return to the TGN from the plasma membrane, because the two signals in the peripheral vesicles in double-labeled cells (e.g., as in Figure 5) when carefully compared do not show any colocalization, in contrast to their colocalization in the TGN (data not shown). In all probability, the numerous sorting signals in the cytosolic domain of furin, including, in addition to YKGL, its acidic cluster motif, which undergoes phosphorylation and dephosphorylation (Molloy et al., 1999), define its unique trafficking pathway. The fact that UCE contains a second known endocytosis motif in the NPFKD sequence (Tan et al., 1996) at its C terminus suggests that motif may also direct some sorting step in the pathway of UCE cycling between the TGN and the plasma membrane. The present results suggest that NPFKD plays a modest role in the rate of internalization at the plasma membrane. Further studies will be necessary to determine whether, for example, Eps 15, which can bind NPF motifs through its N-terminal EH domains (Salcini et al., 1997) and bind the α subunit of AP2 through its C terminus (Benmerah et al., 1996), may facilitate the interaction of the cytosolic domain of UCE with AP2 and the plasma membrane endocytosis machinery. The unexpected observation that the GFP-UCE Y486 stop mutant was retained in the TGN indicates that UCE, like TGN 38 (Ponnambalam et al., 1994), has a TGN retention signal in its transmembrane domain as well as a tyrosine-based retrieval signal in its cytosolic tail. It further suggests that what the Y486 stop mutant may lack is a TGN exit signal that allows UCE to move to the plasma membrane. Work is currently underway to explore this possibility. Interestingly, another class of Golgi proteins, namely, glycosyltransferases that are type II membrane-spanning proteins, contain Golgi retention signals in their transmembrane domains (Colley, 1997) but do not cycle to the plasma membrane. Thus, UCE is unique among the known oligosaccharide-modifying enzymes in possessing a complex intracellular trafficking pattern that appears to be related to its function in the biogenesis of lysosomes.
ACKNOWLEDGMENTS
We thank Wang Sik Lee for his help with the methyl β-cyclodextrin experiments and we thank Stuart Kornfeld and Anja Schweizer for suggestions and comments. This work was funded in part by National Institutes of Health grant CA08759 and a Professor Dr. Max Cloeffa fellowship to J.R.
Abbreviations used:
- BFA
brefeldin A
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- Man 6-P
mannose 6-phosphate
- mAb
monoclonal antibody
- MPRs
mannose 6-phosphate receptors
- PCR
polymerase chain reaction
- phosphotransferase
UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase
- TGN
trans-Golgi network
- UCE
“uncovering” enzyme
REFERENCES
- Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of α-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Berger EG, Aegerter E, Mandel T, Hauri HP. Monoclonal antibodies to soluble, human milk galactosyltransferase (lactose synthase A protein) Carbohydr Res. 1986;149:23–33. doi: 10.1016/s0008-6215(00)90366-5. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher SL, Creek KE, Merion M, Hirschberg CB. Subractionation of rat liver Golgi apparatus: separation of enzyme activities involved in the biosynthesis of the phosphomannosyl recognition marker in lysosomal enzymes. Proc Natl Acad Sci USA. 1983;80:3938–3942. doi: 10.1073/pnas.80.13.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer F, von Figura K. Phosphorylation of arylsulphatase A occurs through multiple interactions with the UDP-N-acetylglucosamine-1-phosphotransferase proximal and distal to its retrieval site by the KDEL receptor. Biochem J. 1999;340:729–736. [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Downs MA, Traub LM. Epsin binds to clathrin by associating directly with the clathrin terminal domain: evidence for cooperative binding through two discrete sites. J Biol Chem. 2000;275:6479–6489. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- Goldberg DE, Kornfeld S. The phosphorylation of β-glucuronidase oligosaccharides in mouse P388D cells. J Biol Chem. 1981;256:13060–13067. [PubMed] [Google Scholar]
- Goldberg DE, Kornfeld S. Evidence for extensive subcellular organization of asparagine-linked oligosaccharide processing and lysosomal enzyme phosphorylation. J Biol Chem. 1983;258:3159–3165. [PubMed] [Google Scholar]
