Abstract
Background:
Gastric carcinoma is the second most common cause of cancer-related death in Iran. It is well-known that atrophic gastritis is a major risk factor for gastric cancer, which leads to variations in the serum levels of gastrin 17 (G-17), pepsinogen I (P-I), and pepsinogen II (P-II). The aim of this study was to investigate the diagnostic accuracy of these serum biomarkers in the early detection of atrophic gastritis.
Materials and Methods:
A total of 132 dyspeptic patients underwent upper endoscopy and biopsies were taken. The biopsy specimens were evaluated as the gold standard according to operative link for gastritis assessment staging system. Serum levels of G-17, P-I, and P-II were investigated using enzyme-linked immunosorbent assay. Receiver operating characteristic (ROC) analysis was used to calculate the diagnostic indices and optimal cut-off values using Statistical Package for the Social Sciences SPSS statistical software.
Results:
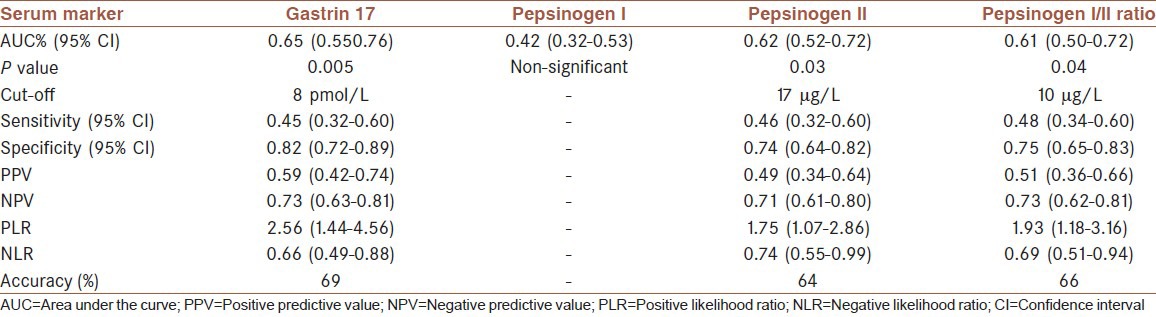
A total of 67 men and 65 women were analyzed, among which 48 (36.4%) had atrophic gastritis. The mean age was 45.8 (±15.8) years. ROC curve analysis demonstrated that the biomarkers (including pepsinogen I/II [P-I/II] ratio), except for P-I, are diagnostically significant in detecting gastric atrophy. The area under the curve (95% confidence interval [CI]) for G-17, P-I, P-II, and P-I/II ratio were 0.65 (0.55-0.76), 0.42 (0.32-0.53), 0.62 (0.52-0.72), and 0.61 (0.50-0.72), respectively. However, the diagnostic indices were low (sensitivity <50%, specificity < 90%). The prevalence of Helicobacter pylori infection was significantly higher in patients with atrophy against those without atrophy (75.0% vs. 57.4%, P value < 0.0001).
Conclusion:
In the studied population, the serum biomarkers of atrophic gastritis are not useful screening tests due to their low sensitivity.
Keywords: Atrophic gastritis, dyspepsia, gastrin 17, pepsinogen I, pepsinogen II
INTRODUCTION
Gastric carcinoma is the second most common cause of cancer-related death in Iran.[1] The most important advancement in the epidemiology of gastric adenocarcinoma is the identification of its relationship with Helicobacter pylori infection which has been reported by several well-designed cohort studies. Persistent H. pylori infection usually results in chronic gastritis followed by gastric atrophy, metaplasia, dysplasia, and subsequently malignancy. Therefore, atrophic gastritis is an extremely important precancerous phenomenon and its early diagnosis is essential in order to stop its progress by performing prompt treatment and surveillance.[2,3,4]
Atrophic gastritis is defined as a loss in the gastric glands that logically leads to a reduction in their productions. For instance, corpus and antral atrophy mainly affects pepsinogen I (P-I) and gastrin secretion, respectively. In addition, P-II is released from all parts of the stomach and impacted wherever the atrophy occurs.[4]
Recent studies have shown that decreased serum levels of these biomarkers may be valuable in screening for gastric atrophy.[5,6,7,8] Moreover, these serological methods are easy, inexpensive, and noninvasive compared to other routine methods such as endoscopy and histologic investigations.
