Human herpesvirus infections are generally asymptomatic in immune-competent hosts; however, immune dysfunction can unveil inherent oncogenic properties associated with Epstein-Barr virus (EBV) and Kaposi’s sarcoma herpesvirus (KSHV) infection. Although these viruses were first discovered in human tumor specimens in 1964 and 1994, respectively, it was discovered later on that large proportions of the human population are persistently infected with EBV and to a lesser extent KSHV. To colonize immune-competent hosts, EBV and KSHV drive proliferation of newly infected cells by latent transcription programs. EBV infection is principally limited to B cells, and few infected epithelial cells in the oral mucosa that produce infectious virus for host–host transmission via the saliva. In contrast, KSHV has a broader spectrum of target cells while transmission and life cycle are less well understood. However, these viruses share a host-colonization and persistence strategy that involves activation of NF-κB, a critical survival factor for developing human B cells. Deregulated NF-κB activation can cause uncontrolled B-cell proliferation and secretion of proinflammatory factors that support cancer development. Apart from protumorigenic soluble factors, many tumor cells release endosome-derived vesicles, called exosomes, which mediate intercellular communication. In PNAS, Meckes et al. describe that EBV- and KSHV-infected lymphoma cells secrete exosomes with distinct repertoires of cellular proteins (1). In light of recent studies demonstrating that tumor cell-derived exosomes exert protumorigenic signaling properties (2), the data presented by Meckes et al. suggest that virus and tumor cells share common strategies in shaping a permissive microenvironment by modulating the cargo of secreted exosomes (Fig. 1).
Fig. 1.
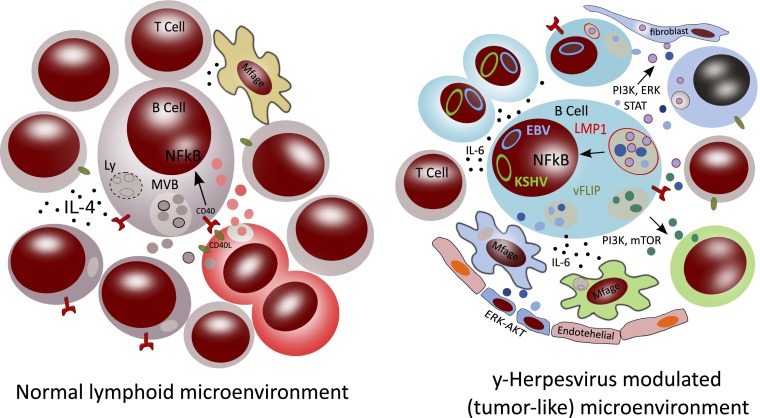
Possible effects on the human B-cell lymphoid microenvironment by EBV- and KSHV-modified exosomes. In this schematic representation of “normal” B-cell activation, the left image represents a human B-cell that is activated by CD40 ligand (CD40L) carrying T cells. This leads to NF-κB transcription (black arrow) in the nucleus of the B cells, which is also a signal for the production and release of IL-4 and exosomes from specialized endosomes known as MVBs (16). The released B cell exosomes may be internalized by other cell types, such as macrophages, or could “decorate” T cells by binding to the surface. In the B-/T-cell contact area (immunological synapse), responding T cells polarize and may proliferate, secreting exosomes that seem to be preferentially internalized by the activated B cells (17). The right schematic represents a coinfected B cell carrying latent EBV and KSHV (represented by the circular blue and green DNA episomes in the nucleus, respectively). EBV-encoded LMP1 and KSHV-encoded vFLIP activate NF-κB without the external involvement of T cells (6). LMP1 localizes to the limiting membrane of MVBs (represented in red), and presumably activates NF-κB from this site. As a consequence, LMP1 can be sorted into ILVs inside these compartments. Besides LMP1-associated vesicles, distinct types of vesicles carrying different proteins, lipids, or RNAs may also be produced (indicated by light and dark blue color variations). It is currently unknown whether the trafficking, localization, and intracellular sorting of vFLIP follows a similar path as LMP1. The infected cells with activated NF-κB produce IL-6, which serves as an autocrine growth factor promoting B-cell proliferation and could contribute to a sustained positive inflammatory feedback loop (18). The infected B cells release virus-modified exosomes, of which some carry LMP1 (indicated with a red membrane) when derived from LMP1+ MVBs. These exosomes can activate signaling PI3K, ERK, and STAT signaling pathways in target cells, either upon internalization or alternatively by activating surface receptors on the target cell (12). Both EBV and KSHV impact the proteome of the produced exosomes that are distinct from exosomes produced by noninfected counterparts (represented as brown vesicles in the left) that may primarily function in immune cell–cell interactions. However, the EBV and KSHV vesicles (indicated by blue and green vesicles, respectively) are suspected to render the behavior of distinct target cells in the vicinity, including endothelial cells, macrophages, and T cells that, as a whole, provide a permissive environment for virus-infected tumor cells.