- Hasilik A, Klein V, Waheed A, Strecker G, von Figura K. Phosphorylated oligosaccharides in lysosomal enzymes: identification of α-N-acetylglucosamine(1) phospho(6) mannose diester groups. Proc Natl Acad Sci USA. 1980;77:7074–7078. doi: 10.1073/pnas.77.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille-Rehfeld A. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim Biophys Acta. 1995;1241:177–194. doi: 10.1016/0304-4157(95)00004-b. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kain R, Angata K, Kerjaschki D, Fukuda M. Molecular cloning and expression of a novel human trans-Golgi network glycoprotein, TGN51, that contains multiple tyrosine-containing motifs. J Biol Chem. 1998;273:981–988. doi: 10.1074/jbc.273.2.981. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EPC, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Bao M, Brewer K, Noll C, Canfield WM. Purification and multimeric structure of bovine α-N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase. J Biol Chem. 1998;273:23203–23210. doi: 10.1074/jbc.273.36.23203. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Bao M, Brewer K, Noll C, Canfield W. Molecular cloning and functional expression of two splice forms of human α-N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase. J Biol Chem. 1999;274:32778–32785. doi: 10.1074/jbc.274.46.32778. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987;1:462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Lazzarino DA, Gabel CA. Biosynthesis of the mannose 6-phosphate recognition marker in transport-impaired mouse lymphoma cells: demonstration of a two-step-phosphorylation. J Biol Chem. 1987;263:10118–10126. [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and TGN suggest a general mechanism of regulating organelle structure, and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Mallet WG, Maxfield FR. Chimeric forms of furin and TGN 38 are transported from the plasma membrane to the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- Mullis KG, Ketcham CM. The synthesis of substrates and two assays for the detection of α-N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase (uncovering enzyme) Anal Biochem. 1992;205:200–207. doi: 10.1016/0003-2697(92)90424-6. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyataki S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Pohlmann R, Waheed A, Hasilik A, von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982;257:5323–5325. [PubMed] [Google Scholar]
- Ponnambalam S, Girotti M, Yaspo M-L, Owen CE, Perry ACF, Suganuma T, Nilsson T, Fried M, Banting G, Warren G. Primate homologues of rat TGN38: primary structure, expression and functional implications. J Cell Sci. 1996;109:675–685. doi: 10.1242/jcs.109.3.675. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S, Rabouille C, Luzio JP, Nilsson T, Warren G. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J Cell Biol. 1994;125:253–268. doi: 10.1083/jcb.125.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radons J, Isidoro C, Hasilik A. Brefeldin A prevents uncovering but not phosphorylation of the recognition marker in cathepsin D. Biol Chem Hoppe Seyler. 1990;371:567–573. doi: 10.1515/bchm3.1990.371.2.567. [DOI] [PubMed] [Google Scholar]
- Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt B vD, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Johnson KF, Kornfeld S. A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J Cell Biol. 1995;130:1297–1306. doi: 10.1083/jcb.130.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Minenkova O, Cesareni G, Pellicci PG, DiFiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;17:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, Varki A, Freeze HH. The spectrum of incomplete N-linked oligosaccharides synthesized by endothelial cells in the presence of brefeldin A. J Biol Chem. 1992;267:4440–4455. [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Bachi T, Ginsel L, Hauri HP. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemering KR, Golbik R, Sever R, Haseloff J. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr Biol. 1996;6:1653–1663. doi: 10.1016/s0960-9822(02)70789-6. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Kornfeld S. Biosynthetic intermediates of β-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980;255:6633–6639. [PubMed] [Google Scholar]
- Tan PK, Howard JP, Payne GS. The sequence of NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1789–1800. doi: 10.1083/jcb.135.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Kornfeld S. Identification of rat liver α-N-acetylglucosaminyl phosphodiesterase capable of removing “blocking”: α-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J Biol Chem. 1980;255:8398–8401. [PubMed] [Google Scholar]
- Varki A, Kornfeld S. Purification and characterization of rat liver α-N-acetylglucosaminyl phosphodiesterase. J Biol Chem. 1981;256:9937–9943. [PubMed] [Google Scholar]
- Varki A, Sherman W, Kornfeld S. Demonstration of the enzymatic mechanisms of α-N-acetyl-d-glucosaminidase (formerly called α-N-acetylglucosaminylphosphodiesterase) and lysosomal α-N-acetylglucosaminidase. Arch Biochem Biophys. 1983;222:145–149. doi: 10.1016/0003-9861(83)90511-8. [DOI] [PubMed] [Google Scholar]
- Vladutiu GD, Rattazzi M. Excretion-reuptake route of β-hexosaminidase in normal and I-cell disease cultured fibroblasts. J Clin Invest. 1979;63:595–601. doi: 10.1172/JCI109341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A, Hasilik A, von Figura K. Processing of the phosphorylated recognition marker in lysosomal enzymes: characterization and partial purification of a microsomal α-N-acetylglucosaminyl phosphodiesterase. J Biol Chem. 1981;256:5717–5721. [PubMed] [Google Scholar]
- Wong SH, Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]