There is a well-known diversity in gastric cancer epidemiology between different geographical areas. For instance, the relative risk (95% CI) of gastric atrophy for gastric cardia cancer is 2.72 (1.67-4.44) and 3.07 (1.95-4.83) for studies from Asia and Europe/the USA, respectively.[9] Bearing in mind these differences, we hypothesized that the adequacy of serum biomarkers of gastric atrophy may also show geographical difference. To our knowledge, only two relevant studies had been conducted in Iran. In the first, Haj-Sheykholeslami et al. concluded that the biomarkers are not useful for determining premalignant lesions, which contrasts with the general universal expectation.[10] In the more recent study, Nasrollahzadeh et al. investigated fundic atrophy and non-atrophic pan-gastritis and found the biomarkers as stable screening tests.[11] These opposing findings clarify that the topic of serum biomarkers of gastritis is still a point of debate in the Iranian population. Therefore, this study aimed to investigate the diagnostic accuracy of serum biomarkers in detecting atrophic gastritis in Iranian dyspeptic patients living in North-East Iran.
MATERIALS AND METHODS
Samples
A total of 132 consecutive dyspeptic patients entered the study. The patients were referred to the gastroenterology outpatient clinic of Ghaem University Hospital in Mashhad, Iran, during 2010. All the participants were dyspeptic patients in need of an upper endoscopic investigation and gastric biopsy. They were excluded if they had any progressive systemic disease or previous gastrectomy. The use of proton pump inhibitors and H2 blockers were prohibited 1 week and 48 h before endoscopy, respectively. Regarding the operative link for gastritis assessment (OLGA) proposal, five biopsy samples were taken from the greater and lesser curvatures of the distal antrum, the lesser curvature at the incisura angularis, and the anterior and posterior walls of the proximal corpus.
The study was conducted according to the Ethical Standards Committee of Mashhad University of Medical Sciences, which complies with the provisions of the World Medical Association's Declaration of Helsinki. Mashhad University of Medical Sciences supported and financed the project (#89083). Informed consents were obtained from the study participants.
Procedures
Endoscopic biopsy specimens were fixed in 10% formalin, embedded in paraffin, cut in serial sections, and stained with hematoxylin and eosin. The histologic examination was performed by a well-experienced pathologist as the gold standard method of detecting atrophy. The pathologist was single blinded and not informed of the patients’ serum marker levels. The OLGA protocol was used to define and score the severity of atrophy. In OLGA staging, the atrophy could be either metaplastic or non-metaplastic.[12]
After fasting, 5 ml of blood was obtained from the brachial vein centrifuged, and serum samples were analyzed for gastrin 17 (G-17), P-I, P-II, and anti-helicobacter antibody. Whenever storing was necessary, the samples were kept in −20°C. All measurements were carried out using enzyme-linked immunosorbent assay kits purchased from Biohit (Helsinki, Finland).
Statistical analysis
The Kolmogorov–Smirnov and Levene tests were applied to verify normal distribution and the equality of variances, respectively. Student t-test or its nonparametric counterpart (Mann–Wittney U-test) was used to compare the mean among groups. Chi-square test was performed to find the associations between qualitative variables. Receiver operating characteristic (ROC) analysis was used to calculate the diagnostic indices and optimal cut-off values for G-17, P-I, P-II, and P-I/II ratio. All analyses were performed using SPSS v. 13 (SPSS Inc, Chicago, USA). The significance level was set at 0.05.