Because KSHV and EBV are lymphotropic gamma herpesviruses associated with B-cell lymphomas, similar mechanisms may underlie their oncogenicity. EBV infects naïve resting B cells in vitro, leading to immortalized proliferating lymphoblastiod cell lines (LCLs), but KSHV in isolation does not immortalize lymphocytes. Only B cells from EBV-infected hosts and subsequent KSHV infection can promote LCLs that harbor both viral genomes (3). Thus, the KSHV latency program is not immortalizing in vitro and presumably requires one or more cofactors to transform B cells. Studies in transgenic mouse models showed that the EBV-encoded latent-membrane protein 1 (LMP1) is critical for B-cell lymphoma development (4), but FLICE-inhibitory protein (vFLIP) acts as an important driver of KSHV-induced lymphomagenesis (5). Both proteins constitutively activate NF-κB (6), the function of which is frequently deregulated in human cancer-mediating prosurvival functions and tumor-associated inflammation (7).
So how can it be that 90% of the world population carries EBV apparently without problems, and few will develop EBV-driven B-cell lymphomas? Interestingly, LMP1 itself also activates an immune surveillance mechanism in B cells, as if EBV evolved to protect its host from developing lymphomas (4). One EBV-persistence model suggests that the main function of LMP1 in EBV-persistence is to mimic CD40 signaling, a protein crucial for timely transition of proliferating B cells, through a germinal center reaction, into memory B cells (8). This model is consistent with the finding that LMP1 modulation of NF-κB is governed by the germinal center-specific TNF receptor-associated factor 2 and NF-κB–inducing kinase (9). A crucial disparity between CD40 and LMP1 is that CD40-signaling in B cells is controlled by an external ligand expressed on T cells (CD40L). LMP1 lacks such a regulatory element and, in addition, the dominant pool of LMP1 protein in LCL localizes to late endosomes (10). LMP1 is sorted into intraluminal vesicles (ILVs), the presumed precursors of exosomes that are formed within multivesicular bodies (MVBs) (11). Meckes et al. previously demonstrated that release of LMP1 via exosomes can activate critical signaling pathways in uninfected target cells, suggesting important messenger functions of virus-modified exosomes (12).
In their latest work, Meckes et al. aim to decipher how EBV and KSHV modulate the proteome carried by exosomes and whether these viruses have a similar or distinct impact that could give new clues into the physiological function of herpesvirus-modified exosomes (1). To this end, the exosomal proteome of 10 EBV and KSHV (co)infected B-cell lines was determined using mass-spectrometry, leading to the identification of 871 proteins, many of them shared between all cell-types, as expected. However, when the exosomal-protein profiles of uninfected B cells were compared with exosomes from virus-infected cells, interesting observations could be made with computational analysis. EBV and KSHV influence the proteome of B-cell exosomes in largely similar fashion, suggesting the virus-modified exosomes have a role in activating signaling pathways associated with cell death and survival, ribosome function, protein synthesis, and mammalian target of rapamycin (mTOR) signaling. KSHV-modified exosomes seemed most likely to impact the cellular metabolism of recipient cells, but EBV exosomes may be better equipped to activate cellular signaling pathways in target cells.