RESULTS
A total of 67 men (50.8%) and 65 women (49.2%) entered the study. The mean age (±SD) of the study patients was 45.8 (±15.8) years, ranging from 17 years to 82 years.
Histologic examination showed that 48 (36.4%) patients had atrophic gastritis, among which 22 had concurrent metaplasia, mostly in the antrum (81.8%). The antral atrophy and pan-atrophy (involving all parts of the stomach) were seen in 79.2% and 18.8% of the patients, respectively. Only one patient (2.1%) had funus/corpus-predominant atrophy and was excluded from the analysis. The percentage of involved patients illustrated a decreasing trend as the OLGA stage increased (58.3%, 27.1%, 8.3%, and 6.3% in stages I, II, III, and IV, respectively).
The average age of patients with atrophy was higher than that of patients without atrophy (53.7 years vs. 41.3 years, P = 0.001). Thus, there was a positive association between age and atrophy. No significant association between gender and atrophy was detected. The mean (±SD) serum levels of G-17, P-I, P-II, and the P-I/II ratio were 13.1 ± 6.1, 162.2 ± 44.8, 22.0 ± 10.6, and 9.2 ± 5.7, respectively. Summary statistics for serum biomarkers by different clinicopathologic conditions are shown in Table 1.
Table 1.
Serum level of some markers according to different status of patients
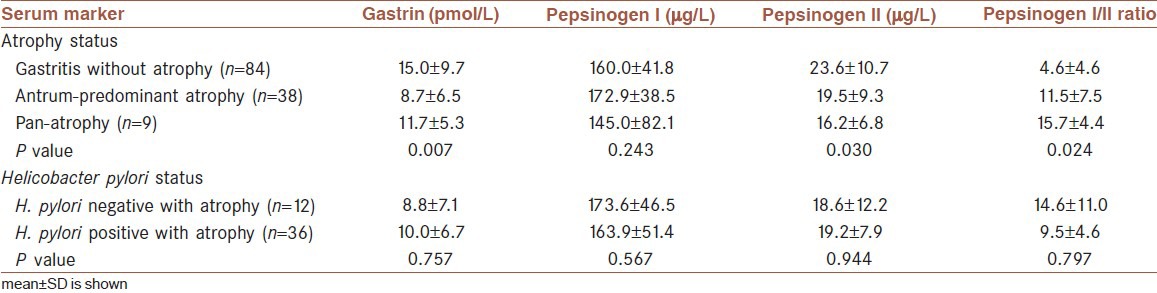
Comparing the mean levels of serum biomarkers in atrophic and non-atrophic patients, there was no significant difference in P-I between the two groups, whereas the differences were significant for G-17, P-II, and P-I/II (P = 0.005, P = 0.02, and P = 0.04, respectively).
Helicobacter pylori infection
The prevalence of H. pylori infection was 57.4%. Patients with atrophy were significantly more affected with the infection (36 of 48, P = 0.0001). There was also a significant positive association between the severity of infection and atrophy (P = 0.001), as well as between the severity of infection and metaplasia (P = 0.035).
In H. pylori negative patients, the mean (±SD) serum levels of G-17, P-I, P-II, and the P-I/II ratio were 14.2 ± 9.6, 161.6 ± 42.4, 23.0 ± 11.0, and 9.1 ± 6.0, respectively. There were no significant differences between infected and non-infected patients in different serum biomarkers. Moreover, there were no significant differences between infected and noninfected patients with atrophy in terms of different biomarkers.
Receiver operating characteristic curve analysis
ROC curves were drawn for G-17, P-I, P-II, and P-I/II ratio [Figure 1]. The results of ROC analysis and the corresponding diagnostic indices are summarized in Table 2.
Figure 1.