Focusing on viral factors that may influence the exosomal proteome, perhaps not
Meckes et al. aim to decipher how EBV and KSHV modulate the proteome carried by exosomes.
surprisingly, LMP1-expressing cells secreted EBV-modified exosomes with a defined protein cargo, as judged by computational analysis. Although the proteomic studies suggest that virus-infected cells modulate
the proteome of exosomes, the assumption that KSHV and EBV exosomes as a consequence transmit distinct messages to neighboring or distal cells needs further confirmation. It would be interesting to know whether LMP1, for example by affecting membrane domain formation or through direct associations, influences the exosomal proteome, as may be expected from recent proteomic studies focusing on HLA-DR (13). In addition, could it be that host–pathogen interactions mediated by exosomes are largely restricted to persistent viral infections? Importantly, besides proteins, exosomes consist of lipids and carry RNA molecules, including (viral) microRNAs and presumably messenger RNA (12, 14, 15). In this regard the true properties of exosomes are ideally studied in situ, as it is most likely the combinatorial effect of exosome-associated material that dictates how various target cells may respond (11). Unfortunately for EBV, this may prove a daunting task, because accurate animal models for herpesvirus infections do not exist and sensitive tools for studying exosomes' communication in vivo are still lacking.
Despite current limitations, the work by Meckes et al. (1) in PNAS represents a significant step into the deciphering of the mixed signals that are transmitted by exosomes produced in virus-infected tumor cells. Although the convergence of viral and exosomal pathways has been proposed previously, our knowledge on the function of virus-modified exosomes in vivo remains at the beginning stages. Herpesvirus coevolution with the human host may have been the driving force behind exosome-cargo modifications in order to shape a microenvironment that supports viral persistence. However, tumor cells infected with EBV/KSHV may exploit the properties of virus-modulated exosomes to establish a tumor-permissive microenvironment.
Footnotes
The author declares no conflict of interest.
See companion article on page E2925.
References
- 1.Meckes DG, Jr, et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci USA. 2013;110:E2925–E2933. doi: 10.1073/pnas.1303906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliche S, Kremmer E, Hammerschmidt W, Koszinowski U, Haas J. Persistent infection of Epstein-Barr virus-positive B lymphocytes by human herpesvirus 8. J Virol. 1998;72(10):8143–8149. doi: 10.1128/jvi.72.10.8143-8149.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell. 2012;148(4):739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballon G, Chen K, Perez R, Tam W, Cesarman E. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. J Clin Invest. 2011;121(3):1141–1153. doi: 10.1172/JCI44417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oliveira DE, Ballon G, Cesarman E. NF-kappaB signaling modulation by EBV and KSHV. Trends Microbiol. 2010;18(6):248–257. doi: 10.1016/j.tim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roughan JE, Thorley-Lawson DA. The intersection of Epstein-Barr virus with the germinal center. J Virol. 2009;83(8):3968–3976. doi: 10.1128/JVI.02609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shkoda A, et al. The germinal center kinase TNIK is required for canonical NF-κB and JNK signaling in B-cells by the EBV oncoprotein LMP1 and the CD40 receptor. PLoS Biol. 2012;10(8):e1001376. doi: 10.1371/journal.pbio.1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verweij FJ, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011;30:2115–2129. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Meckes DG, Jr, et al. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA. 2010;107(47):20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buschow SI, et al. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88(8):851–856. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- 14.Jochum S, Ruiss R, Moosmann A, Hammerschmidt W, Zeidler R. RNAs in Epstein-Barr virions control early steps of infection. Proc Natl Acad Sci USA. 2012;109(21):E1396–E1404. doi: 10.1073/pnas.1115906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arita S, et al. B cell activation regulates exosomal HLA production. Eur J Immunol. 2008;38(5):1423–1434. doi: 10.1002/eji.200737694. [DOI] [PubMed] [Google Scholar]
- 17.Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA. 2011;108(4):1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]