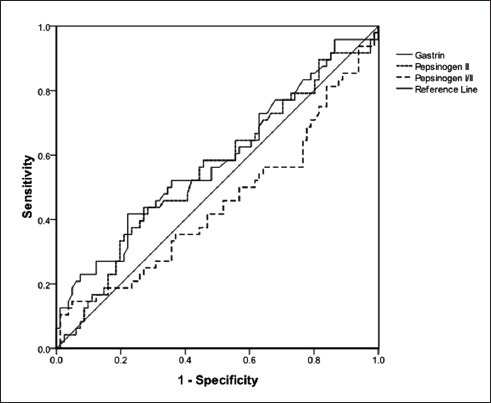
Receiver operating characteristic curves generated with gastrin 17 (line), pepsinogen II (upper dashed line), and pepsinogen I/II ratio (lower dashed line) for detecting gastric atrophy in dyspeptic patients
Table 2.
Summary of the receiver operating characteristic curve analysis for atrophy identification by the serum biomarkers
DISCUSSION
In the studied population, there was a significant association between gastric atrophy and serum levels of P-II, P-I/II ratio, and G-17. However, P-I serum level was not associated with atrophy.
Cao et al., investigating 458 participants in China, found similar results regarding G-17 and P-I/II, but not P-II. On the contrary, they reported a meaningful relationship for P-I serum level.[13] Likewise, Pimenov and coworkers from Russia reported a negative correlation between the severity of gastric atrophy and serum P-I levels, as well as P-I/II ratio. However, their result was not statistically significant for G-17.[14]
In two other studies, one by Germana et al. from Italy and the other by Storskrubb et al. from Sweden, the investigators reported P-I, P-II, P-I/II, and G-17 as powerful diagnostic tests for early detection of atrophy, which was in accordance with our results except for P-I serum level.[7,15]
The dissimilarity among these findings might be the result of differences in methods used for selection of participants and/or the possible differences in the severity of atrophy among the patients.
Most of the studies have reported that P-I serum level is associated with atrophy, which is in contrast with our results. This may be due to the small size of the participants with pan-atrophy or fundus/body-predominant atrophy in the current study. The only similar study conducted on Iranian population is carried out by Haj-Sheykholeslami et al. in Tehran, the capital of Iran. They investigated 481 first-degree relatives of gastric cancer patients, as a high-risk population. They demonstrated that the patients with gastric atrophy had higher P-II and lower P-I/II levels compared to normal ones. Nonetheless, P-I and G-17 were not significantly different between groups, except for a higher G-17 in patients with corpus atrophy. They also reported similar findings for metaplasia.[10] Their study was mostly concentrated on gastric inflammation and corpus-predominant atrophy and metaplasia, whereas our study was mainly focused on antral atrophy. In fact, only one corpus-predominant and nine pan-atrophic specimens were present in the current study. In addition, all the patients in our study had some degree of gastritis. In addition, their patients were asymptomatic relatives of gastric cancer patients, but our patients were all nonrelative dyspeptic patients from the general population.
The overall accuracy of G-17, P-II, and P-I/II ratio in discriminating atrophic from non-atrophic specimens was 69%, 64%, and 66%, respectively. Similar studies show controversies and some geographical variations. For instance, in a study from Japan, Urita et al. reported a sensitivity of 72% and a specificity of 67% in detecting intestinal metaplasia among participants,[16] whereas in another study from Japan, by Ijima et al., high accuracy, sensitivity, and specificity (94%, 95%, and 93%, respectively) in discriminating atrophy have been reported.[17] The higher diagnostic indices in the Ijima's study were possibly because of the more advanced technology used for the detection of the biomarkers’ serum levels.
Storskrubb et al. from Sweden reported a sensitivity and specificity of 71% and 98%, respectively.[7] In another study, Zhang et al. from China reported that a combination of serum anti-Helicobacter antibody, gastrin, and pepsinogen has a sensitivity and specificity of 87.5% and 100%, respectively.[8]
On the contrary, some researchers have found non-significant or weak results. Among them is a study by Ricci et al. from Italy in which they reported that serum gastrin (total and G-17) and P-I and P-II (and their ratio) are not able to discriminate patients without atrophy from those with antrum-predominant atrophic gastritis. Likewise, Colarossi et al. from Peru demonstrated that none of the biomarkers could discriminate between patients with and without atrophy.[18] These findings are in contrast with our results as well as most of other studies.[19] This discrepancy is probably related to the selection of participants who have been chosen among asymptomatic patients. In another study by Sita et al. from the United Kingdom, a very low sensitivity of about 20% was reported, which renders the biomarkers practically useless.[20]
Regarding the studies from Iran, including our study in Mashhad and those by Haj-Sheykholeslami et al. in Tehran, it appears that the sensitivities of G-17, P-II, and P-I/II in the Iranian population are lower than that in other studies from different geographical areas (lower than 50%). Thus, these biomarkers may not be adequate screening tests for practical purposes in the Iranian population. In a more recent study from Iran, Nasrollahzadeh et al. investigated fundic atrophy. They found P-I (sensitivity: 61.9%, specificity: 94.8%) and P-I/II ratio (sensitivity: 75.0%, specificity: 91.0%) are useful in screening fundic atrophy. They also found that P-II was 84.2% sensitive and 45.4% specific to distinguish non-atrophic pan-gastritis. Their study lacks diagnostic data on antral atrophy.[11]
In the current study, the mean serum levels of all the studied biomarkers were higher than the expected levels provided by the manufacturer of the testing kit. This difference may be due to nutritional or genetic differences between Iranian and the manufacturer's population. Moreover, all of our patients had gastric inflammation which has a positive effect on serum levels of these biomarkers, as indicated by Haj-Sheykholeslami et al.[10]
There was a high prevalence of H. pylori infection (>50%) among dyspeptic patients, which was in accordance to the results of other studies.[1,5,8,20,21] However, the prevalence reported by Cao and colleagues from China was much higher (>85%), which may be partly related to their investigated subjects who were patients with ulcer, gastric atrophy, or cancer.[13] The prevalence of H. pylori infection among atrophic patients in the current study was about 80%, which is similar to the Cao et al. report.[13] There was also a significant positive relationship between the stage of metaplasia or atrophy and the severity of H. pylori infection. Many other studies confirm this finding.[12,20] In our study, there were no significant associations between H. pylori infection and different serum biomarkers.
CONCLUSION
P-I, P-II, P-I/II ratio, and G-17 are potential serologic biomarkers for screening atrophic gastritis, but convincing evidence is not still available to support their routine clinical use among the Iranian population.
This study lacks information about the fundal-type atrophy. It is justified to conduct similar studies including fundal-type atrophy.
Footnotes
Source of Support: Mashhad University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: Epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 2.Fleming SL. 1st ed. New York, USA: InfoBase Publishing; 2007. Helicobacter pylori; pp. 85–8. [Google Scholar]
- 3.Lee EL, Feldman M. Gastritis and gastropathies. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 9th ed. Philadelphia: Sanduers; 2010. pp. 845–8. [Google Scholar]
- 4.Washington MK, Peek RM. Gastritis and gastropathy. In: Yamada T, Alper DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 5th ed. Chichester, UK: Wiley-Blackwell; 2009. pp. 1005–25. [Google Scholar]
- 5.Valle Muñoz J, Artaza Varasa T, López Pardo R, Rodríguez Merlo R, Pérez Grueso MJ, Martín Escobedo R, et al. Serological diagnosis of atrophic gastritis with a combination of pepsinogen I and II, gastrin-17 and anti-Helicobacter pylori antibodies. Gastroenterol Hepatol. 2007;30:567–71. doi: 10.1157/13112584. [DOI] [PubMed] [Google Scholar]
- 6.Day DW, Jass JR, Price AB, Shepherd NA, Sloan JM, Talbot NJ, et al. Gastritis and related conditions. In: Day DW, Jass JR, Price AB, Shepherd NA, Sloan JM, Talbot NJ, editors. Morson and Dawson's Gastrointestinal Pathology. 4th ed. Massachusetts, USA: Blackwell Science Ltd; 2003. pp. 104–131. [Google Scholar]
- 7.Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, Talley NJ, et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43:1448–55. doi: 10.1080/00365520802273025. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Sun L, Gong YH, Wang XG, Zhang M, Yuan Y. Factors affecting the serum gastrin 17 level: An evidence-based analysis of 3906 serum samples among Chinese. J Dig Dis. 2007;8:72–6. doi: 10.1111/j.1443-9573.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 9.Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma: A systematic review and meta-analysis. Ann Oncol. 2011;22:754–60. doi: 10.1093/annonc/mdq411. [DOI] [PubMed] [Google Scholar]
- 10.Haj-Sheykholeslami A, Rakhshani N, Amirzargar A, Rafiee R, Shahidi SM, Nikbin B, et al. Serum pepsinogen I, pepsinogen II, and gastrin 17 in relatives of gastric cancer patients: Comparative study with type and severity of gastritis. Clin Gastroenterol Hepatol. 2008;6:174–9. doi: 10.1016/j.cgh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Shakeri R, Persson EC, Islami F, et al. Accuracy and cut-off values of pepsinogens I, II and gastrin 17 for diagnosis of gastric fundic atrophy: Influence of gastritis. PLoS One. 2011;6:e26957. doi: 10.1371/journal.pone.0026957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, et al. OLGA staging for gastritis: A tutorial. Dig Liver Dis. 2008;40:650–8. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Cao Q, Ran ZH, Xiao SD. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis. 2007;8:15–22. doi: 10.1111/j.1443-9573.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 14.Pimenov SI, Makarenko EV. Potentialities of a serological method in diagnosis of atrophic gastritis. Ter Arkh. 2008;80:15–21. [PubMed] [Google Scholar]
- 15.Germaná B, Di Mario F, Cavallaro LG, Moussa AM, Lecis P, Liatoupolou S, et al. Clinical usefulness of serum pepsinogens I and II, gastrin-17 and anti-Helicobacter pylori antibodies in the management of dyspeptic patients in primary care. Dig Liver Dis. 2005;37:501–8. doi: 10.1016/j.dld.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Urita Y, Hike K, Torii N, Kikuchi Y, Kanda E, Sasajima M, et al. Serum pepsinogens as a predicator of the topography of intestinal metaplasia in patients with atrophic gastritis. Dig Dis Sci. 2004;49:795–801. doi: 10.1023/b:ddas.0000030091.92379.91. [DOI] [PubMed] [Google Scholar]
- 17.Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, Sipponen P, et al. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853–9. doi: 10.3748/wjg.15.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colarossi A, Inga R, Prochazka R, Reyes U, Bussalleu A, Barúa RL. Pepsinogen and gastrin in the noninvasive diagnosis of gastric atrophy. A case-control study in Peruvian population. Rev Gastroenterol Peru. 2011;31:110–5. [PubMed] [Google Scholar]
- 19.Ricci C, Vakil N, Rugge M, Gatta L, Perna F, Osborn JF, et al. Serological markers for gastric atrophy in asymptomatic patients infected with Helicobacter pylori. Am J Gastroenterol. 2004;99:1910–5. doi: 10.1111/j.1572-0241.2004.40614.x. [DOI] [PubMed] [Google Scholar]
- 20.Sitas F, Smallwood R, Jewell D, Millard PR, Newell DG, Meuwissen SG, et al. Serum anti-Helicobacter pylori IgG antibodies and pepsinogens A and C as serological markers of chronic atrophic gastritis. Cancer Epidemiol Biomarkers Prev. 1993;2:119–23. [PubMed] [Google Scholar]
- 21.Sipponen P, Ranta P, Helske T, Kääriäinen I, Mäki T, Linnala A, et al. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: An observational case-control study. Scand J Gastroenterol. 2002;37:785–91. [PubMed] [Google Scholar]


